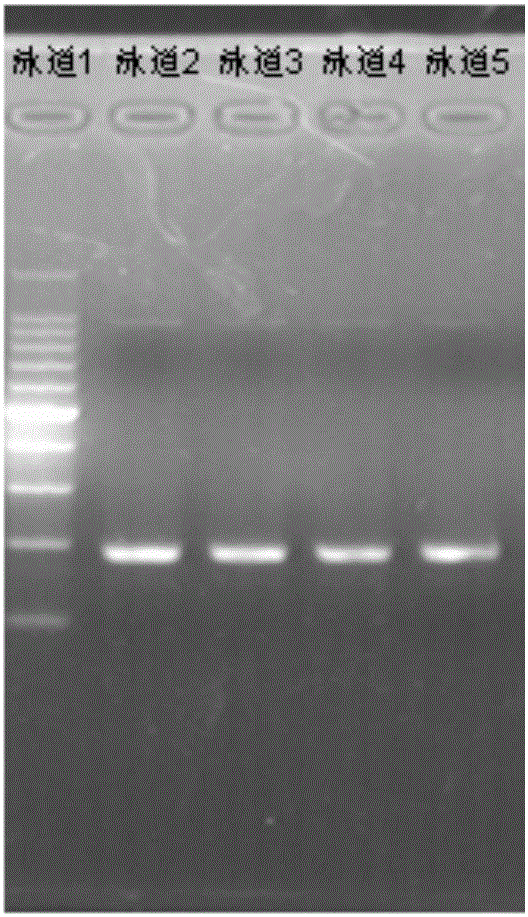
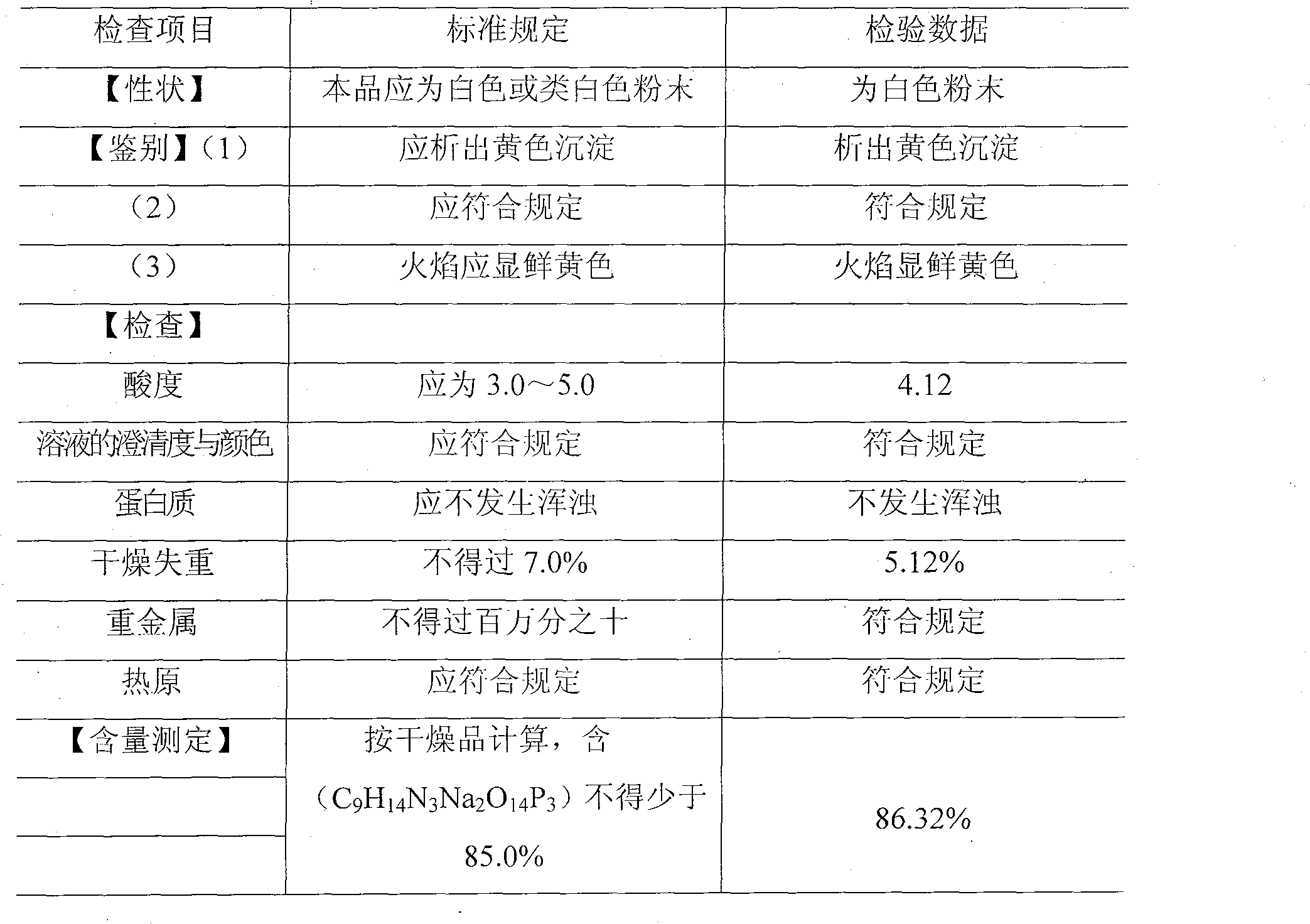
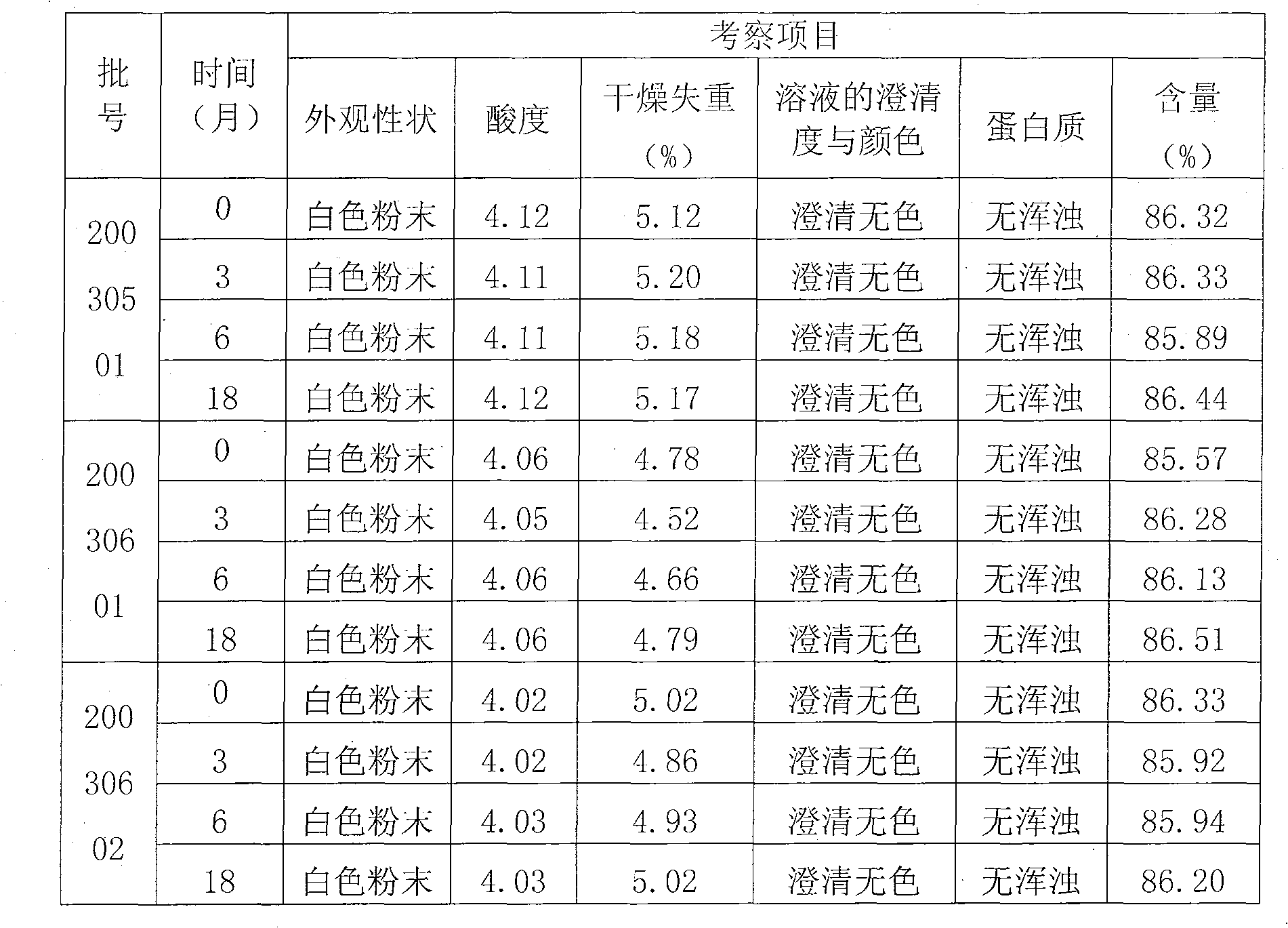
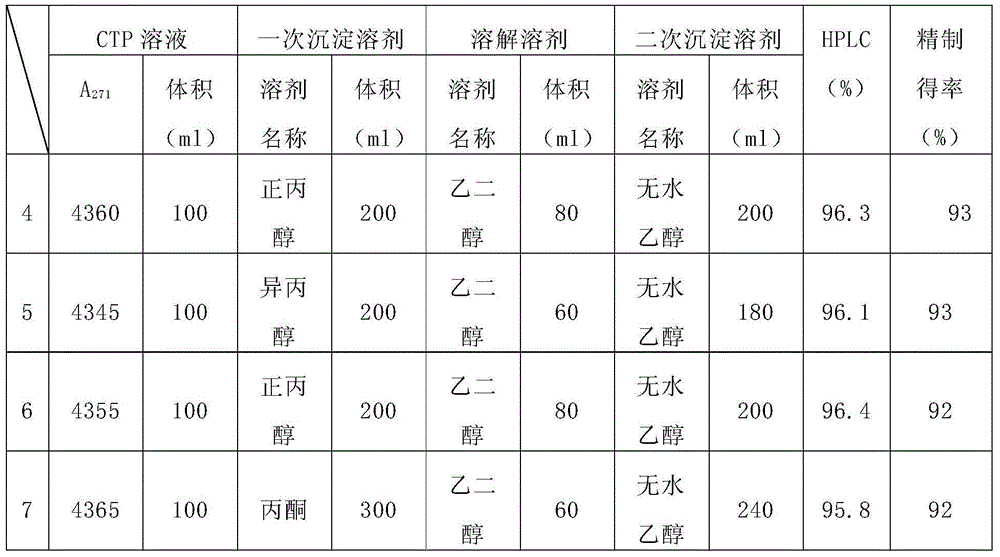
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Cytidine diphosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
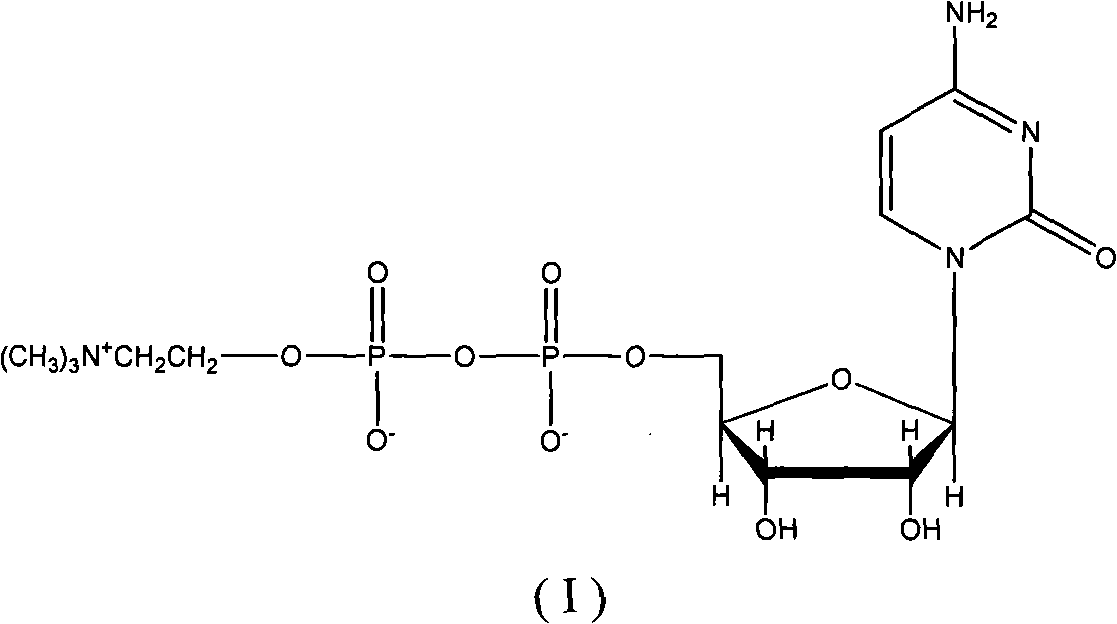
Cytidine diphosphate, abbreviated CDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside cytidine. CDP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase cytosine.
Nutritional compositions containing a neurologic component and uses thereof
InactiveUS20140199265A1Promoting neurologicalPromote brainBiocideHydroxy compound active ingredientsDeveloping nervous systemCytidine diphosphate
The present disclosure relates to nutritional compositions comprising a neurologic component, wherein, the neurologic component may promote brain and nervous system development and further provide neurological protection and repair. The neurologic component may include phosphatidylethanolamine, sphingomyelin, cytidine diphosphate-choline, ceramide, uridine, at least one ganglioside, and mixtures thereof. The disclosure further relates to methods of promoting brain and nervous system health by providing said nutritional compositions to target subjects, which includes pediatric subjects.
Owner:MEAD JOHNSON NUTRITION
Process for producing cytidine diphosphate choline
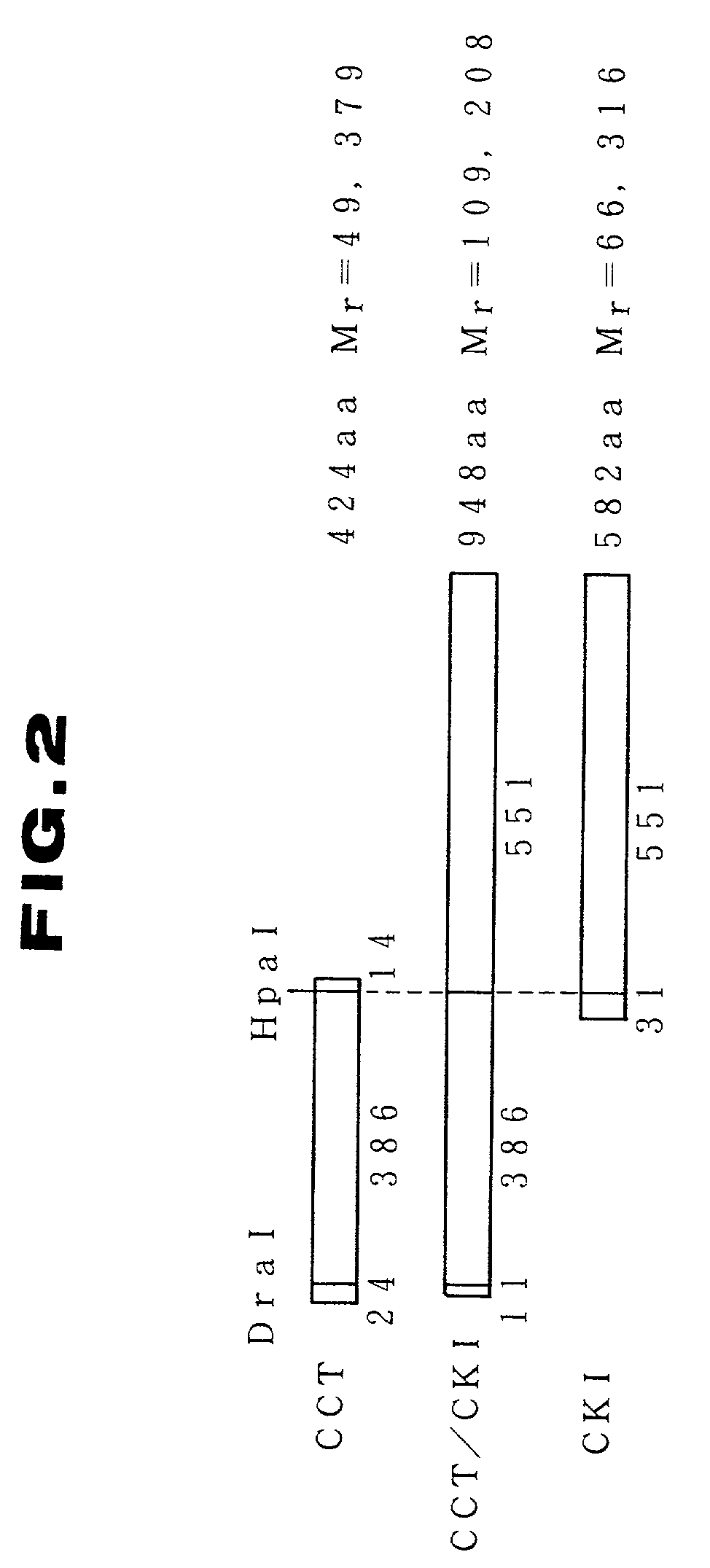
A substantially pure culture of a microorganism having enzyme activities of pyrG and CCT and carrying a recombinant DNA composed of a DNA fragment containing genes encoding pryG and CCT and a vector.
Owner:KYOWA HAKKO BIO CO LTD
Rhodamine fluorescent probe with pseudo nucleic acid base as recognition site and preparation thereof and application to nucleotide image
ActiveCN103540312ASensitive imaging effectImaging effect is highly sensitiveOrganic chemistryBiological testingAdenosinePhosphoric acid
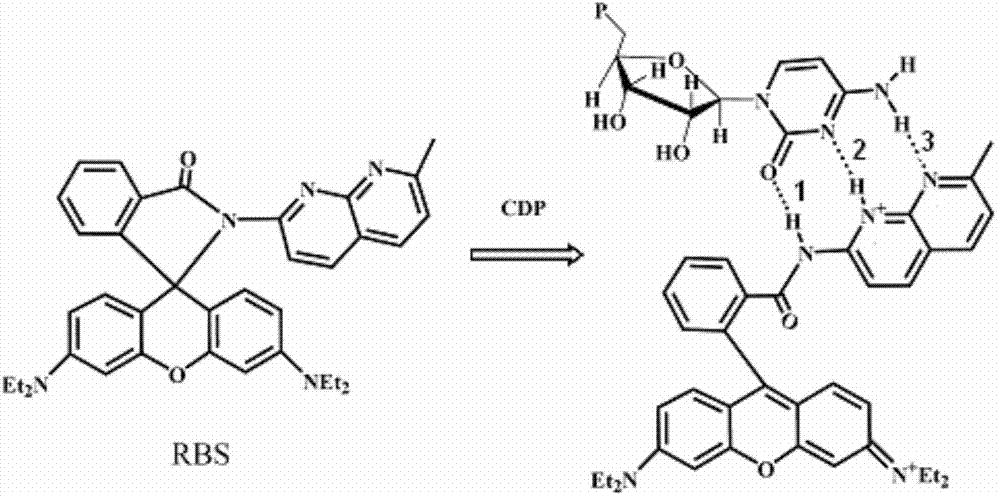
The invention relates to a fluorescent probe, which belongs to the technical field of fine chemical engineering, and discloses a rhodamine fluorescent probe with a pseudo nucleic acid base as a recognition site and preparation thereof and application to a nucleotide image. The ring opening of rhodamine lactone is induced between amino naphthyridine and nucleic acid base through a base analogue complementary hydrogen bond effect, and the selective response of specific nucleotide is realized by using the weakness of electrostatic interaction between imine positive ions generated by a ring opening product and phosphoric acid link negative ions with proper length. The probe shows good selectivity to cytidine diphosphate (CDP) and adenosine triphosphoric acid (ATP). Hela cell copolymerization focal imaging experiments show that the probe can produce a sensitive imaging effect on the nucleotide in the cell, the cell state is good after and before imaging, and the probe has small cell toxicity. Since the probe has good bioavailability and a high-sensitivity imaging effect, the probe is widely applied to medical fluorescent imaging.
Owner:DALIAN UNIV OF TECH
Method for separating purified cytidine diphosphate choline by hydrophobic chromatography
ActiveCN101906126AEasy to separateBrief lossSugar derivativesSugar derivatives preparationInorganic saltsCentrifugation
The invention discloses a method for separating purified cytidine diphosphate choline by hydrophobic chromatography. The method comprises the following steps of: (1), performing enzyme inactivation, centrifugation and ultra-filtration of a cytidine diphosphate choline conversion liquid; (2), after adjusting the pH of the pre-processed cytidine diphosphate choline conversion liquid to between 2.0 and 14.0, adding an inorganic salt to prepare column-loading liquid which contains 0.01 to 5mol / L of inorganic salt and 0.1 to 40g / L of cytidine diphosphate choline with the cytidine diphosphate choline conversion liquid, absorbing the column-loading liquid with a hydrophobic chromatography resin and eluting with pure water of which the pH value is 2.0 to 14.0; and (3), nano-filtering, desalinating and concentrating the eluate, and obtaining the cytidine diphosphate choline through crystallization. In the method, the separation process is simple; through regeneration, the resin after elution can be repeatedly used; the separation cost is low; the product is easily crystallized; and the CDP-choline product with high purity and high yield can be obtained.
Owner:NANJING UNIV OF TECH +1
Nutritional compositions containing a neurologic component and uses thereof
InactiveCN104883909APromotes Nerve HealthGood for healthHydroxy compound active ingredientsAmide active ingredientsNutritionCholine Phosphate
The present disclosure relates to nutritional compositions comprising a neurologic component, wherein, the neurologic component may promote brain and nervous system development and further provide neurological protection and repair. The neurologic component may include phosphatidylethanolamine, sphingomyelin, cytidine diphosphate-choline, ceramide, uridine, at least one ganglioside, and mixtures thereof. The disclosure further relates to methods of promoting brain and nervous system health by providing said nutritional compositions to target subjects, which includes pediatric subjects.
Owner:MJN U S HLDG LLC
Method of preparing cytidine disodium triphosphate and application
The invention relates to a manufacturing method of disodium cytidine triphosphate, which comprises the following steps: proceeding enzymatic conversation with CMP material; proceeding ion exchange column chromatography; separating and purifying. The enzymatic conversation reaction principle is that: brewers' yeast gets the needed enzyme liquid of the enzymatic conversation reaction; brewers' yeast produces a plurality of biological enzyme as sugar glycolysis enzyme and phosphorylase with high sugar glycolysis ability; the biological energy produced by glycolysis glucose with sugar glycolysis enzyme is transferred to cytidine monophosphate by ATP; cytidine monophosphate and phosphate radical combines to form cytidine triphosphate with the effect of phosphorylase. The method shapes low cost, simple operation, high production, high product purity by optimization, which comprises the following steps: getting enzyme liquid; proceeding enzymatic conversation by adding the main material cytidine monophosphate, monobasic potassium phosphate of proper quantity and glucose; getting product by deposition, ion exchange column chromatography, segregation, purification, refining and vacuum drying.
Owner:BEIJING SL PHARMA
Process for preparing cytidine diphosphate choline
ActiveCN102586383AFast metabolismReduce manufacturing costMicroorganism based processesFermentationMicroorganismPh control
The invention relates to a process for preparing cytidine diphosphate choline. According to the method, denovo synthesis of pyrimidine is utilized as a basic reaction and choline materials are subjected to a reaction at the reaction temperature ranging from 20 DEG C to 38 DEG C for 10 to 72 hours with pH controlled at 5 to 7.5 in the presence of culture solution of microorganism A or further in the presence of culture solution of microorganism B or treatment so as to accumulatively produce cytidine diphosphate choline. Simple starting materials of carbon and nitrogen sources are utilized, in culture solution containing the choline materials, precursor of the cytidine diphosphate choline, namely cytidine monophosphate is synthesized by means of metabolic process, and cytidine diphosphate choline is cumulatively produced in the reaction solution. According to the method, expensive cytidine monophosphate or the precursor materials are avoided so that the preparing cost of the cytidine diphosphate choline is greatly reduced.
Owner:QILU PHARMA HAINAN +1
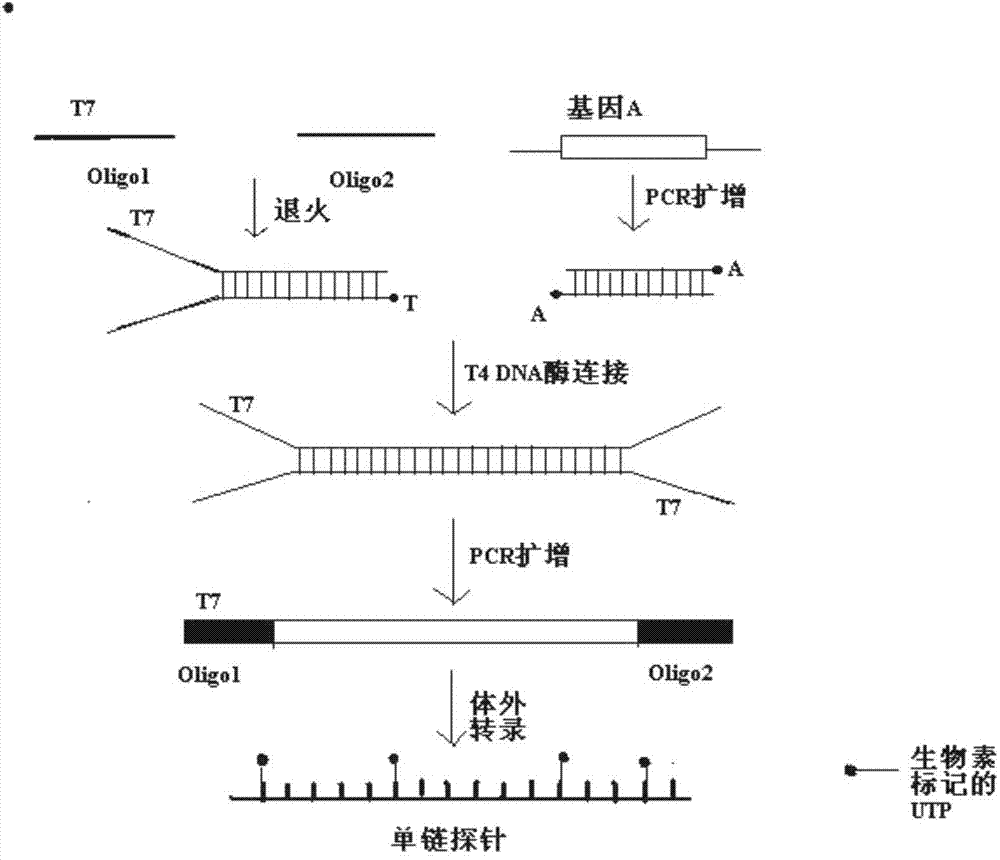
Preparation method of gene DNA (Deoxyribose Nucleic Acid) sequence capture probe
ActiveCN103898210AEfficient preparationImprove efficiencyMicrobiological testing/measurementDNA preparationDeoxyadenosine monophosphateBiotin
The invention discloses a preparation method of a gene DNA (Deoxyribose Nucleic Acid) sequence capture probe. According to the technical scheme, the method comprises the following steps: 1) designing two partially complementary primers and carrying out annealing treatment so as to form a partially double-chain structured split joint, and carrying out a coupled reaction between the split joint and an ordinary PCR (Polymerase Chain Reaction) amplification product (A1) of a target gene so as to obtain a target gene (A2) two ends of which are provided with joints; 2) designing corresponding primers according to the sequences of the joints and amplifying the target gene (A2) so as to obtain an amplification product (A3) with a T7 promoter; and 3) quantifying the product (A3), and adding NTP (Nucleotide Triphosphate) with biotin labels (wherein biotins are labeled on the NTP is labeled with biotins but ATP (Adenosine Triphosphate), CTP (Cytidine Triphosphate) and DTP (Deoxyadenosine Triphosphate) are not labeled with biotins) into the product (A3) under a certain condition so as to carry out an in-vitro transcription reaction, thereby finally obtaining an RNA (Ribose Nucleic Acid) probe with biotin labels (gene DNA sequence capture probe).
Owner:HANGZHOU D A GENETIC ENG
Artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol synthase coding sequence
InactiveCN101665793AIncrease contentAchieve mass productionEnzymesFermentationAgricultural scienceTransformation cell
The invention relates to an artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol synthase coding sequence in the technical field of gene engineering. The sequence is a sequence generated after one or a plurality of codons in nucleotide are replaced by degenerate codons of amino acid with same coding, as shown in 18-position to 926-position of SEQ ID NO: 3 or shown in 18-position to926-position of SEQ ID NO: 3. The method for improving the content of plant artemisinin by using the coding sequence includes the steps of: connecting the coding sequence to a plant expression regulatory sequence and constructing a plant expression carrier containing the coding sequence; transferring the expression carrier in step 1 to agrobacterium rhizogenes, then transferring agrobacterium rhizogenes to artemisia apiacea; through antibiotic screening, obtaining a transformation cell containing the coding sequence and regenerating a transgenosis plant. The invention relates to a polypeptide, and the sequence is shown in SEQ ID NO: 4. The coding sequence can improve the content of artemisinin in artemisia apiacea and the content of isoprene compound of other plants.
Owner:SUZHOU TANGJI BIOTECH CO LTD
Preparation method of uridine-5'-monophosphate disodium
ActiveCN104447922ASimple preparation processEasy to operateSugar derivativesSugar derivatives preparationAlcoholOrganic solvent
The invention discloses a preparation method of uridine-5'-monophosphate disodium. The method comprises the following steps: (1) mixing cytidine monophosphate (CMP) or sodium salt, sodium nitrite and deionized water, dropwise adding acid and / or acid anhydride, continuously reacting for 0-6 hours after completing dropwise adding to obtain reaction solution 1; (2) if residual quantity of CMP is lower than 1%, adjusting the pH value of the reaction solution 1 to 6.4-7.2 to obtain reaction solution 2; (3) mixing the reaction solution 2 and 95v / v% of ethyl alcohol, crystallizing and filtering to obtain a uridine-5'-monophosphate disodium crude product; (4) mixing the uridine-5'-monophosphate disodium crude product and water, and adjusting the pH value to 7.0-8.5 to obtain reaction solution 3; and (5) mixing the reaction solution 3 and an organic solvent, recrystalizing and filtering to obtain the uridine-5'-monophosphate disodium.
Owner:美亚药业海安有限公司
Amphipathic Eu (III) complex and application thereof in detection for cytidine triphosphate
InactiveCN105277523AEasy to prepareMild reaction conditionsFluorescence/phosphorescenceDodecaneNitrogen
The invention discloses an amphipathic Eu (III) complex as well as a preparation method and application thereof in detection for cytidine triphosphate. The structural formula of the complex is as shown in the specification. The amphipathic Eu (III) complex is formed by matching a ligand with Eu (III), wherein a compound which takes octadecyl as hydrophobic tail part and takes 1,4,7,10-tetraazacyclododecane as a hydrophobic head part serves as the ligand. The preparation method is simple and the reaction condition is mild. The amphipathic Eu (III) complex provided by the invention has the characteristics of long fluorescent lifetime, large stokes shift and unsaturated coordination; a super-molecule sensing interface is assembled and formed in a water phase; the energy donor alpha-naphthyl phosphate sodium is introduced into the system and the amphipathic Eu (III) complex serves as an energy receptor for receiving energy and emitting Eu (III) characteristic light; when the cytidine triphosphate is contained in a to-be-detected sample, the alpha-naphthyl phosphate sodium on the surface of the self-assembly body is replaced by the cytidine triphosphate, so that the fluorescence is quenched and the selective identification for the cytidine triphosphate is realized.
Owner:SHAANXI NORMAL UNIV
Cytidine disodium triphosphate injection liquid and preparation method thereof
InactiveCN102335127AFormulation stabilityQuality improvementOrganic active ingredientsNervous disorderCytidine diphosphateCytidine triphosphate
The invention relates to a medicinal preparation of a compound, and especially relates to a cytidine disodium triphosphate injection liquid, and a preparation method thereof. The preparation comprises cytidine disodium triphosphate, propylene glycol, and injection water. The preparation method comprises steps that: a small amount of injection water (with a temperature cooled to below 20 DEG C), propylene glycol and cytidine disodium triphosphate are fetched; cytidine disodium triphosphate is added to injection water, and is completely dissolved; propylene glycol is added to the solution, and is well-mixed by stirring; a 4% sodium hydroxide solution is slowly dropped into the solution, until the pH value of the solution is 8.6-.8.9; injection-use active carbon which takes 0.1% (g / ml) of the solution by volume is added to the solution, and the solution is stirred for 15 minutes; the solution is filtered, and injection water is added until a full dose is reached; and the solution is then processed through filtering, filling, sealing, lamp inspecting, and packaging. The injection liquid provided by the invention has advantages of simple formulation, simple technology, high production efficiency, strong medicine liquid stability, and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Genetic engineering preparation method of cytidine triphosphate
InactiveCN102199644AFermentation is easy to controlReaction conditions are easy to controlMicroorganism based processesFermentationMicroorganismCytidine diphosphate
The invention discloses a genetic engineering preparation method of cytidine triphosphate. In the method, a culture or a treatment substance of single genetic engineering strain is used as an enzyme source; during catalysis, ammonium chloride, saratin and the like are used for reaction, so that the cytidine triphosphate is generated in a reaction solution and accumulated and is extracted from the reaction solution. The invention has the benefits that: (1) the reaction is easier to control due to the adoption of the microbial catalytic reaction ; (2) the cost of a substrate is lower and the cytidine triphosphate can be produced at low cost; and (3) the reaction is quick and the conversion rate is higher. The genetic engineering preparation method can be widely applied to preparation of the cytidine triphosphat.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Preparation method of poly i-c
ActiveCN110256516AEfficient removalEasy to removeSugar derivativesFermentationInosine DiphosphatePhosphoric acid
The invention belongs to a preparation method of poly i-c and belongs to the technical field of biomedicine. The preparation method includes: utilizing inosine diphosphate to prepare polyinosinic acid, and using cytidine diphosphate to prepare polycytidylic acid; utilizing the prepared polyinosinic acid to react with the polycytidylic acid to obtain a high-purity poly i-c product. Poly i-c is prepared through self-developed high-purity polyinosinic acid and polycytidylic acid, so that prepared poly i-c is high in purity, and treatment difficulty in the process of preparing is lowered.
Owner:宋龙飞
Preparation method of gene dna sequence capture probe
ActiveCN103898210BEfficient preparationImprove efficiencyMicrobiological testing/measurementDNA preparationBiotinCytidine diphosphate
The invention discloses a preparation method of a gene DNA (Deoxyribose Nucleic Acid) sequence capture probe. According to the technical scheme, the method comprises the following steps: 1) designing two partially complementary primers and carrying out annealing treatment so as to form a partially double-chain structured split joint, and carrying out a coupled reaction between the split joint and an ordinary PCR (Polymerase Chain Reaction) amplification product (A1) of a target gene so as to obtain a target gene (A2) two ends of which are provided with joints; 2) designing corresponding primers according to the sequences of the joints and amplifying the target gene (A2) so as to obtain an amplification product (A3) with a T7 promoter; and 3) quantifying the product (A3), and adding NTP (Nucleotide Triphosphate) with biotin labels (wherein biotins are labeled on the NTP is labeled with biotins but ATP (Adenosine Triphosphate), CTP (Cytidine Triphosphate) and DTP (Deoxyadenosine Triphosphate) are not labeled with biotins) into the product (A3) under a certain condition so as to carry out an in-vitro transcription reaction, thereby finally obtaining an RNA (Ribose Nucleic Acid) probe with biotin labels (gene DNA sequence capture probe).
Owner:HANGZHOU D A GENETIC ENG
Artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence
InactiveCN101665796BIncrease contentAchieve mass productionTransferasesFermentationAgricultural scienceTransformation cell
The invention relates to an artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence in the technical field of gene engineering. The sequence is a sequence generated after one or a plurality of codons in nucleotide are replaced by degenerate codons of amino acid with same coding, as shown in 15-pisition to 1214-position of SEQ ID NO: 3 or shown in 15-position-1214-position of SEQ ID NO: 3. The method for improving the content of plant artemisinin by using the coding sequence includes the steps of: connecting the coding sequence to a plant expression regulatory sequence and constructing a plant expression carrier containing the artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence; transferring the expression carrier in step 1 to agrobacterium rhizogenes, then transferring agrobacterium rhizogenes to artemisia apiacea; through antibiotic screening, obtaining a transformation cell containing the artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence and regenerating a transgenosis plant. The coding sequence can improve the content of artemisinin in artemisia apiacea and the content of isoprene compound of other plants.
Owner:SUZHOU TANGJI BIOTECH CO LTD
Method of preparing cytidine disodium triphosphate and application
The invention relates to a manufacturing method of disodium cytidine triphosphate, which comprises the following steps: proceeding enzymatic conversation with CMP material; proceeding ion exchange column chromatography; separating and purifying. The enzymatic conversation reaction principle is that: brewers' yeast gets the needed enzyme liquid of the enzymatic conversation reaction; brewers' yeast produces a plurality of biological enzyme as sugar glycolysis enzyme and phosphorylase with high sugar glycolysis ability; the biological energy produced by glycolysis glucose with sugar glycolysis enzyme is transferred to cytidine monophosphate by ATP; cytidine monophosphate and phosphate radical combines to form cytidine triphosphate with the effect of phosphorylase. The method shapes low cost, simple operation, high production, high product purity by optimization, which comprises the following steps: getting enzyme liquid; proceeding enzymatic conversation by adding the main material cytidine monophosphate, monobasic potassium phosphate of proper quantity and glucose; getting product by deposition, ion exchange column chromatography, segregation, purification, refining and vacuum drying.
Owner:BEIJING SL PHARMA
Preparation method of powdery cytidine triphosphate or sodium salt of cytidine triphosphate
InactiveCN104558078AEasy accessEliminates the grinding stepSugar derivativesSugar derivatives preparationOrganic solventCytidine diphosphate
The invention discloses a preparation method of powdery cytidine triphosphate or a sodium salt of cytidine triphosphate. The preparation method comprises the following steps: mixing and precipitating an acid solution containing cytidine triphosphate or the sodium salt of cytidine triphosphate with methanol of which the volume is 5-20 times of that of the acid solution so as to obtain powdery cytidine triphosphate or the sodium salt of powdery cytidine triphosphate, or the preparation method comprises the following steps: (1) mixing the acid solution containing cytidine triphosphate or the sodium salt of cytidine triphosphate with a first organic solvent of which the volume is 2-20 times of that of the acid solution so as to obtain a first precipitate; (2) mixing the first precipitate with ethanediol or glycerin of which the volume is 2-20 times of that of the first precipitate so as to obtain a dissolved solution; (3) mixing and precipitating the dissolved solution with a second organic solvent of which the volume is 2-20 times of that of the dissolved solution so as to obtain powdery cytidine triphosphate or the sodium salt of powdery cytidine triphosphate.
Owner:SHANGHAI YOUKESAI BIOTECH
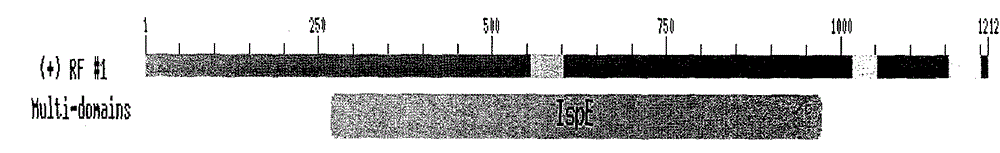
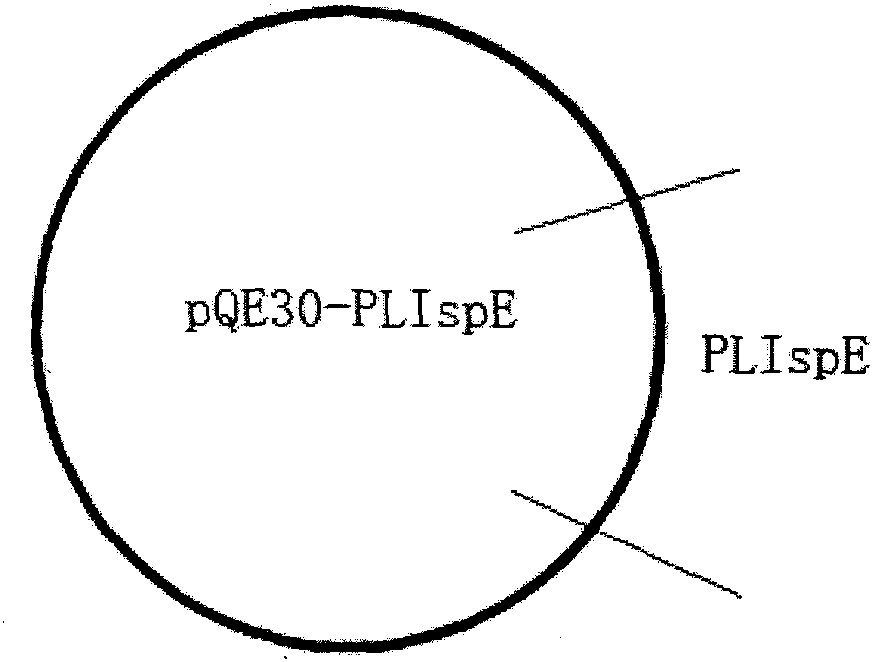
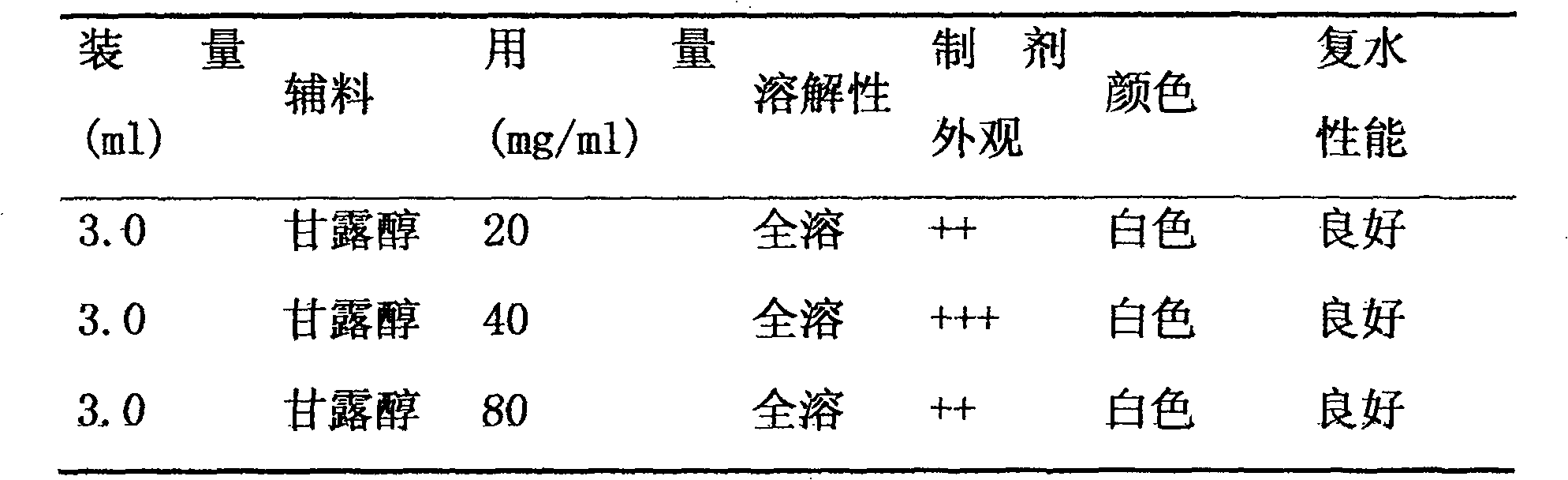
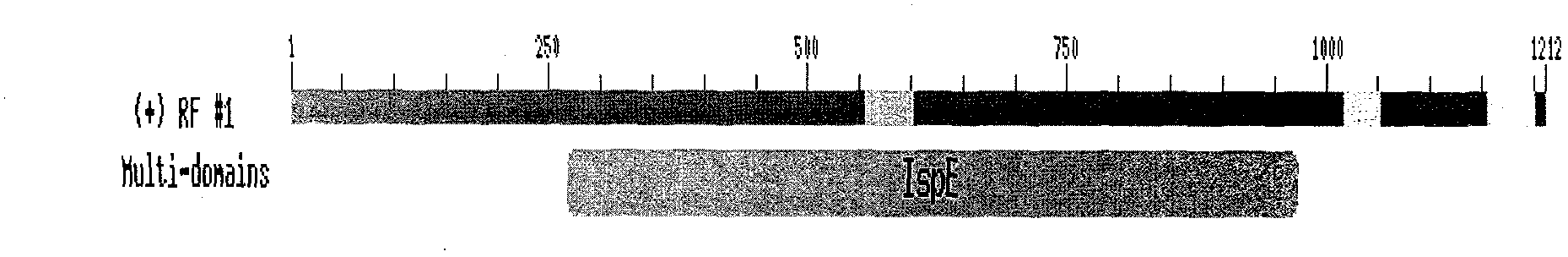
Peony 4-diphosphate cytidine-2-c-methylerythrosekinase (plispe) gene and its coding product and application
The invention discloses a PLIspE gene and a coded protein and application thereof. The gene is cloned from Paeonia lactiflora by constructing a cDNA library for the first time, so a gap in separation and cloning of a 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase gene from the traditional Chinese medicine--Paeonia lactiflora is filled in. The PLIspE gene provided by the invention has a nucleotide sequence shown in SEQ ID NO. 1, or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of nucleotides of the nucleotide sequence shown in SEQ ID NO. 1, or an allele of the gene or a nucleotide sequence derived from the allele. The protein coded by the gene has an amino acid sequence shown in SEQ ID NO. 2 or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of amino acids of the amino acid sequence shown in SEQ ID NO. 2. The PLIspE gene can increase the content of metabolites of 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol in Paeonia lactiflora through biotechnology and has good application prospects in aspects like quality improvement of the medicinal material Paeonia lactiflora and synthetic biology of active components of Paeonia lactiflora.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Cytidine disodium triphosphate and arginine composition and preparation method thereof
ActiveCN100528174CImprove stabilityExtended storage timeOrganic active ingredientsPowder deliveryArginineFreeze-drying
Owner:山东北大高科华泰制药有限公司
Paeonia lactiflora 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (PLIspE) gene, and coded product and application thereof
The invention discloses a PLIspE gene and a coded protein and application thereof. The gene is cloned from Paeonia lactiflora by constructing a cDNA library for the first time, so a gap in separation and cloning of a 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase gene from the traditional Chinese medicine--Paeonia lactiflora is filled in. The PLIspE gene provided by the invention has a nucleotide sequence shown in SEQ ID NO. 1, or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of nucleotides of the nucleotide sequence shown in SEQ ID NO. 1, or an allele of the gene or a nucleotide sequence derived from the allele. The protein coded by the gene has an amino acid sequence shown in SEQ ID NO. 2 or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of amino acids of the amino acid sequence shown in SEQ ID NO. 2. The PLIspE gene can increase the content of metabolites of 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol in Paeonia lactiflora through biotechnology and has good application prospects in aspects like quality improvement of the medicinal material Paeonia lactiflora and synthetic biology of active components of Paeonia lactiflora.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Liponucleotide-based therapy for ards
InactiveCN109475554ASugar derivativesCarbohydrate active ingredientsCDP-DiacylglycerolCDP ethanolamine
Compositions and method are therefore disclosed for treating ARDS. In particular, disclosed a composition that contains one, two, or more cytidine diphosphate (CDP)-conjugated precursors selected fromthe group consisting of CDP-choline, CDP-ethanolamine, and CDP-diacylglycerol (CDP-DAG) in a pharmaceutically acceptable carrier for use in treating ARDS.
Owner:OHIO STATE INNOVATION FOUND
Cordyceps sinensis ctp synthetase, coding gene and application thereof
ActiveCN104651324BIncrease productionEnhance expressive abilityBacteriaMicroorganism based processesBiosynthetic genesCytidine diphosphate
The invention relates to CTP (Cytidine Triphosphate) synthetase from Bailing producing strain cordyceps sinensis hirsutella sinensis participated in synthesis of metabolic cytidine triphosphate from uridine triphosphate, a gene for coding the synthetase and application of the CTP synthetase; the CTP synthetase has an amino acid sequence represented by SEQ ID No.2 and with the homology of more than 90%; the coding gene has a nucleotide sequence represented by SEQ ID No.4 and with the homology of more than 90%; a metabolic pathway of synthesis of cytidine triphosphate by uridine triphosphate is researched in a detailed manner in principle; cloned DNA of the nucleotide sequence provided by the invention can be transferred into engineering bacteria through transduction, conversion and conjugal transfer methods; by adjusting expression of a biosynthetic gene of cytidine triphosphate, the high expressivity of host cytidine triphosphate is given; an effective way is provided for increasing the yield of cytidine triphosphate; and therefore, the CTP synthetase has great application prospect.
Owner:ZHEJIANG UNIV OF TECH +2
A kind of preparation method of polymyocyte
ActiveCN110256516BEfficient removalEasy to removeSugar derivativesSugar derivatives preparationInosinic acidPolymer science
The invention belongs to a preparation method of poly i-c and belongs to the technical field of biomedicine. The preparation method includes: utilizing inosine diphosphate to prepare polyinosinic acid, and using cytidine diphosphate to prepare polycytidylic acid; utilizing the prepared polyinosinic acid to react with the polycytidylic acid to obtain a high-purity poly i-c product. Poly i-c is prepared through self-developed high-purity polyinosinic acid and polycytidylic acid, so that prepared poly i-c is high in purity, and treatment difficulty in the process of preparing is lowered.
Owner:保定乐研生物科技有限公司
Cordyceps sinensis ctp synthase, encoding gene and its application
ActiveCN103031280BIncrease productionEnhance expressive abilityBacteriaMicroorganism based processesCytidine diphosphateBiology
The invention relates to a CTP (Cytidine Triphosphate) synthetase which is from Bailing production bacterium cordyceps sinensis hirsutella sinensis and takes part in synthesis of metabolic cytidine triphosphate from uridine triphosphate, a gene encoding the synthetase and an application of the synthetase. The CTP synthetase has more than 90% of homology of an amino acid sequence represented by SEQ ID No.1 or SEQ ID No. 2, and the encoding gene of the synthetase has more than 0% of homology of a nucleotide sequence represented by SEQ ID No. 3 or SEQ ID No.4. According to the invention, a metabolic pathway for synthesizing the cytidine triphosphate from the uridine triphosphate is deeply studied in principle. Cloned DNA (Deoxyribonucleic Acid) comprising the nucleotide sequence provided by the invention can be transferred into engineering bacteria by transduction, conversion and transconjunction, and the expression of a biological synthesis gene of the cytidine triphosphate is regulated to ensure high expressivity of the host cytidine triphosphate, so that an effective way for enlarging the output of the cytidine triphosphate is provided, and the prospect in application is great.
Owner:ZHEJIANG UNIV OF TECH +1
Genetic engineering preparation method of cytidine triphosphate
InactiveCN102199644BFermentation is easy to controlReaction conditions are easy to controlMicroorganism based processesFermentationMicroorganismCytidine diphosphate
The invention discloses a genetic engineering preparation method of cytidine triphosphate. In the method, a culture or a treatment substance of single genetic engineering strain is used as an enzyme source; during catalysis, ammonium chloride, saratin and the like are used for reaction, so that the cytidine triphosphate is generated in a reaction solution and accumulated and is extracted from the reaction solution. The invention has the benefits that: (1) the reaction is easier to control due to the adoption of the microbial catalytic reaction ; (2) the cost of a substrate is lower and the cytidine triphosphate can be produced at low cost; and (3) the reaction is quick and the conversion rate is higher. The genetic engineering preparation method can be widely applied to preparation of the cytidine triphosphat.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
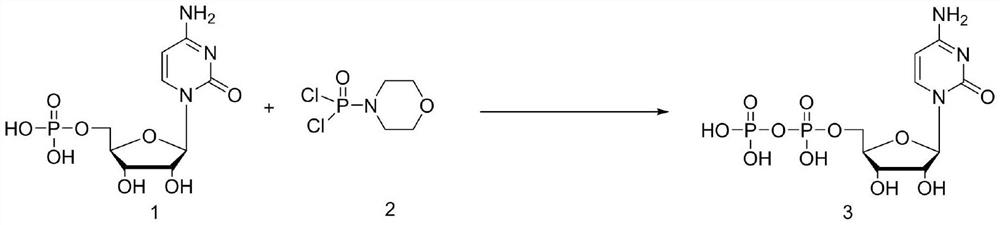
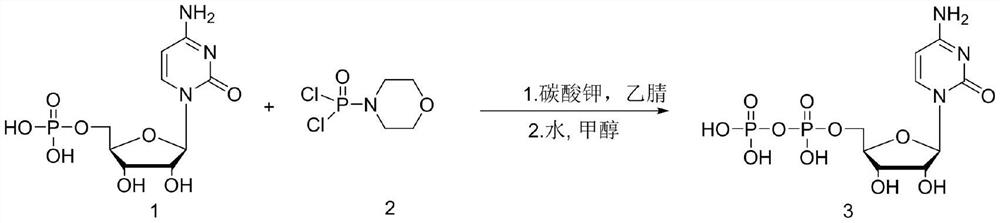
Method for synthesizing cytidine diphosphate
ActiveCN113999270AReduce usageReduce generationSugar derivativesSugar derivatives preparationAlcoholMorpholine
The invention discloses a method for synthesizing cytidine diphosphate, and belongs to the field of nucleoside synthesis in organic chemistry. The method comprises the following reaction steps that cytidine monophosphate and dichlorophosphoryl morpholine serve as raw materials, condensation is conducted under the base catalysis effect, then a crude product cytidine diphosphate is obtained through acyl chloride hydrolysis, recrystallization is conducted through an alcohol / water mixed system, and a finished cytidine diphosphate product with the HPLC purity larger than 95% is obtained. The whole reaction only comprises two steps, reaction operation is simple, and the total yield reaches 70% or above.
Owner:TUOXIN GROUP +1
Artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol synthase coding sequence
InactiveCN101665793BIncrease contentAchieve mass productionEnzymesFermentationAgricultural scienceTransformation cell
The invention relates to an artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol synthase coding sequence in the technical field of gene engineering. The sequence is a sequence generated after one or a plurality of codons in nucleotide are replaced by degenerate codons of amino acid with same coding, as shown in 18-position to 926-position of SEQ ID NO: 3 or shown in 18-position to 926-position of SEQ ID NO: 3. The method for improving the content of plant artemisinin by using the coding sequence includes the steps of: connecting the coding sequence to a plant expression regulatory sequence and constructing a plant expression carrier containing the coding sequence; transferring the expression carrier in step 1 to agrobacterium rhizogenes, then transferring agrobacterium rhizogenes to artemisia apiacea; through antibiotic screening, obtaining a transformation cell containing the coding sequence and regenerating a transgenosis plant. The invention relates to a polypeptide, and the sequence is shown in SEQ ID NO: 4. The coding sequence can improve the content of artemisinin in artemisia apiacea and the content of isoprene compound of other plants.
Owner:SUZHOU TANGJI BIOTECH CO LTD
Paeonia lactiflora 2-C-methyl-D-erythritol-4-cyclodiphaophate synthase (PLIspF) gene, and coded product and application thereof
The invention discloses a PLIspF gene and a coded protein and application thereof. The gene is cloned from Paeonia lactiflora by constructing a cDNA library for the first time, so a gap in separation and cloning of a 2-C-methyl-D-erythritol-4-cyclodiphaophate synthase gene from the traditional Chinese medicine--Paeonia lactiflora is filled in. The PLIspF gene provided by the invention has a nucleotide sequence shown in SEQ ID NO. 1, or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of nucleotides of the nucleotide sequence shown in SEQ ID NO. 1, or an allele of the gene or a nucleotide sequence derived from the allele. The protein coded by the gene has an amino acid sequence shown in SEQ ID NO. 2 or a homologous sequence obtained through addition, substitution, insertion or deletion of one or a plurality of amino acids of the amino acid sequence shown in SEQ ID NO. 2. The PLIspF gene can increase the content of metabolites of 2-phospho-4-(cytidine 5'-phospho)-2-C-methyl-D-erythritol in Paeonia lactiflora through biotechnology and has good application prospects in aspects like quality improvement of the medicinal material Paeonia lactiflora and synthetic biology of active components of Paeonia lactiflora.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence
InactiveCN101665796AIncrease contentAchieve mass productionTransferasesFermentationCytidine diphosphateGenetic engineering
The invention relates to an artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence in the technical field of gene engineering. The sequence is a sequence generatedafter one or a plurality of codons in nucleotide are replaced by degenerate codons of amino acid with same coding, as shown in 15-pisition to 1214-position of SEQ ID NO: 3 or shown in 15-position-1214-position of SEQ ID NO: 3. The method for improving the content of plant artemisinin by using the coding sequence includes the steps of: connecting the coding sequence to a plant expression regulatory sequence and constructing a plant expression carrier containing the artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence; transferring the expression carrier in step 1 to agrobacterium rhizogenes, then transferring agrobacterium rhizogenes to artemisia apiacea; through antibiotic screening, obtaining a transformation cell containing the artemisia apiacea4-(5'-cytidine diphosphate)-2-C-methyl-D-erythritol kinase coding sequence and regenerating a transgenosis plant. The coding sequence can improve the content of artemisinin in artemisia apiacea and the content of isoprene compound of other plants.
Owner:SUZHOU TANGJI BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com