Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Cytidine monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
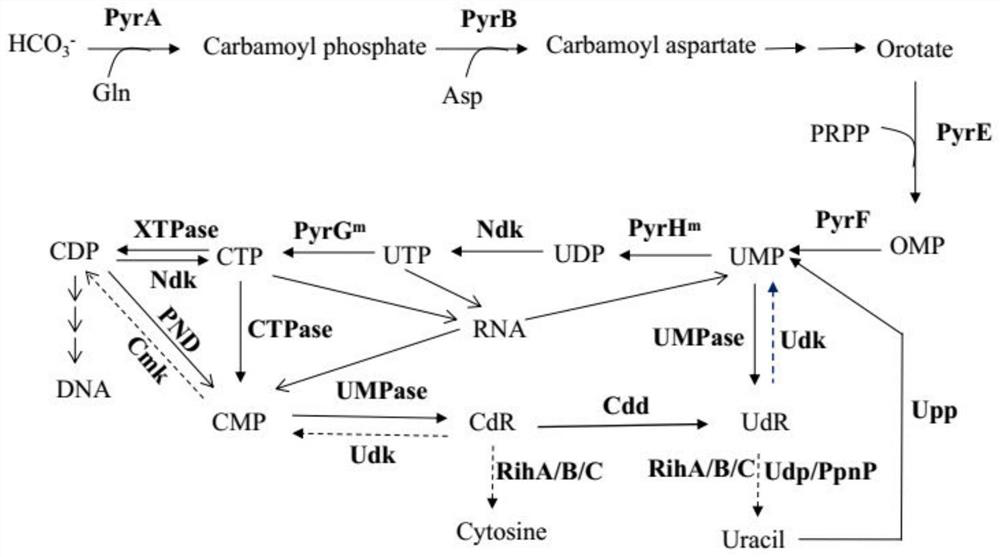
Cytidine monophosphate, also known as 5'-cytidylic acid or simply cytidylate, and abbreviated CMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside cytidine. CMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase cytosine; hence, a ribonucleoside monophosphate. As a substituent it takes the form of the prefix cytidylyl-.
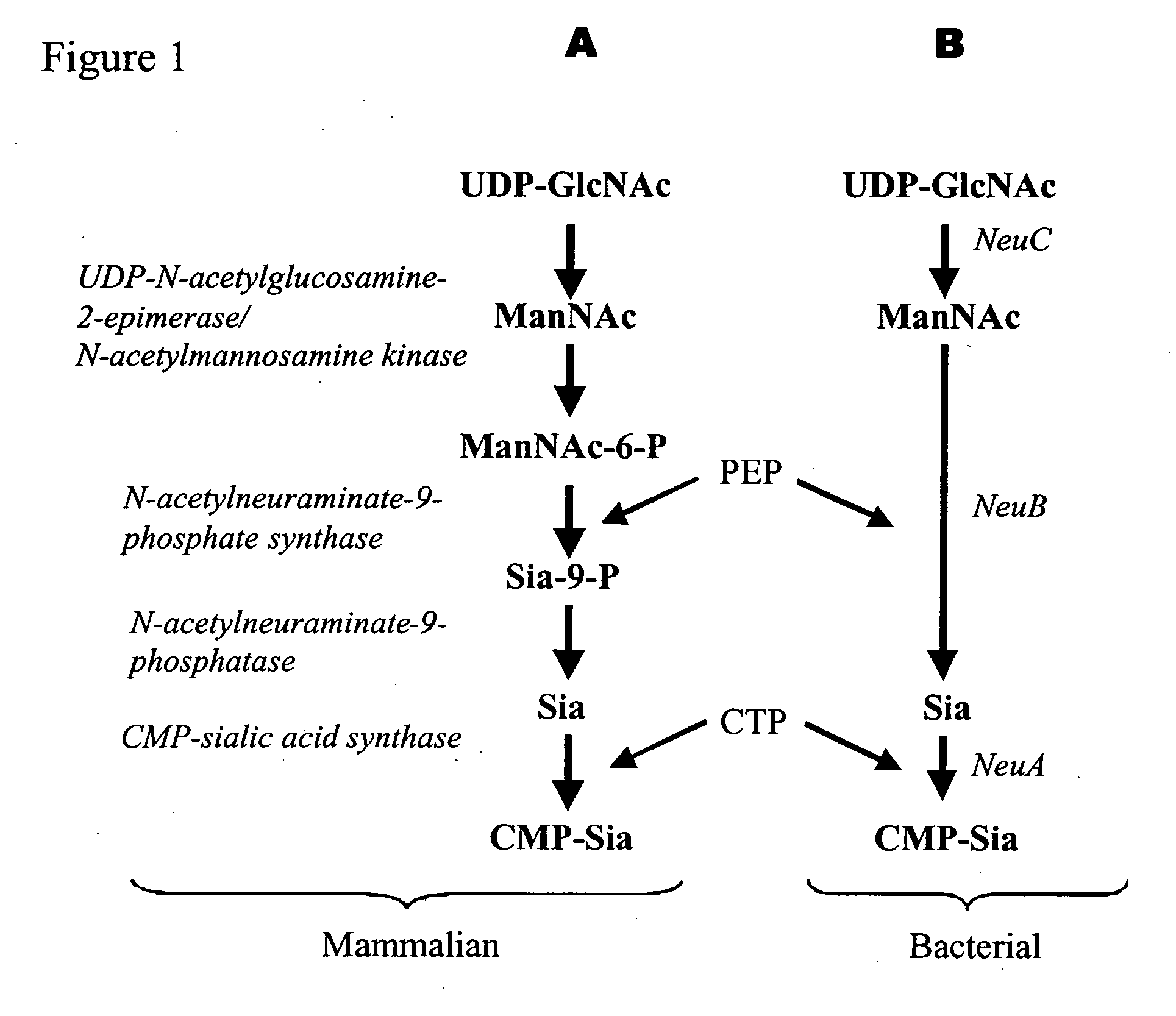
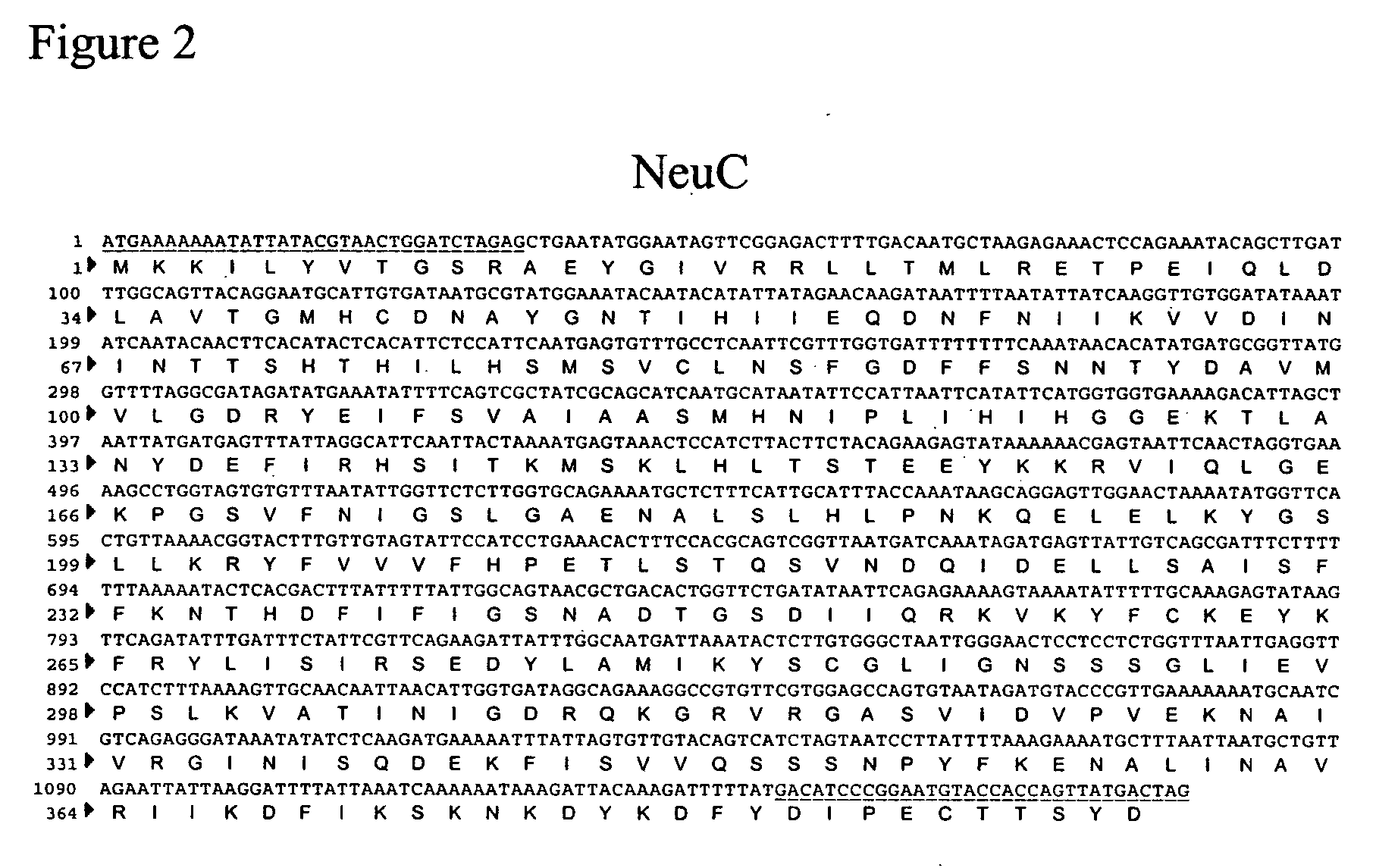
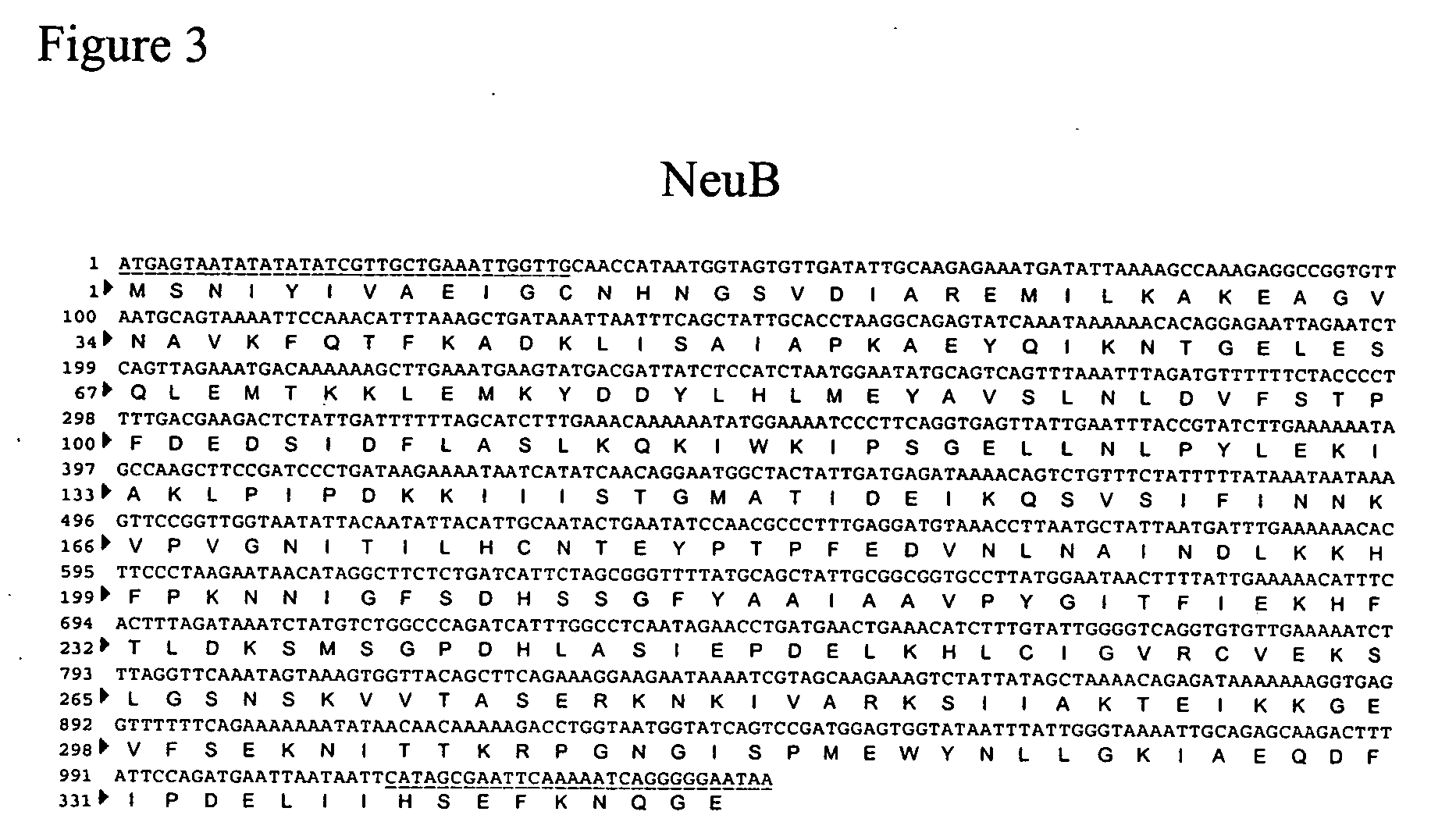
Method of engineering a cytidine monophosphate-sialic acid synthetic pathway in fungi and yeast
The present invention provides methods for generating CMP-sialic acid in a non-human host which lacks endogenous CMP-Sialic by providing the host with enzymes involved in CMP-sialic acid synthesis from a bacterial, mammalian or hybrid CMP-sialic acid biosynthetic pathway. Novel fungal hosts expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided.
Owner:GLYCOFI
Weaned piglet nucleotide feed additive
InactiveCN102987169AImprove antioxidant capacityEnhance immune functionAnimal feeding stuffBiotechnologyGuanosine monophosphate
The invention discloses a weaned piglet nucleotide feed additive. The weaned piglet nucleotide feed additive comprises, by weight, 0.8 to 1.3 of adenosine monophosphate 5'-AMP, 0.3 to 0.8 of cytidine monophosphate 5'-CMP, 1.1 to 1.5 of guanosine monophosphate 5'-GMP and 0.7 to 1.4 of uridine monophosphate 5'UMP. The invention further discloses a preparation method and an application of the feed additive. According to the weaned piglet nucleotide feed additive, stress reaction of weaned piglets can be effectively relieved, the piglet grazing rate and the feed utilization efficiency are improved, the oxidation resistance and the immune function of weaned piglets are improved apparently, the organic resistance is enhanced, and accordingly, important significances are provided for modern scientific, intensive and large-scale pig production.
Owner:NANJING BIOTOGETHER
Ubelin manufacturing technique
InactiveCN101130797ASolve pollutionHigh activityMicroorganism based processesFermentationUltrafiltrationCholine Phosphate
The present invention relates to a production process of citicoline. It is characterized by that said production process includes the following steps: firstly, adding water and glucose into a reaction tank, then adding yeast, making fermentation, after fermentation adding inorganic salt and choline phosphate, the adding cytidine monophosphate solution, an adding cane sugar, heating, then quickly-cooling by using water, pressing reaction liquid, washing by using water, removing salt from obtained clear liquor; then pressing said clear liquor and making said clear liquor be fed into a carbon column, rinsing said carbon column by using pure water, then using ethyl alcohol solution to make elution, collecting citicoline sodium; then vaporizing and concentrating eluent, diluting concentrate and making it be fed into a macroporous ion exchange resin column, then washing said column by using water and making elution, concentrating eluent, heating the collected citicoline sodium solution, removing impurity by utilizing ultrafiltration device, decolouring, adding ethyl alcohol and stirring them, storing the above-mentioned material in a refrigeration house and staying overnight; then making crystal solution undergo the processes of centrifugal separation, vacuum drying, bagging, weighing, sealing and dry-storage.
Owner:张剑
Genetically engineered bacterium of colon bacillus for producing arabinoside-cytidine monophosphate lipoid A, and application thereof
The invention discloses a genetically engineered bacterium of a colon bacillus for producing arabinoside-cytidine monophosphate lipoid A. The genetically engineered bacterium is characterized in that a deletion mutation deactivation is carried out on lacz genes in a genome of the genetically engineered bacterium; and express exogenous pagL and lpxE and pagP genes are implanted into the lacz genes. According to the invention, the formed bacterial strain cannot produce a pathogenic bacterium and is free of the use of an inductive agent during a production process. Therefore, the genetically engineered bacterium is suitable for mass production of MPL adjuvants in earlier stages.
Owner:JIANGNAN UNIV
Method for preparing citicoline
InactiveCN101538598AIncrease profitAvoid investmentMicroorganism based processesFermentationPhosphate ionPhosphorylation
The invention discloses a method for preparing citicoline. The method comprises the following steps of taking choline chloride, phosphate ions and cytidine monophosphate or a precursor thereof as substrates, taking glucose, fructose, sucrose or maltose as an energy donor, adding a small-molecule chemical effect substance and utilizing the yeast-cell whole cells with permeability to catalyze and prepare the citicoline. By building a metabolic network model and metabolic flow analysis, adopting the small-molecule chemical effect substance to regulate and control metabolic flow so as to improve the efficiency of energy self-coupling and choline phosphorylation and utilizing the yeast cells with permeability to efficiently prepare the citicoline, the method has the advantages of greatly increasing product concentration and raising the utilization ratio of the substrates.
Owner:NANJING UNIV OF TECH
Mixed nucleotide which comes from yeast and contains uridylic acid and preparing method and application thereof
The invention relates to the industrial field of animal feed, in particular to mixed nucleotide which comes from yeast and contains uridylic acid and a preparing method and application thereof. The mixed nucleotide contains, by weight, 43-80 parts of cytidine monophosphate, 116-200 parts of adenylate, 95-150 parts of guanylic acid, 400-800 parts of uridylic acid and 1 part of inosinic acid. The invention further provides a yeast autolysate containing the mixed nucleotide, wherein the weight percentage of the mixed nucleotide in the yeast autolysate is 1.5-2%. By the adoption of the yeast autolysate, the growing speed of piglets can be effectively increased, productivity and feed conversion rate are high, average daily gain of piglets is increased by 9.8-17.4%, and application prospects are broad.
Owner:ANGELYEAST CO LTD
Novel n-acetylglucosamine-2-epimerase and method for producing cmp-neuraminic acid using the same
The present invention relates to a novel N-acetylglucosamine-2- epimerase and a method for preparing CMP-N-acetylneuraminic acid, more specifically, relates to a N-acetylglucosamine-2-epimerase derived from Bacteroides fragilis NCTC 9343, and a method for preparing CMP-N- acetylneuraminic acid using said N-acetylglucosamine-2-epimerase. According to the present invention, CMP-N-acetylneuraminic acid can be produced economically in a large amount through a one-step reaction using cytidine monophosphate and N-acetyl-D-glucosamine which are inexpensive substrates.
Owner:GENECHEM
Nucleotide probiotic capsule for delaying senescence
ActiveCN113615837AAvoid Oxidative DamageExtend your lifeOrganic active ingredientsDigestive systemBiotechnologyAdenosine 5 monophosphate
The invention discloses a nucleotide probiotic capsule for delaying senescence, and belongs to the technical field of medicine and health care. The nucleotide probiotic capsule comprises a capsule shell and a content, wherein the content comprises four exogenous nucleotides and five probiotics; the exogenous nucleotides comprise 5'-cytidine monophosphate (5'-CMP), 5'-adenosine monophosphate (5'-AMP), 5'-disodium uridine monophosphate (5'-UMPNa2) and 5'-disodium guanylate (5'-GMPNa2); the probiotics comprise lactobacillus acidophilus, lactobacillus plantarum, lactobacillus casei and the like; and the content of the probiotics is greater than 1 * 10 <7>CFU / g. The nucleotide probiotic capsule for delaying senescence can remove free radicals in a body, inhibit oxidative damage of the free radicals to the body and prolong the cell life, has multiple health care effects of delaying senescence, prolonging life, improving immunity, regulating intestinal flora and the like, is convenient to carry and take, and is a health care food suitable for middle-aged and elderly people.
Owner:陈玉松
Method for preparing citicoline sodium
ActiveCN104031105ALow costAvoid swappingSugar derivativesSugar derivatives preparationCiticoline sodiumPhosgene
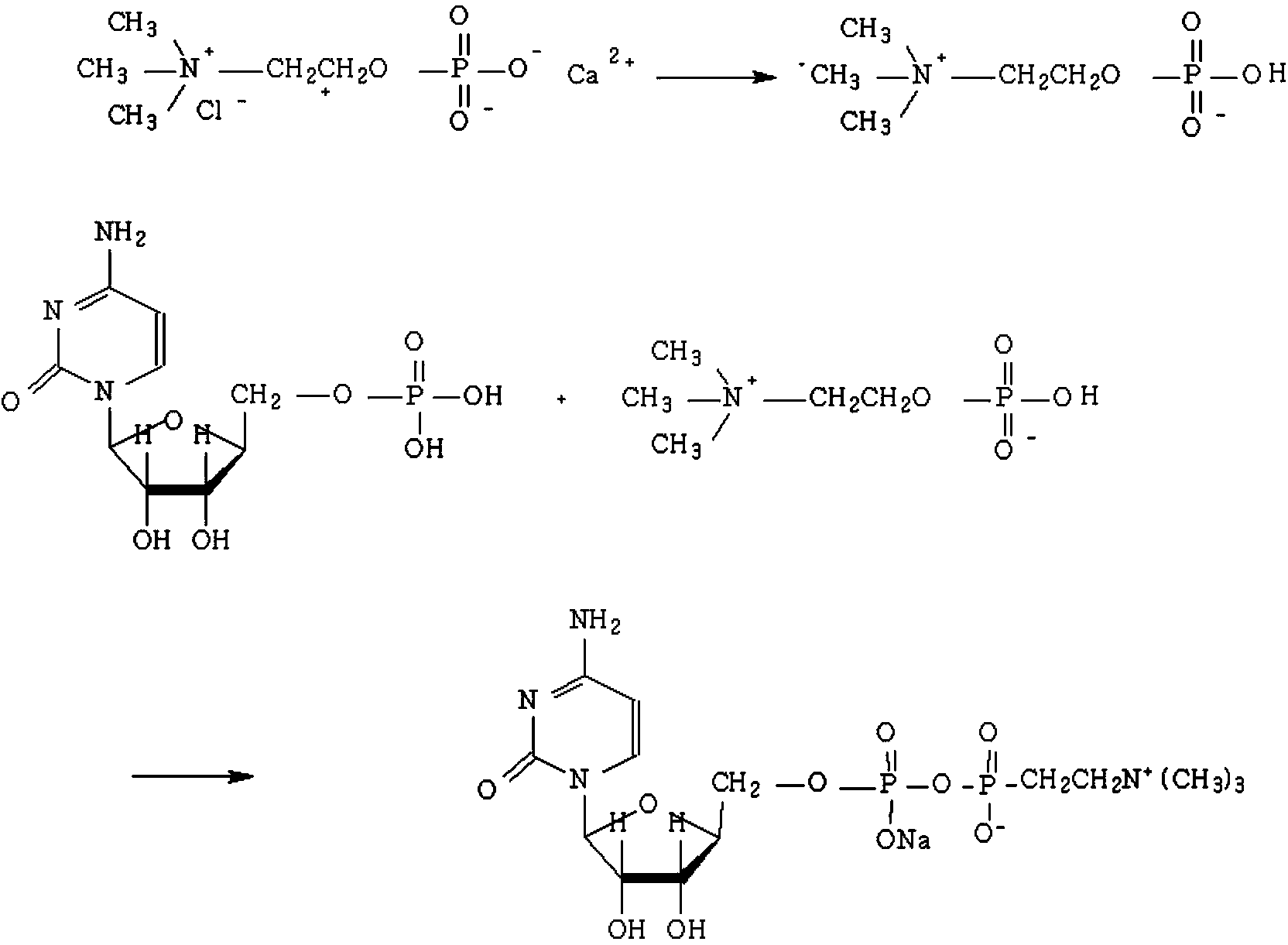
The invention discloses a method for preparing citicoline sodium. The method comprises the following steps: adding oxalic acid into a chlorination calcium water solution, removing the precipitate and water, reacting with cytidine monophosphate under the action of triphosgene, and subsequently purifying the citicoline sodium by using a recrystallization method.
Owner:安庆回音必制药股份有限公司
Recombinant escherichia coli for high yield of cytidine monophosphate and application of recombinant escherichia coli
PendingCN111269870AMeet the requirements of large-scale industrial productionReduce purificationBacteriaTransferasesEscherichia coliEnzyme Gene
The invention discloses recombinant escherichia coli for high yield of cytidine monophosphate and application of the recombinant escherichia coli. According to the invention, cytidine kinase gene is obtained by cloning from a cytidine monophosphate production strain; crude enzyme liquid obtained by crushing recombinant bacteria has good catalytic activity and stability; cytidine, adenosine triphosphate (ATP) and Mg < 2 + > are added, the reaction system is simple, the conditions are mild, the period is short, byproducts are fewew, the method is clean and free of pollution, the application is asimple, rapid and efficient production way, and the conversion rate of substrate cytidine and ATP reaches 85% or above.
Owner:NANJING UNIV OF TECH
Method of preparing cytidine disodium triphosphate and application
The invention relates to a manufacturing method of disodium cytidine triphosphate, which comprises the following steps: proceeding enzymatic conversation with CMP material; proceeding ion exchange column chromatography; separating and purifying. The enzymatic conversation reaction principle is that: brewers' yeast gets the needed enzyme liquid of the enzymatic conversation reaction; brewers' yeast produces a plurality of biological enzyme as sugar glycolysis enzyme and phosphorylase with high sugar glycolysis ability; the biological energy produced by glycolysis glucose with sugar glycolysis enzyme is transferred to cytidine monophosphate by ATP; cytidine monophosphate and phosphate radical combines to form cytidine triphosphate with the effect of phosphorylase. The method shapes low cost, simple operation, high production, high product purity by optimization, which comprises the following steps: getting enzyme liquid; proceeding enzymatic conversation by adding the main material cytidine monophosphate, monobasic potassium phosphate of proper quantity and glucose; getting product by deposition, ion exchange column chromatography, segregation, purification, refining and vacuum drying.
Owner:BEIJING SL PHARMA
Process for preparing cytidine diphosphate choline
ActiveCN102586383AFast metabolismReduce manufacturing costMicroorganism based processesFermentationMicroorganismPh control
The invention relates to a process for preparing cytidine diphosphate choline. According to the method, denovo synthesis of pyrimidine is utilized as a basic reaction and choline materials are subjected to a reaction at the reaction temperature ranging from 20 DEG C to 38 DEG C for 10 to 72 hours with pH controlled at 5 to 7.5 in the presence of culture solution of microorganism A or further in the presence of culture solution of microorganism B or treatment so as to accumulatively produce cytidine diphosphate choline. Simple starting materials of carbon and nitrogen sources are utilized, in culture solution containing the choline materials, precursor of the cytidine diphosphate choline, namely cytidine monophosphate is synthesized by means of metabolic process, and cytidine diphosphate choline is cumulatively produced in the reaction solution. According to the method, expensive cytidine monophosphate or the precursor materials are avoided so that the preparing cost of the cytidine diphosphate choline is greatly reduced.
Owner:QILU PHARMA HAINAN +1
Citicoline and synthetic method thereof
InactiveCN105732752AHigh yieldLess side effectsSugar derivativesSugar derivatives preparationMorpholineChloride
The invention discloses citicoline and a synthetic method thereof.The synthetic method includes subjecting cheap and easily available cytidine, serving as a raw material, and dichlorophosphoryl morpholine to condensation at a 5' position highly selectively so as to obtain 5'-phosphorylmorpholinylcytidine; subjecting the 5'-phosphorylmorpholinylcytidine and phosphocholine chloride calcium to condensation in solvents so as to obtain the citicoline.Total yield is up to 58% only by the two steps.The synthetic method is cheap in and easily available to raw materials, expensive cytidine monophosphate is unused, operation steps are simplified, and reaction scale can be expanded to synthesize 500 g of citicoline.The synthetic method has a potential application prospect by providing a novel citicoline synthetic route.
Owner:XINXIANG UNIV
Citicoline and synthesizing method of citicoline not using phosphocholine chloride calcium
InactiveCN105693798AReduce usageMild reaction conditionsSugar derivativesSugar derivatives preparationMorpholineChloride
The invention discloses a method for synthesizing citicoline and citicoline without using calcium phosphorylcholine chloride. Acylmorpholine is used as raw material, reacts with ethylene glycol to obtain ethylene glycol ester phosphoromorpholine, and then directly condenses with cytidylic acid to obtain ethylene glycol ester phosphoryl cytidylic acid, and finally tributylamine to ethylene glycol ester The ethylene glycol ester of phosphoromorpholine is ring-opened to give citicoline. There are three steps in the reaction, and the total yield is 78%. The method has cheap and easy-to-obtain raw materials, avoids the use of expensive and toxic DCC and calcium phosphorylcholine chloride, and the yield does not decrease significantly when the reaction scale is expanded to a scale of 500 g. The application of the present invention provides a new synthesis route for the synthesis of citicoline, and has potential application prospects.
Owner:XINXIANG UNIV
Method for preparing recombinant glycoproteins with high sialic acid content
ActiveCN102482674AIncreased sialic acid contentThrombopoietinCell receptors/surface-antigens/surface-determinantsArginineTert-leucine
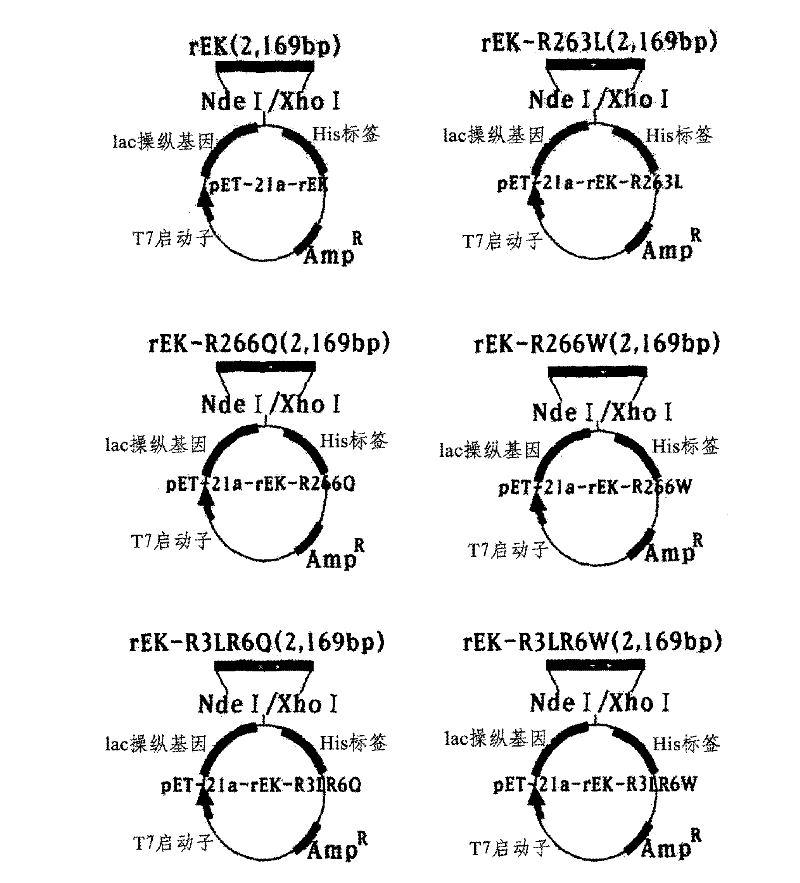
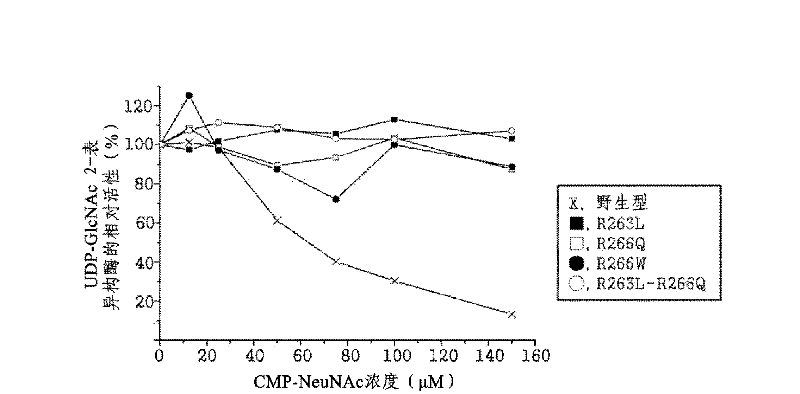
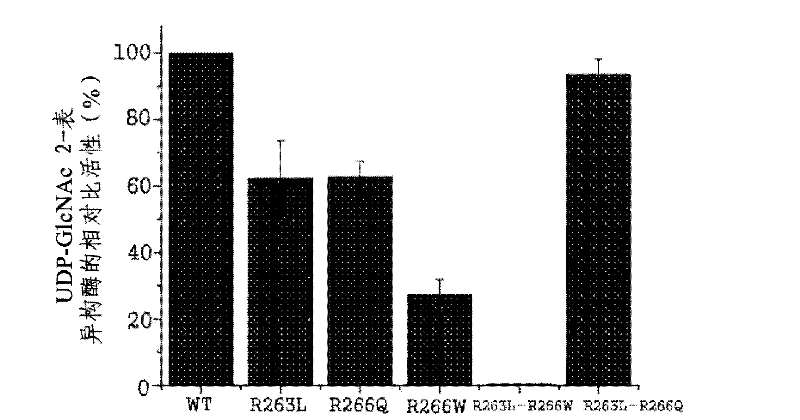
The present disclosure relates to a method for preparing recombinant glycoproteins with high sialic acid content. More specifically, for UDP-GlcNAc 2-epimerase / ManNAc kinase (GNE / MNK) enzyme where point mutation was induced by substituting arginine at position 263 by leucine only or by further substituting arginine at position 266 by glutamine, epimerase activity is constantly maintained, and overexpressed cells thereof experience an increase in intracellular cytidine monophosphate (CMP)-sialic acid content, irrespective of CMP-sialic acid concentration.,Particularly, since in an glycoprotein(such as, erythropoietin and thrombopoietin)-producing host cell where point mutationinduced GNE / MNK, human alpha-2,3-sialyltransferase and a CMP-sialic acid transporter gene are simultaneously overexpressed, intracellular content of CMP-sialic acid and sialic acid in glycoprotein increases in cells, overexpression in a host cell producing a sialylated recombinant glycoprotein the three genes above may be useful for preparing glycoprotein with increased sialic acid content.
Owner:KOREA ADVANCED INST OF SCI & TECH
Application of yeast containing active polysaccharides and nucleotides in dairy cow feed
InactiveCN104855733AEnhance non-specific immunitySolve the pollution of the environmentAnimal feeding stuffRotavirus RNASerum protein albumin
The invention discloses the application of a yeast containing active polysaccharides and nucleotides in a dairy cow feed. The yeast containing active polysaccharides and nucleotides contains such active polysaccharide components as glucan, mannan and mannan oligosaccharide, and the total content of the active polysaccharides is 1-20wt% of that of the yeast; the yeast also contains such nucleotides as adenylate, cytidine monophosphate, guanylic acid and uridylic acid, and the total content of the nucleotides is 1-20wt% of that of the yeast. According to the method, the active feed is added to the dairy cow feed, the serum albumin of a calf can be increased by 140mg / dL by use of the methods of optimizing charging and feeding conditions and the like, and the maximum mutation rate of the rotavirus in the colostrum can be 75%. The application of the yeast containing active polysaccharides and nucleotides in the dairy cow feed has the advantages that 1, the yeast feed is capable of increasing the content of the serum albumin of the calf and boosting the immunity of the calf, and 2, the yeast feed is capable of mutating a large amount of rotavirus in the colostrum and preventing the rotavirus from pathopoiesis.
Owner:NANJING BIOTOGETHER
Recombinant microorganism for producing uridine and method for producing uridine
PendingCN113755414ARealize large-scale industrial productionReduce manufacturing costBacteriaMicroorganism based processesRibonucleosidePhosphorylation
The invention provides a recombinant microorganism for producing uridine and a method for producing uridine by using the recombinant microorganism. Wherein the uridine is degraded and knocked out by using genes, and the genes encode ribonucleoside hydrolase, uridine phosphorylase, nucleoside phosphorylase, cytidine / uridine kinase and nucleoside transporter protein. Meanwhile, overexpression is carried out on key enzymes in a uridine biosynthetic pathway, including cytidine triphosphate pyrophosphorylase for degrading cytidine triphosphate to cytidine monophosphate and uridine monophosphate phosphorylase for catalyzing uridine monophosphate to uridine. In addition, a pyrimidine nucleoside pathway is subjected to genetic engineering modification, and feedback inhibition of a synthetic pathway is relieved. The recombinant strain can reach the uridine yield of 20 g / L or above in a 5-liter fermentation tank through a biological fermentation method, industrial mass production can be achieved, meanwhile, the uridine production cost is low, pollution is reduced, the method is green and environmentally friendly, and the method has high application and popularization value.
Owner:SUZHOU BIOSYNTHETICA CO LTD
Preparation method of uridine-5'-monophosphate disodium
ActiveCN104447922ASimple preparation processEasy to operateSugar derivativesSugar derivatives preparationAlcoholOrganic solvent
The invention discloses a preparation method of uridine-5'-monophosphate disodium. The method comprises the following steps: (1) mixing cytidine monophosphate (CMP) or sodium salt, sodium nitrite and deionized water, dropwise adding acid and / or acid anhydride, continuously reacting for 0-6 hours after completing dropwise adding to obtain reaction solution 1; (2) if residual quantity of CMP is lower than 1%, adjusting the pH value of the reaction solution 1 to 6.4-7.2 to obtain reaction solution 2; (3) mixing the reaction solution 2 and 95v / v% of ethyl alcohol, crystallizing and filtering to obtain a uridine-5'-monophosphate disodium crude product; (4) mixing the uridine-5'-monophosphate disodium crude product and water, and adjusting the pH value to 7.0-8.5 to obtain reaction solution 3; and (5) mixing the reaction solution 3 and an organic solvent, recrystalizing and filtering to obtain the uridine-5'-monophosphate disodium.
Owner:美亚药业海安有限公司
Overexpressed phosphorylcholine cytidine transferase saccharomyces cerevisia genetically engineered bacteria as well as construction method and application thereof
InactiveCN107488603AImprove permeabilityThe biotransformation process is convenient and fastFungiTransferasesBiotechnologyPhosphorylcholine
The invention relates to overexpressed phosphorylcholine cytidine transferase saccharomyces cerevisia genetically engineered bacteria as well as a construction method and application thereof. The construction method comprises the steps of cloning saccharomyces cerevisia sourced gene cct capable of encoding phosphorylcholine cytidine transferase to a pYES2.0-Kanmx carrier so as to construct recombinant plasmid pYES2.0-Kanmx-cct, and transferring the recombinant plasmid pYES2.0-Kanmx-cct into S.cerevisiae HG, so as to obtain the saccharomyces cerevisia genetically engineered bacteria. The yield of a solid matter of the strain to fermentable sugar is 50%-56%, the strain can be used for manufacturing a citicoline product from 5'-cytidine monophosphate and phosphorylcholine through bioconversion, and after the strain reacts for 7 hours, the molar conversion ratio reaches up to 73%. The yield of the strain in a fermentation system for producing and culturing the strain is high. When applied to the production of citicoline, the strain has the advantages of low cost and manufacturing energy consumption, short reaction period and the like.
Owner:NANTONG QIUZHIYOU BIOSCI & BIOTECH
Broiler chicken feedstuff additive
InactiveCN103039765AMeeting nutritional needsEnhance disease resistanceAnimal feeding stuffDiseaseArginine
The invention discloses a broiler chicken feedstuff additive. The feedstuff additive comprises the following components in parts by weight: 12-33 parts of adenylate, 5-24 parts of guanylic acid, 8-27 parts of uridylic acid, 14-39 parts of cytidine monophosphate, 5-22 parts of glutamine and 6-14 parts of arginine. The invention also discloses a preparation method and application of the broiler chicken feedstuff additive. According to the feedstuff additive disclosed by the invention, the appetite of animals can be boosted, the animals can be promoted to take food actively, the nutrient demand of young chickens in a best immune state is satisfied, the disease resistance and the disease prevention capability of the young chickens are enhanced, and the survival rate of the young chickens obviously increases, so that the feedstuff additive has a positive significance to a livestock culture industry.
Owner:NANJING BIOTOGETHER
S. cerevisiae strain for producing citicoline through bioconversion and application of S. cerevisiae strain
The invention belongs to the field of biological medicine and relates to S. cerevisiae strain for producing citicoline through bioconversion and application of the S. cerevisiae strain, namely the S. cerevisiae strain of high activity CMP (cytidine monophosphate) kinase and high activity phosphoryl choline-cytidyltransferase and the application of the S. cerevisiae strain in the production of citicoline through bioconversion. The S. cerevisiae strain provided by the invention has a classification name of S. cerevisiae, and has the Preservation No. of CGMCC7978 in CGMCC (China General Microbiological Culture Collection Center).
Owner:NANTONG QIUZHIYOU BIOSCI & BIOTECH +1
Cigarette filter stick additive capable of reducing harm, as well as preparation and application methods of same
ActiveCN102920019ASimple preparation processReduce the effect of hydrocyanic acid (HCN)Tobacco smoke filtersChemical reactionPotassium
The invention discloses a cigarette filter stick additive capable of reducing harm, as well as an application method of the cigarette filter stick additive. The preparation method comprises the following steps: taking one or more of 5'-cytidine monophosphate, 5'-sodium cytidylate, 5'-potassium cytidylate and poly 5'- cytidine monophosphate as active ingredients; taking silica gel, activated carbon, aluminum oxide and sodium alginate as carriers; loading the active ingredients on surfaces of the carriers by a surface chemical reaction to obtain loaded type materials; adding the prepared loaded type materials into a cigarette filter stick to prepare a composite filter stick; and rolling up the cigarette. The analysis shows that the cigarette with the loaded type materials can effectively reduce content of HCN (hydrogen cyanide) in the smoke by 8-29%. The cigarette filter stick additive capable of reducing harm is safe, stable, simple in preparation method and convenient to industrially produce at large scale.
Owner:CHINA TOBACCO HUNAN INDAL CORP
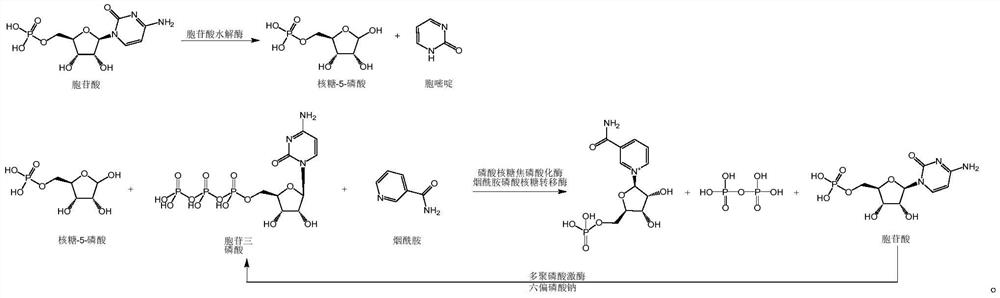
Enzymatic synthesis method of nicotinamide mononucleotide
ActiveCN112961890AGood removal effectAvoid expensiveFermentationBulk chemical productionEnzymatic synthesisNicotinamide mononucleotide
The invention discloses an enzymatic synthesis method of nicotinamide mononucleotide. The enzymatic synthesis method comprises the following steps: S1, taking ribose-5-phosphate, cytidine monophosphate, polyphosphate and nicotinamide as raw materials, and generating nicotinamide mononucleotide under the catalytic action of ribose phosphate pyrophosphorylase, polyphosphate kinase and nicotinamide ribose phosphate transferase. The applicant uses polyphosphate kinase to catalyze cytidine monophosphate to cyclically obtain cytidine triphosphate by using a phosphate group provided by the polyphosphate, and the cytidine triphosphate is used as a pyrophosphoric acid donor of the ribose phosphate pyrophosphorylase to promote the enzymatic reaction of the ribose-5 phosphate and the nicotinamide, thereby avoiding the use of expensive ATP (adenosine triphosphate). Besides, the cytidine monophosphate and the cytidine triphosphate in the enzymatic reaction process provided by the invention are almost insoluble in water under an acidic condition, so that impurities in the product can be quickly, simply and conveniently removed by directly adjusting the pH value after the reaction is finished, the purification process is simplified, and the separation and purification difficulty is reduced.
Owner:深圳希吉亚生物技术有限公司
Preparation method of citicoline sodium
InactiveCN106146590AEasy to removeEasy to operateSugar derivativesSugar derivatives preparationDistillationCiticoline sodium
The invention discloses a preparation method of citicoline sodium, and belongs to the technical field of biopharmacy. The preparation method comprises the steps that phosphorylcholine chloride calcium salt serving as a raw material and benzene are subjected to cbinary azeotrope to remove crystallization water in phosphorylcholine chloride calcium salt; phosphorylcholine chloride calcium salt reacts with sodium carbonate to generate a calcium carbonate precipitate, and filtering is conducted to obtain viscous materials; the viscous materials react with acetylchloride, a product reacts with cytidine monophosphate, and reduced pressure distillation is conducted to obtain residues; crystallization and recrystallizzation are conducted through sodium hydroxide and ethyl alcohol to obtain the product. According to the preparation method, operation is easy, acetylchloride is easy to remove by adding water, all the solvents can be recycled, better environmental friendliness and higher economic benefits are achieved, the purity of the product is high and reaches 98%, and the product is suitable for pharmaceutical purposes.
Owner:陈建峰
Method for evaluating destroy degree on nucleotide of determination method and production technology of free nucleic acid hydrolysate in protein product
The invention discloses a method for determining a free nucleic acid hydrolysate in protein products. The method comprise the following steps: (1) respectively preparing a nucleotide mixed standard solution and a bases mixed standard solution; (2) preparing a solution of a sample to be tested, and using the nucleotide mixed standard solution and the base mixed standard solution described in step 1 as the reference solution, and employing high performance liquid chromatography through washing peak area method comparison to obtain the content of free nucleotides and free base in the sample. According to the invention, high performance liquid chromatography is used for determining 5 kinds of bases in a single-cell protein raw material (cytosine, uracil, guanine, thymine and adenine), 5 kinds of nucleotides (cytidine monophosphate, uridylic acid, guanylic acid, inosinic acid and adenylic acid), and 5 kinds of nucleosides (cytidine, uridine, inosine, guanosine and adenosine); and then the destroy degree on nucleotide of the production process is determined according to t the relationships between the bases and nucleotides, and between the bases and nucleosides.
Owner:江苏征泰饲料有限公司
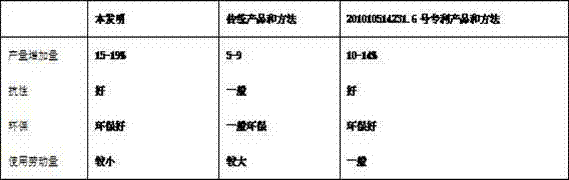
Production method of flos traditional Chinese medicine
ActiveCN103613424AActive ingredient reductionSave raw materialsFertilising methodsFertilizer mixturesFlosMedicinal herbs
The invention discloses a production method of a flos traditional Chinese medicine. The production method is characterized in that nutrients are used during the growth of the flos traditional Chinese medicine, wherein the nutrients include the following components in percentage by weight: 30 to 70% of nucleotide salt and 30 to 70% of medium trace element, and exclude glucose or / and amino acids; the nucleotide salt is the sylvite or sodium salt of four 5'-mixed nucleotides and obtained by hydrolyzing ribonucleic acid alkaline, namely, wherein the four 5'-mixed nucleotides are namely 5'-adenylate dipotassium or sodium, 5'-guanylic dipotassium or sodium, 5'-cytidine monophosphate dipotassium or sodium, 5'-uridylic acid dipotassium or sodium; and the medium trace element includes Mn, B and Fe, namely manganese sulfate, borax and ferrous sulfate. The production method of the flos traditional Chinese medicine has the effect reaching and even exceeding the effect of a patent for invention with publication patent number of CN102079666B and patent number of ZL201010514231.6. The method is economic and simple. The obtained flos traditional Chinese medicine is large and uniform in flower, the uniformity is up to 70 to 90%, the output is 20 to 40% higher than that of flos traditional Chinese medicine produced by the traditional method, the main components account for 10 to 25%, the obtained traditional Chinese medicines can be prepared into quality decoction pieces, or active ingredients can be extracted, and the yield is up to 10 to 22%.
Owner:安徽世茂中药股份有限公司
Production method for flower traditional Chinese medicine
InactiveCN104973910AActive ingredient reductionSave raw materialsFertilising methodsFertilizer mixturesFlosBULK ACTIVE INGREDIENT
Owner:HEFEI KAIGE INFORMATION TECH
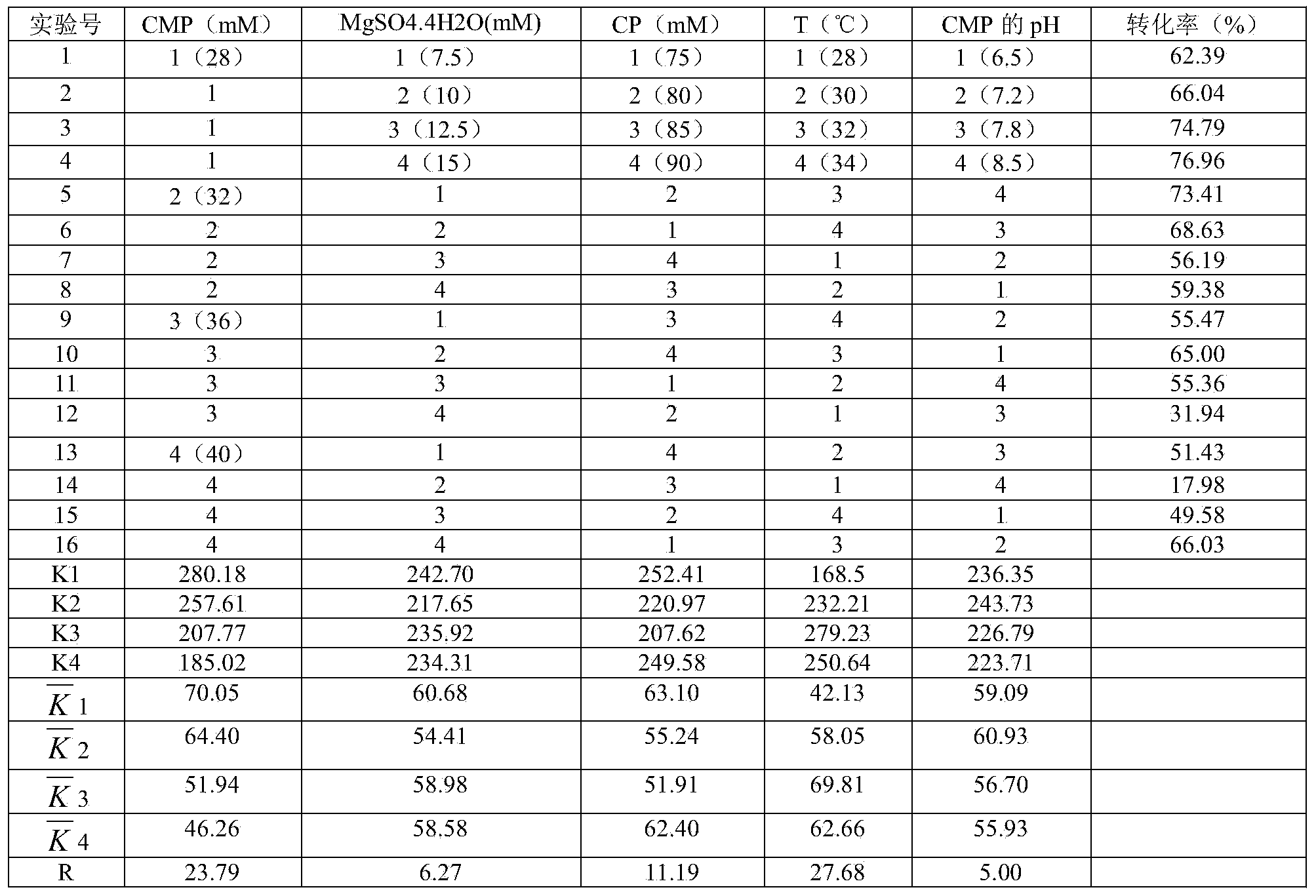
Method for producing 5 '-cytidine monophosphate
ActiveCN114107143AEfficient productionPromote conversionBacteriaHydrolasesEnzyme GenePhosphorylation
The invention discloses a method for producing 5 '-cytidine monophosphate, and belongs to the field of genetic engineering and microbial engineering. A cytidine monophosphate pyrophosphorylase gene ushA and a nucleotide 5 '-monophosphate nucleosidase gene ppnN of escherichia coli are knocked out through a gene knockout technology, and the escherichia coli mutant capable of efficiently accumulating 5'-cytidine monophosphate (5 '-CMP) is obtained. The recombinant Escherichia coli capable of efficiently producing 5 '-CMP is further obtained by overexpressing a uridine kinase gene udk, and the fermentation level of the 5'-CMP can reach 31.8 g / L. The efficient Escherichia coli strain can be used for realizing industrial production of the 5 '-CMP, and is beneficial to reducing the production cost and environmental pollution and realizing green biological manufacturing.
Owner:江苏香地化学有限公司 +1
Enzymatic synthesis process for 5'-cytidine monophosphate
The invention discloses an enzymatic synthesis process for 5'-cytidine monophosphate. The enzymatic synthesis process for 5'-cytidine monophosphate comprises the following steps: synthesis of cytidine: synthesizing the cytidine by using cytosine and D-ribose under the catalytic action of synthetase I, and filtering a solution obtained from reaction to obtain cytidine filtrate; preparation of 5'-cytidine monophosphate mother liquor: synthetizing 5'-cytidine monophosphate from cytidine filtrate and sodium dihydrogen phosphate under catalytic action of synthetase II, stirring obtained reaction liquid for a period of time under the condition of insulating and filtering to obtain 5'-cytidine monophosphate mother liquid; and refined extraction of the 5'-cytidine monophosphate: regulating pH of the 5'-cytidine monophosphate mother liquid, then filtering to obtain a 5'-cytidine monophosphate crude product, and refining the 5'-cytidine monophosphate crude product to obtain the product 5'-cytidine monophosphate. The enzymatic synthesis process for 5'-cytidine monophosphate has the advantages that the production cost of the 5'-cytidine monophosphate can be reduced; the process period is short, and the preparation efficiency of the 5'-cytidine monophosphate can be improved; the total yield of the 5'-cytidine monophosphate can be 90% or above, and the content is greater than 99%; and the 5'-cytidine monophosphate is harmless to the environment, equipment and operators.
Owner:NANTONG SANE BIOLOGICAL
Method of engineering a cytidine monophosphate-sialic acid synthetic pathway in fungi and yeast
The present invention provides methods for generating CMP-sialic acid in a non-human host which lacks endogenous CMP-Sialic by providing the host with enzymes involved in CMP-sialic acid synthesis from a bacterial, mammalian or hybrid CMP-sialic acid biosynthetic pathway. Novel fungal hosts expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided.
Owner:HAMILTON STEPHEN R
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com