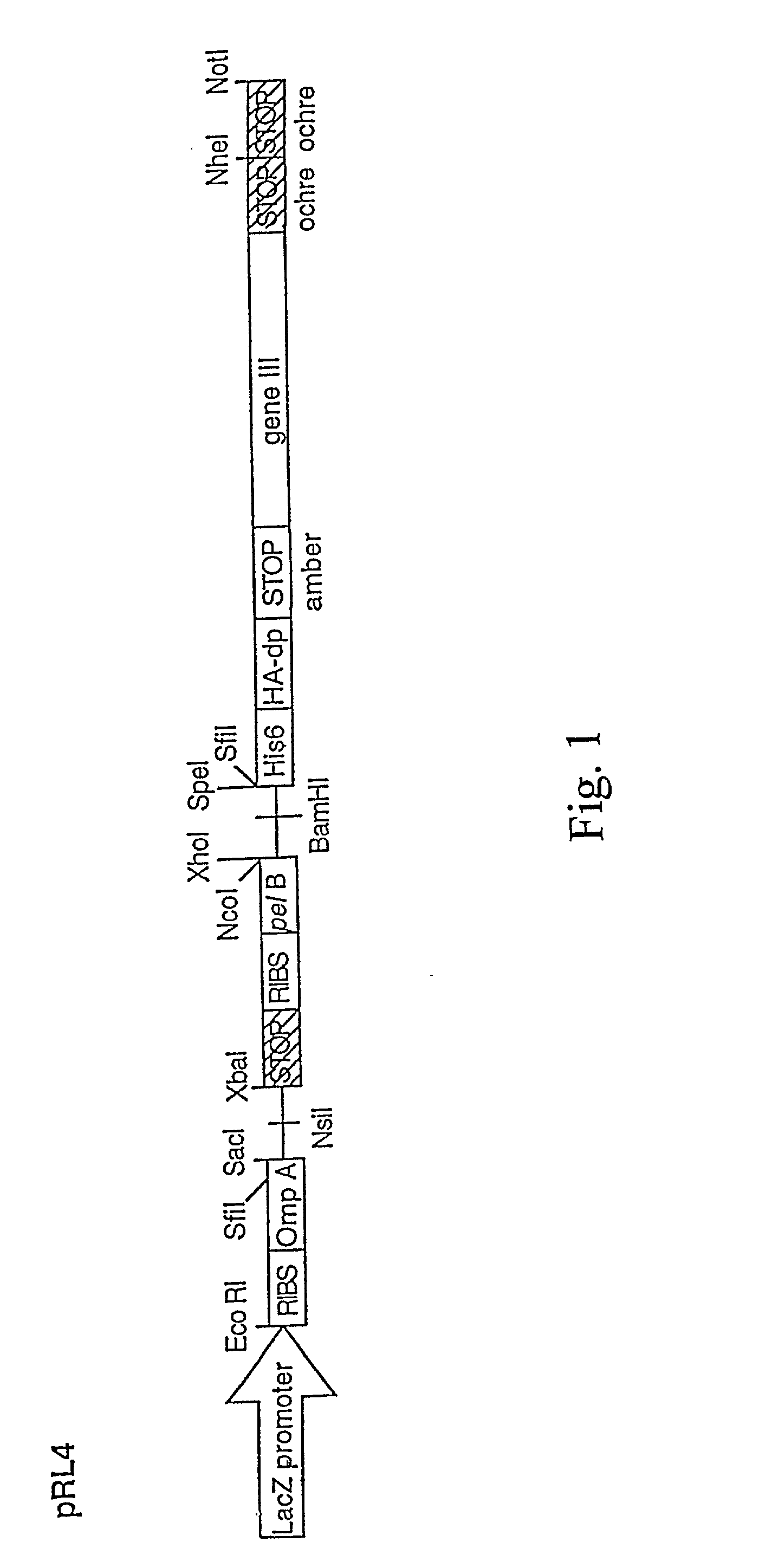
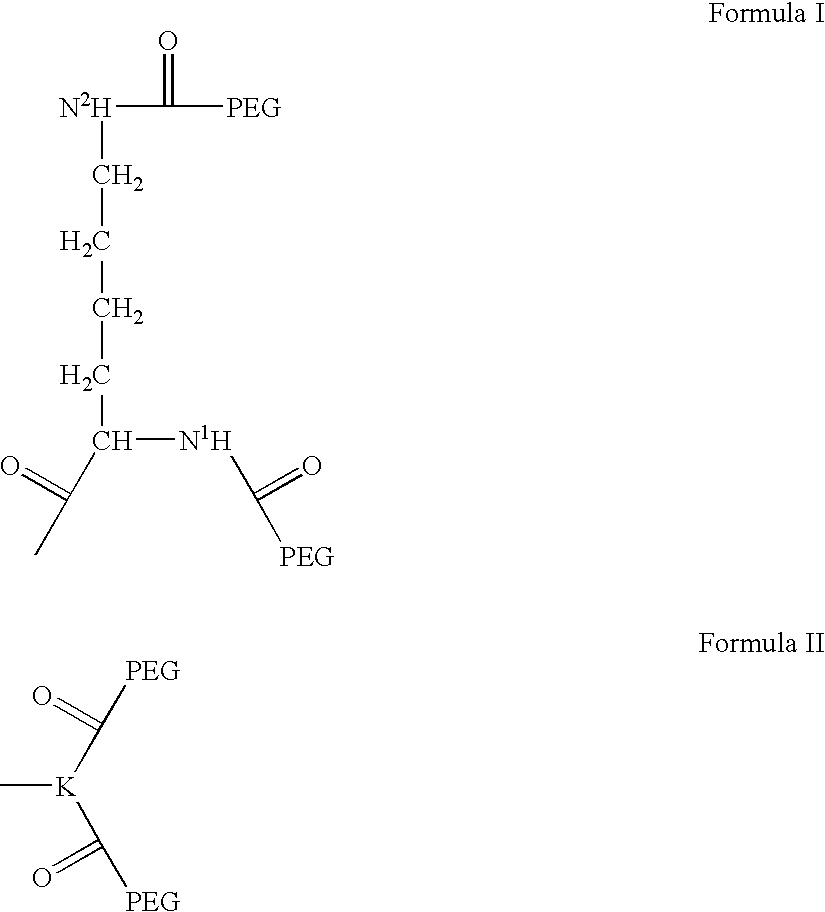
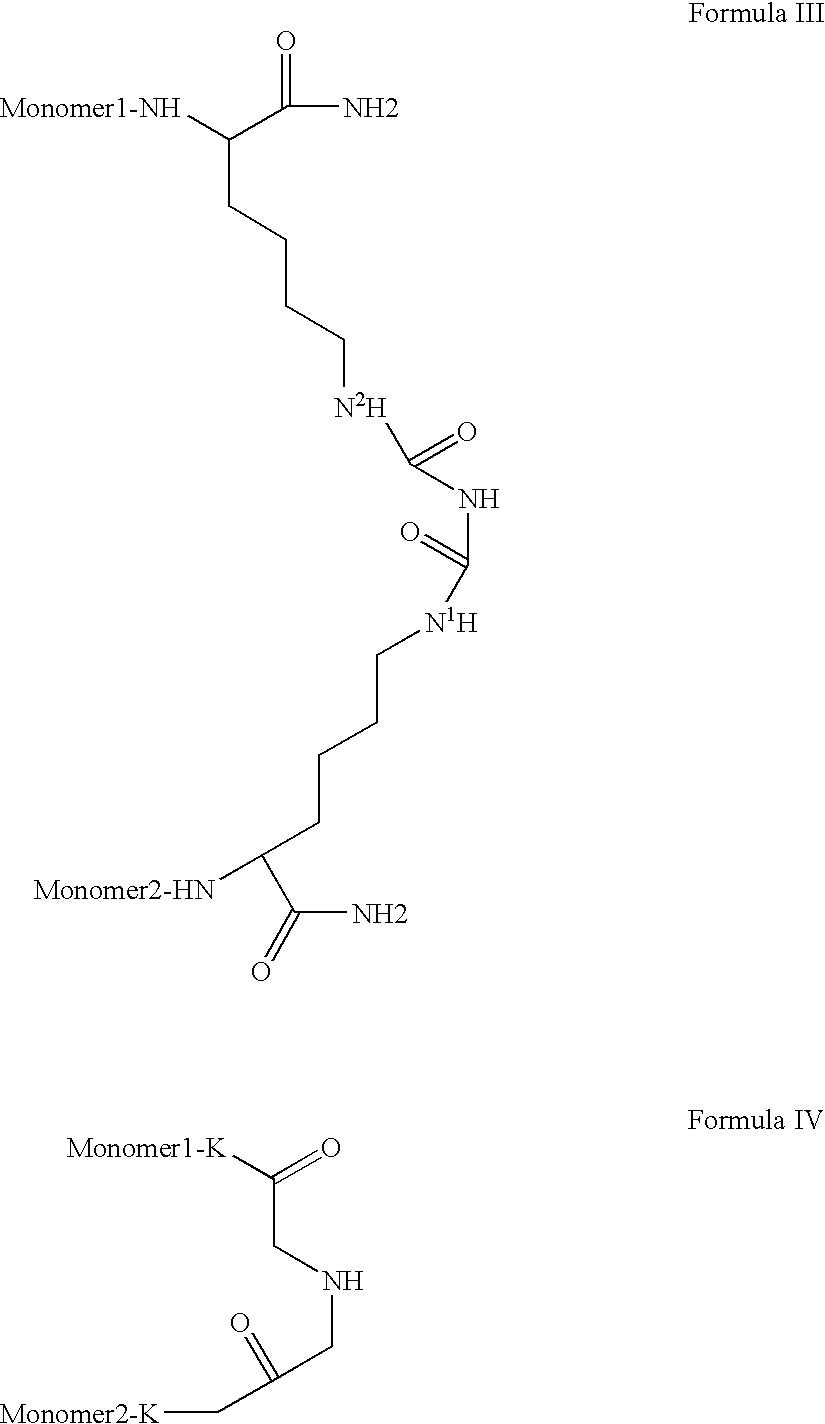
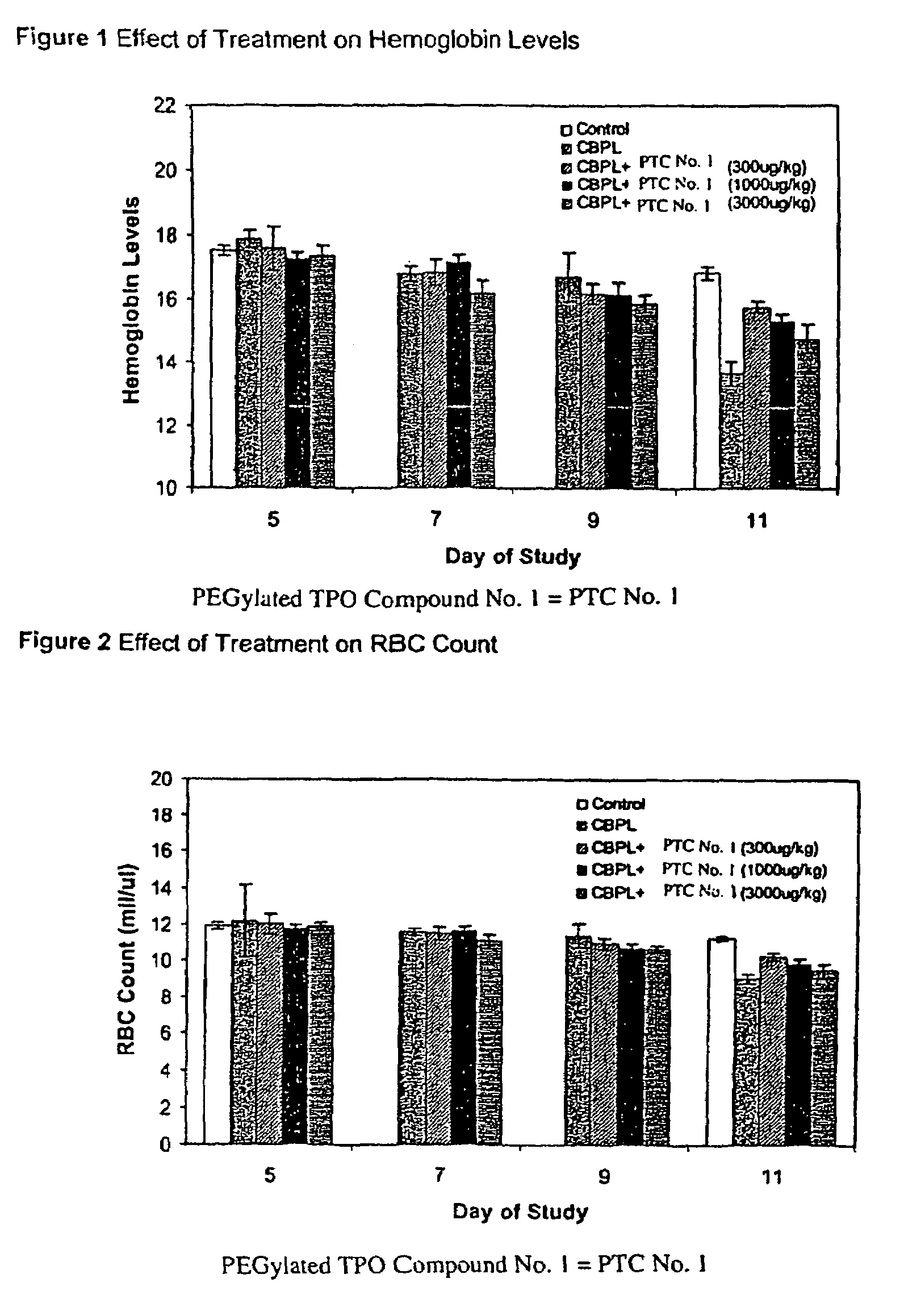
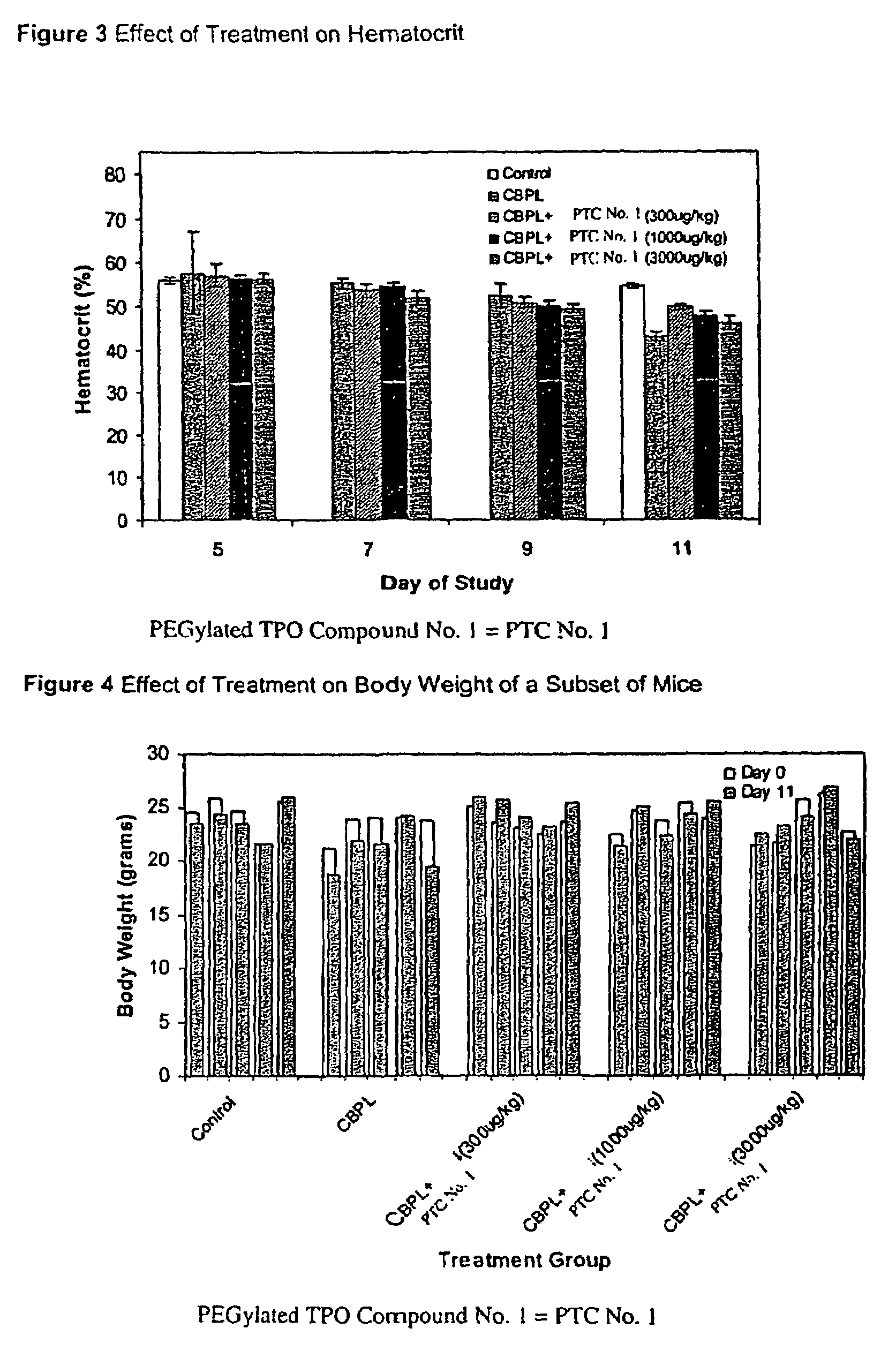
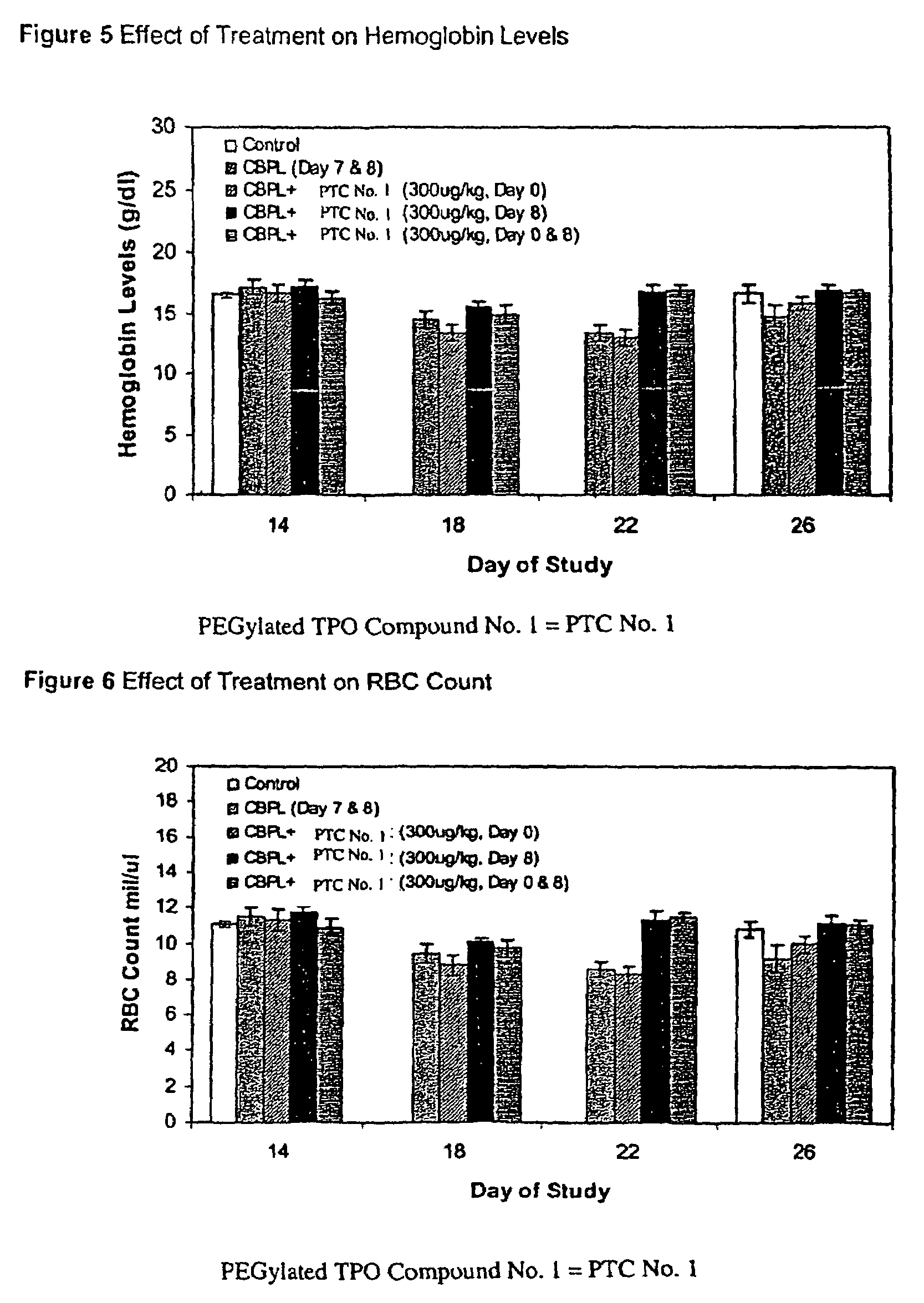
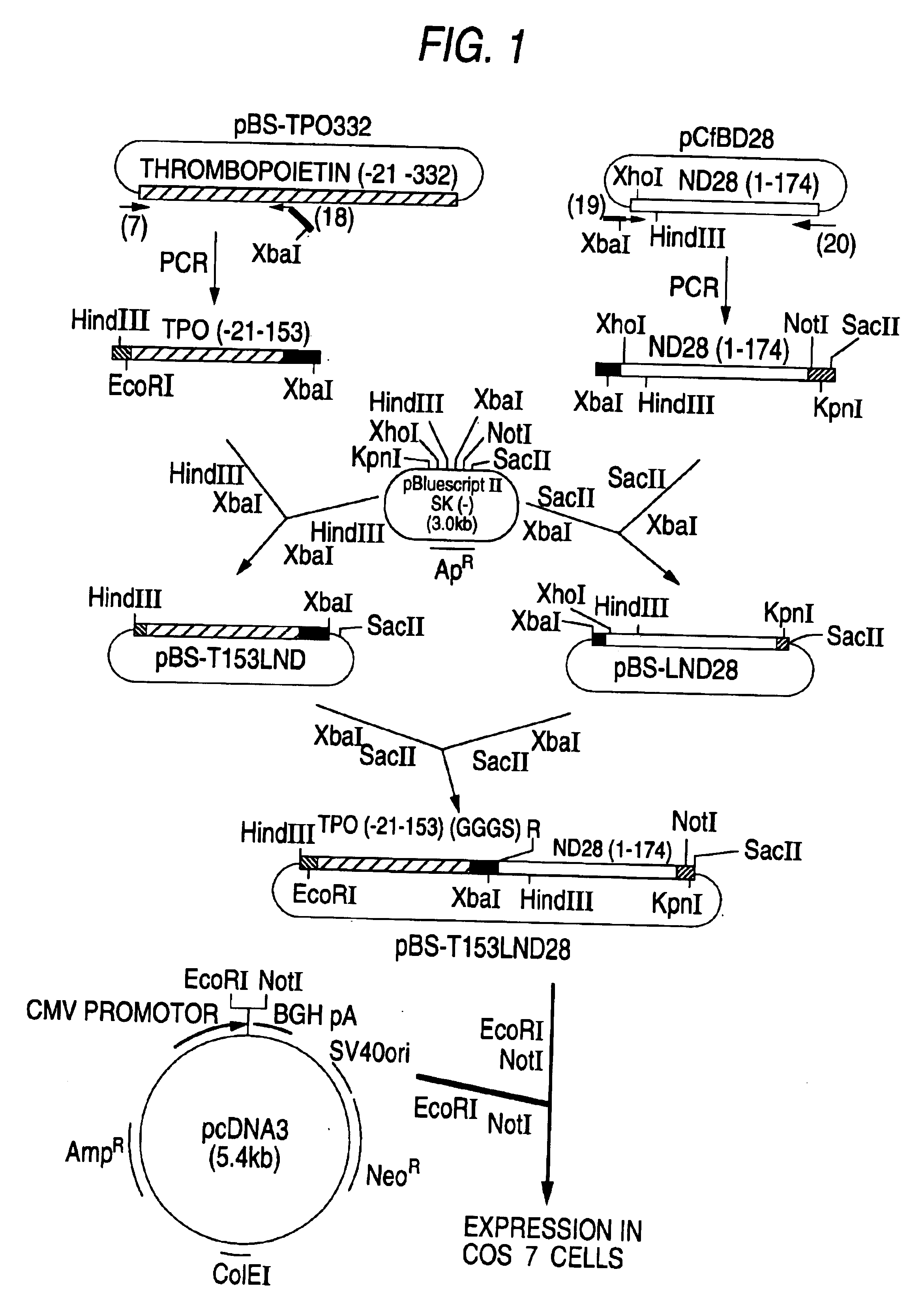
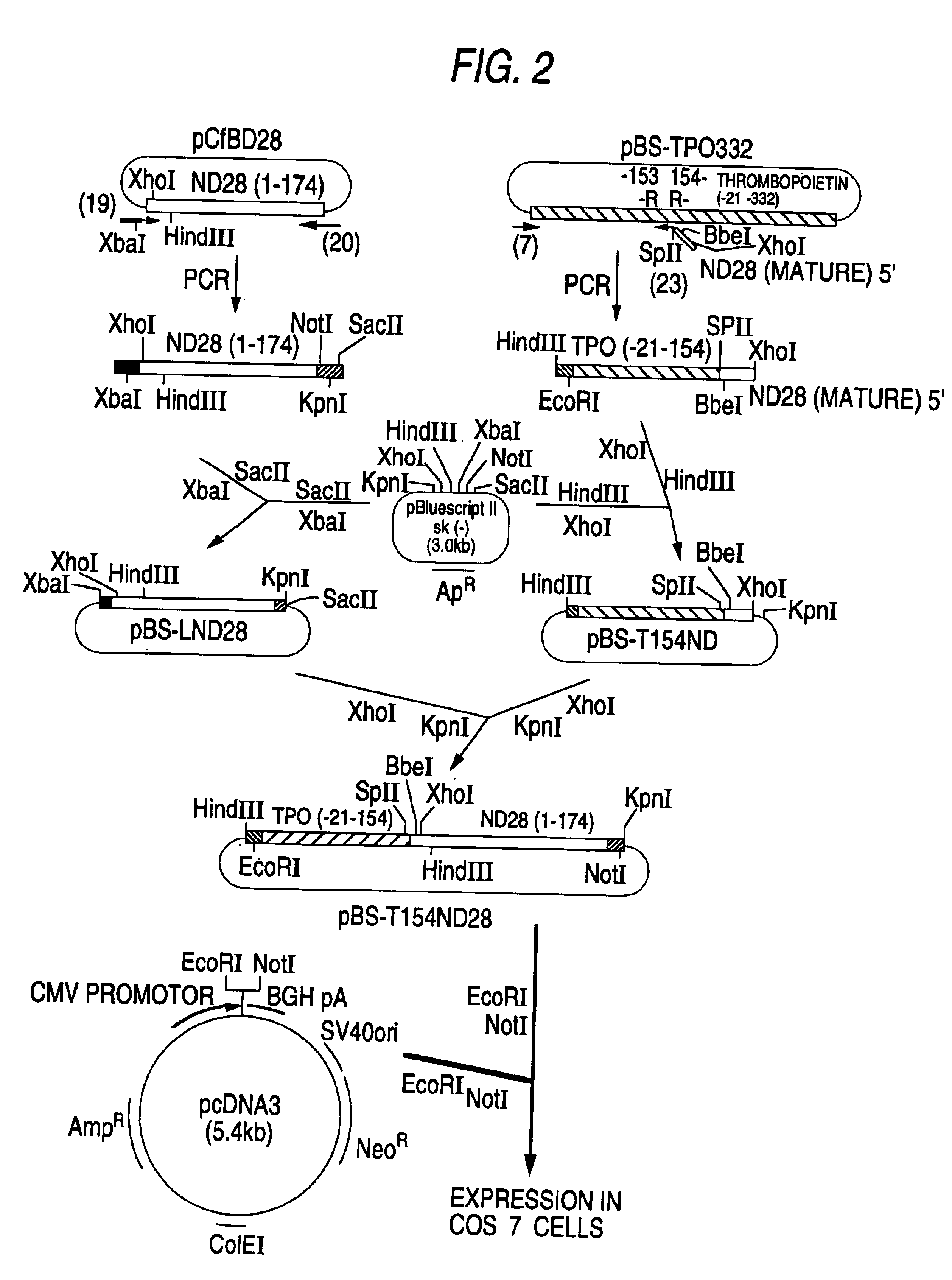
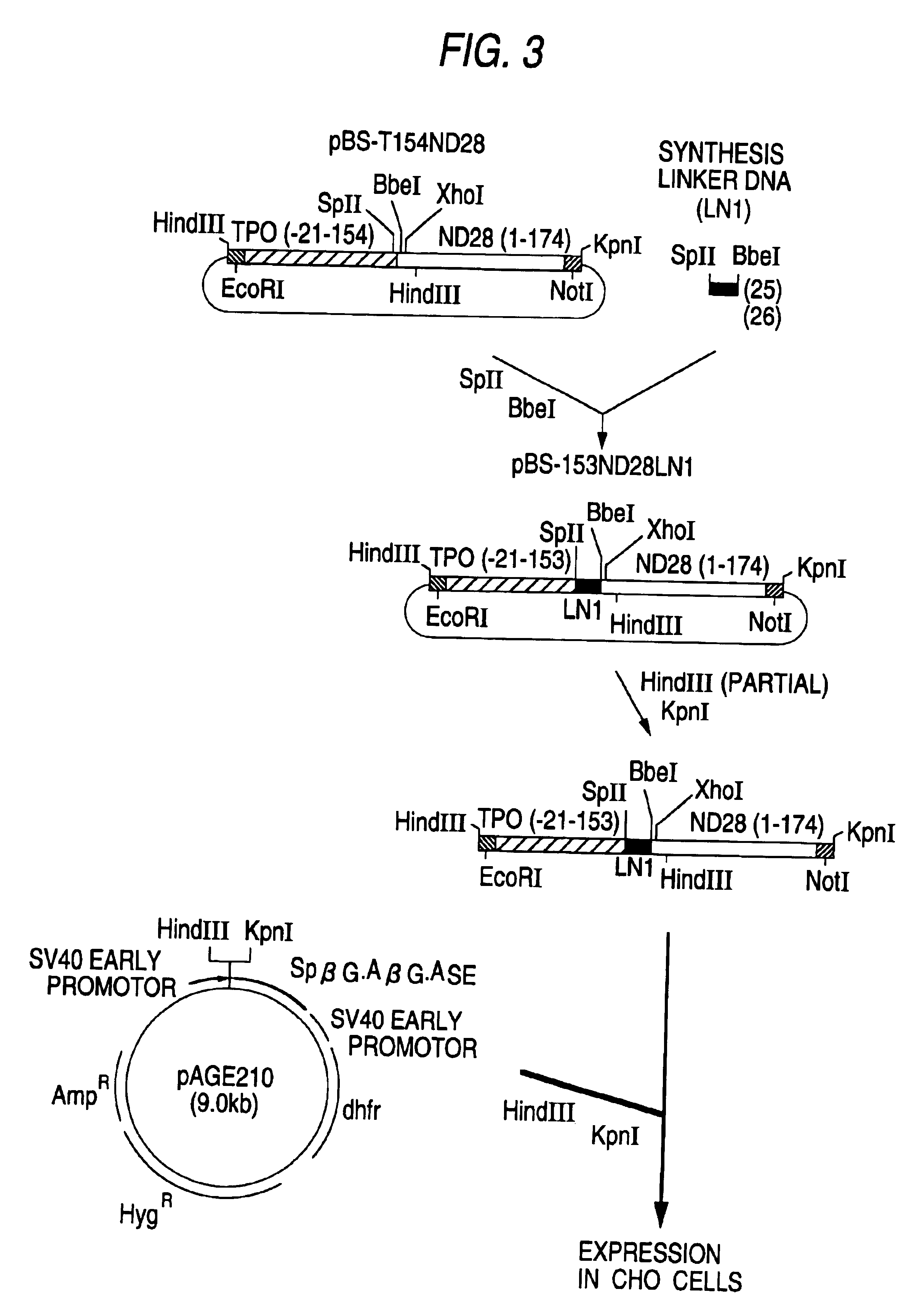
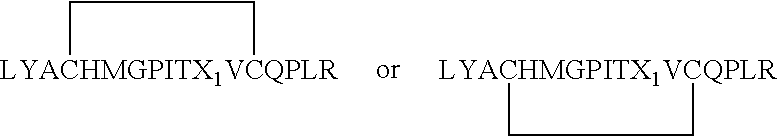Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81results about "Thrombopoietin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rationally designed antibodies
Antibodies or fragments thereof having CDR regions replaced or fused with biologically active peptides are described. Flanking sequences may optionally be attached at one or both the carboxy-terminal and amino-terminal ends of the peptide in covalent association with adjacent framework regions. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
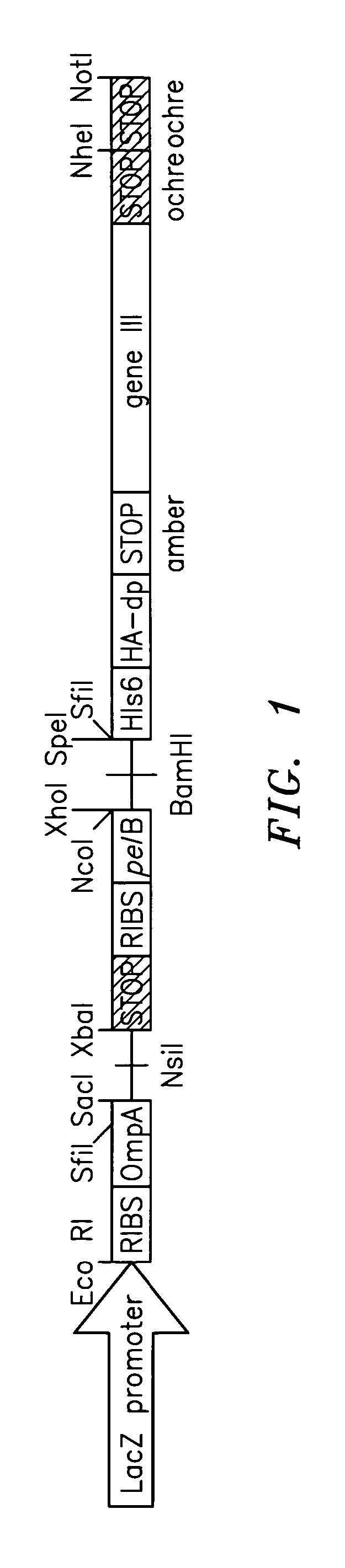
Genetically modified non-human animals and methods of use thereof
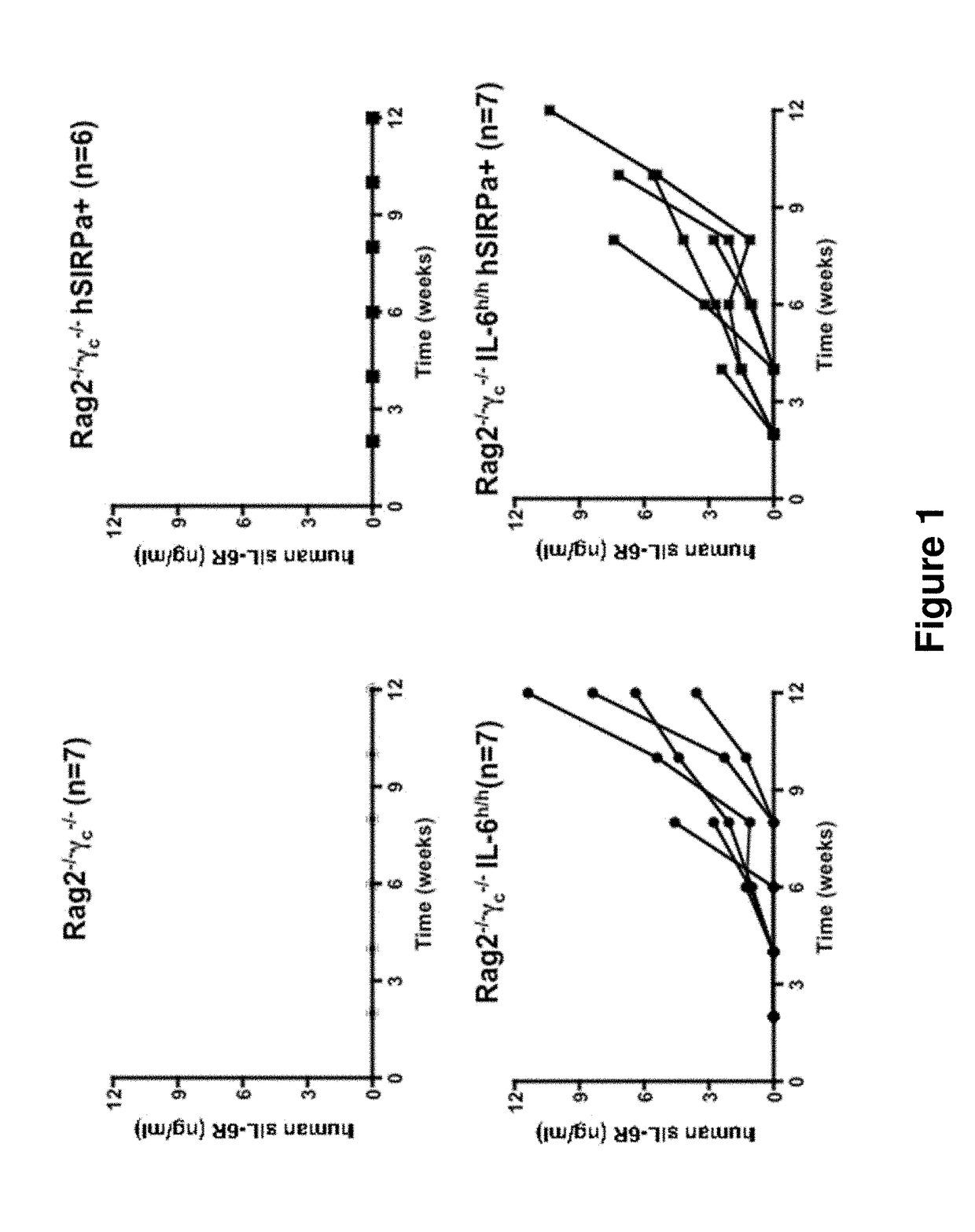
ActiveUS20140134662A1Reduce rateReduced viabilityCompounds screening/testingThrombopoietinDiseaseHematopoietic cell
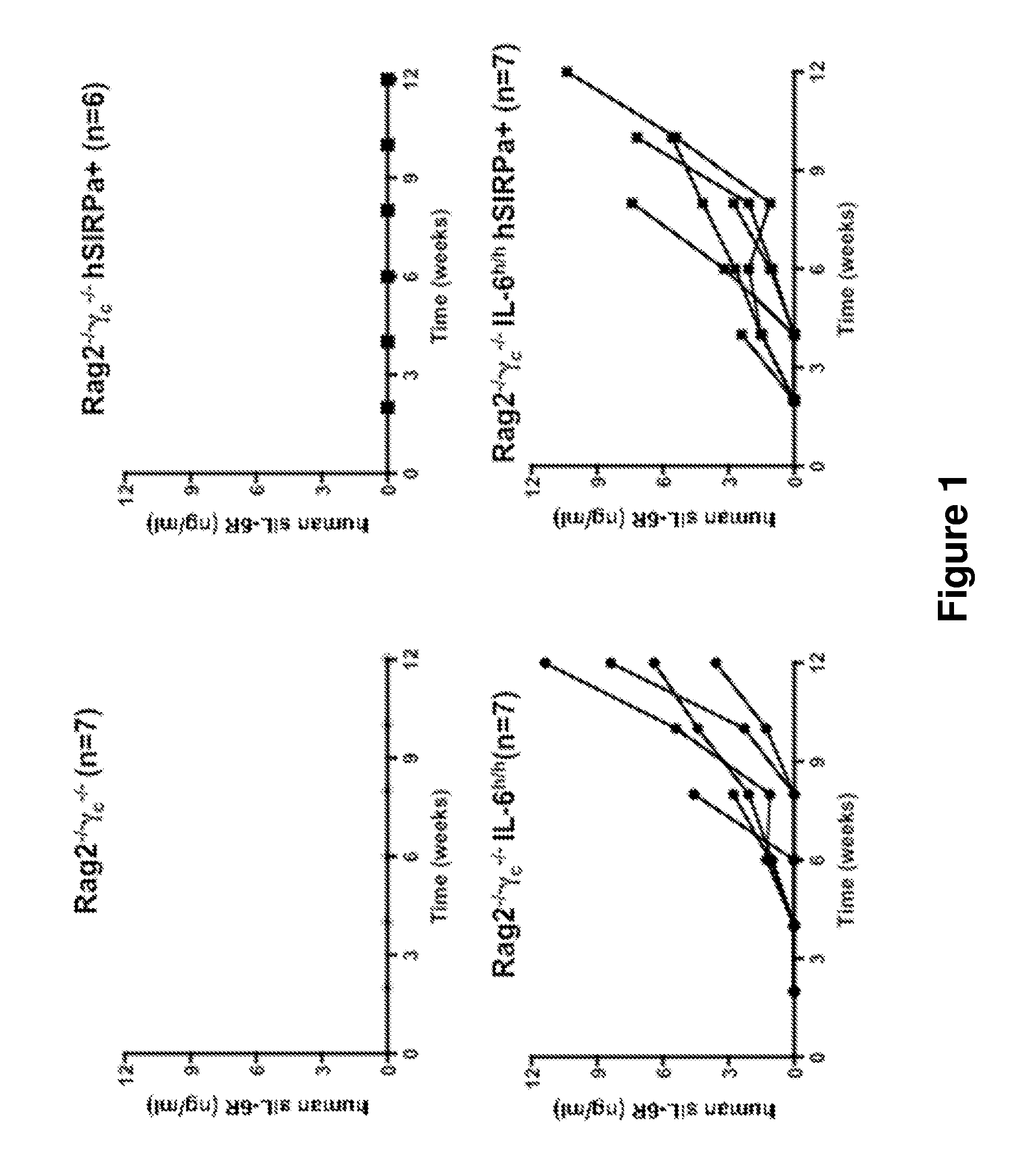
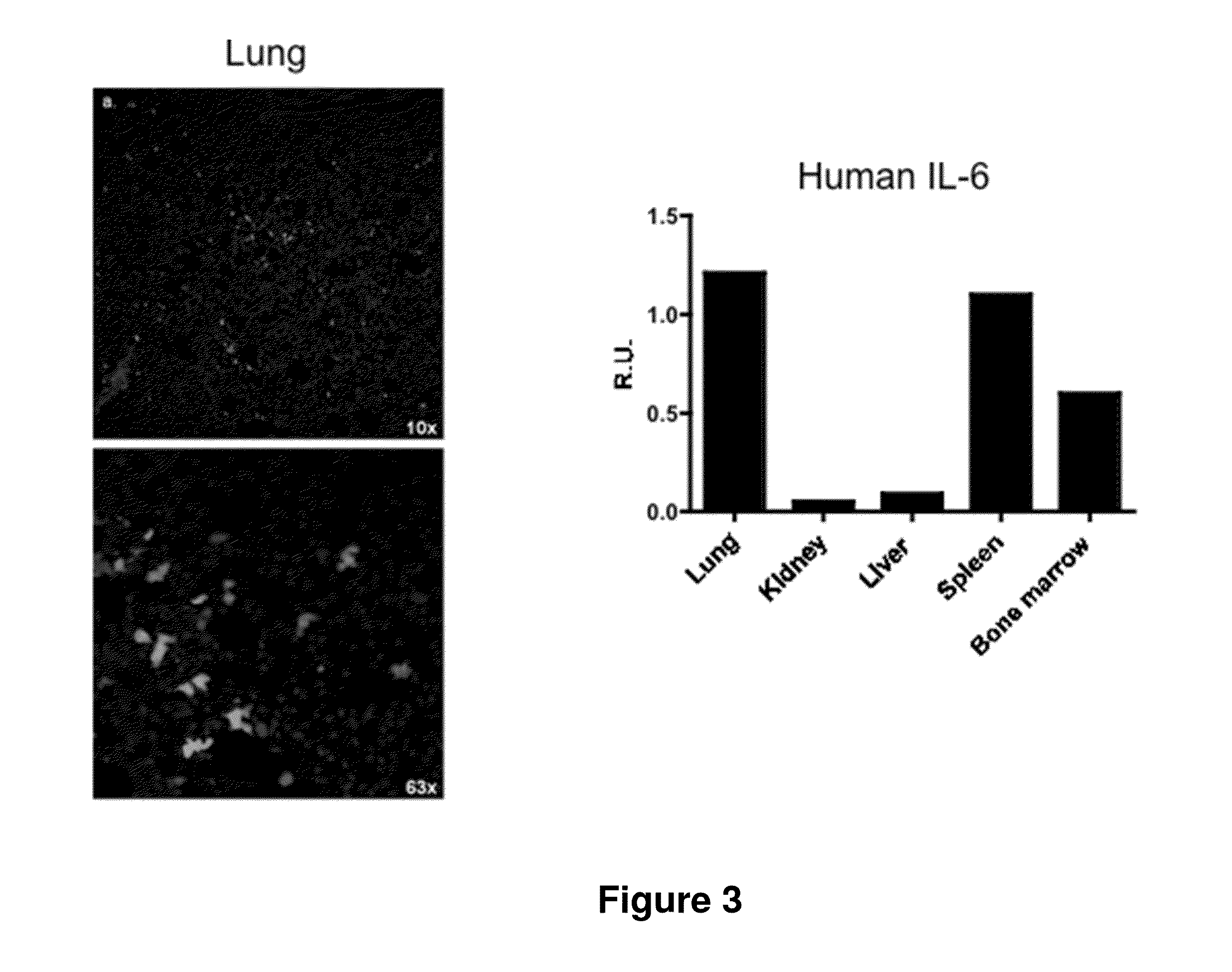
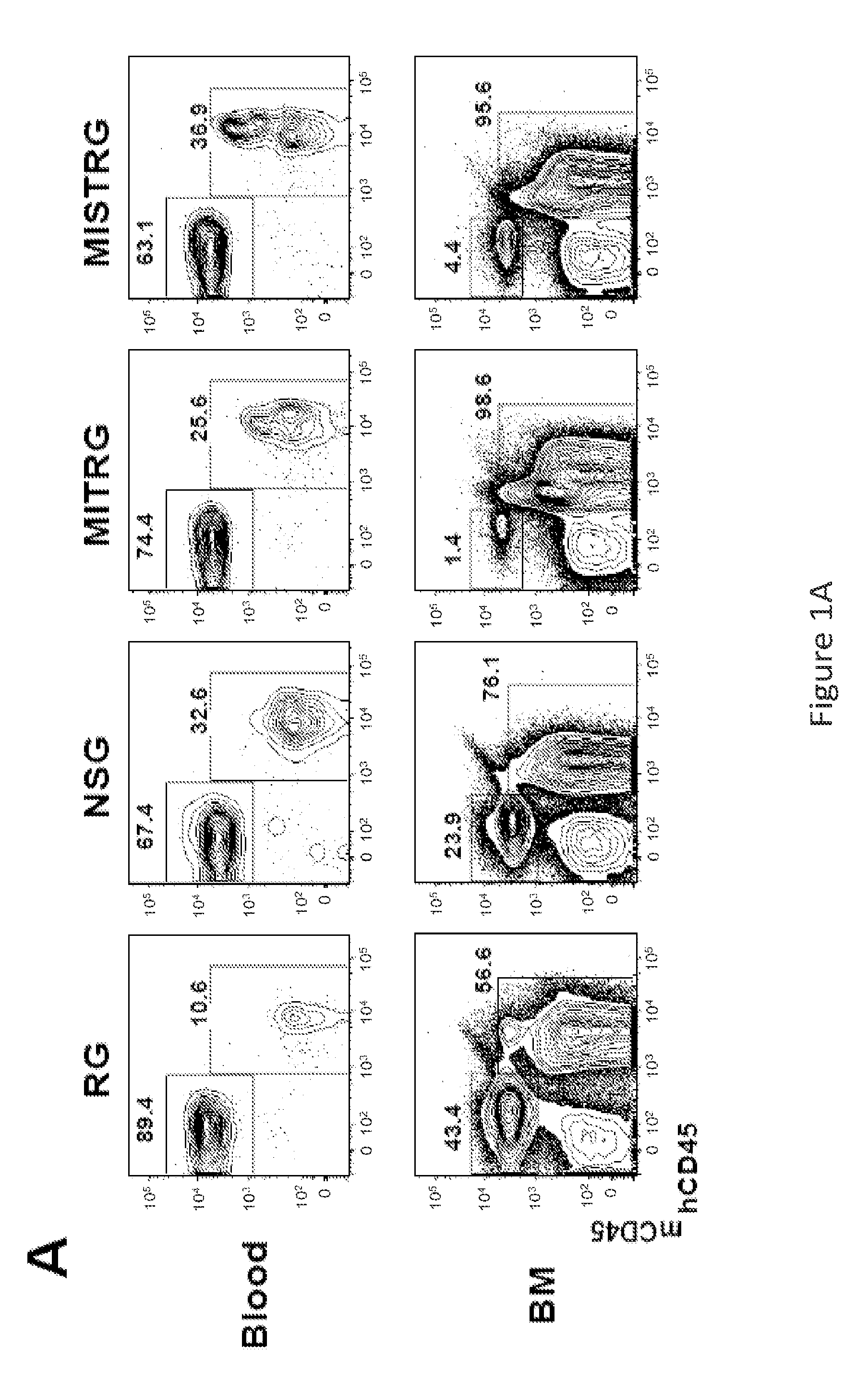
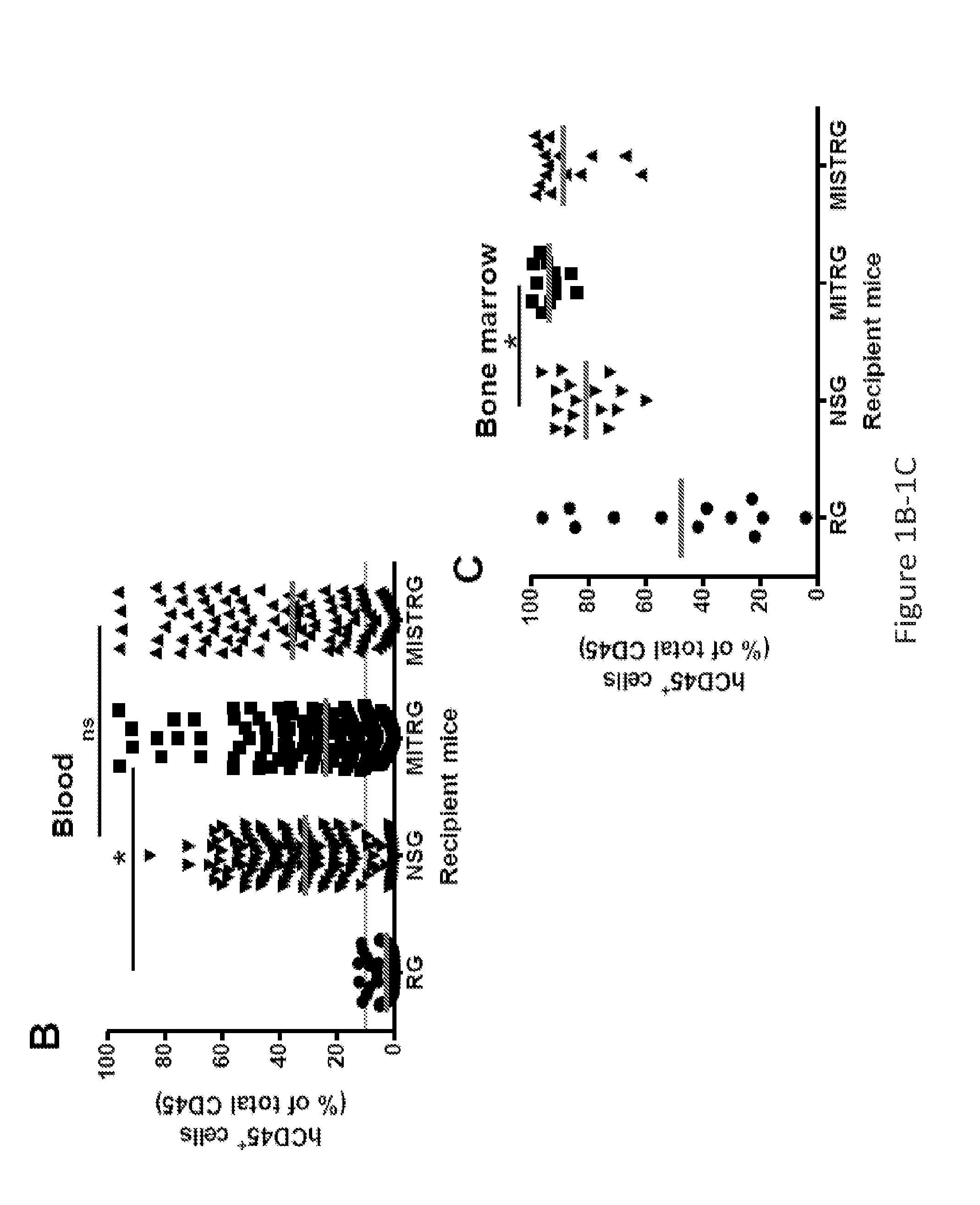
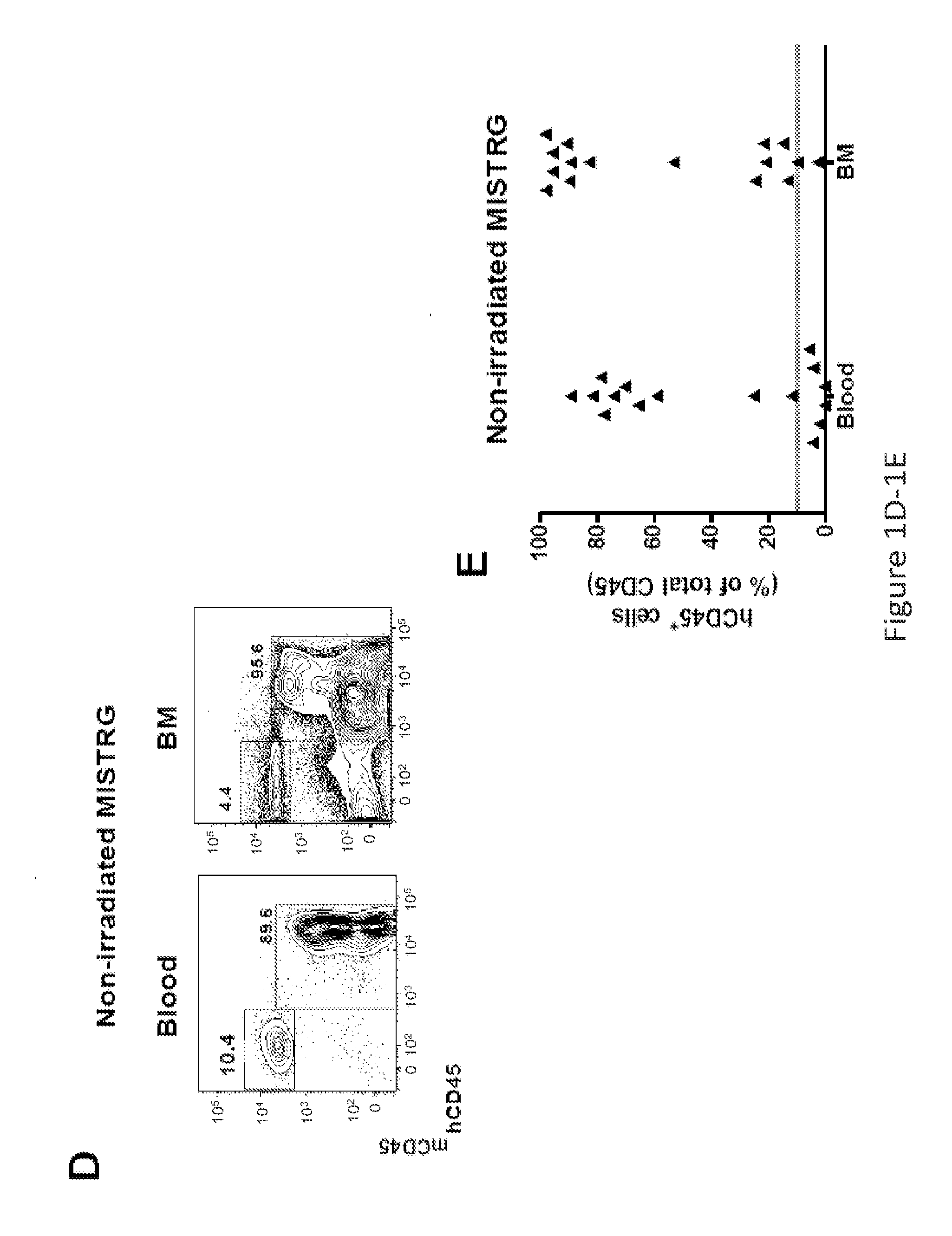
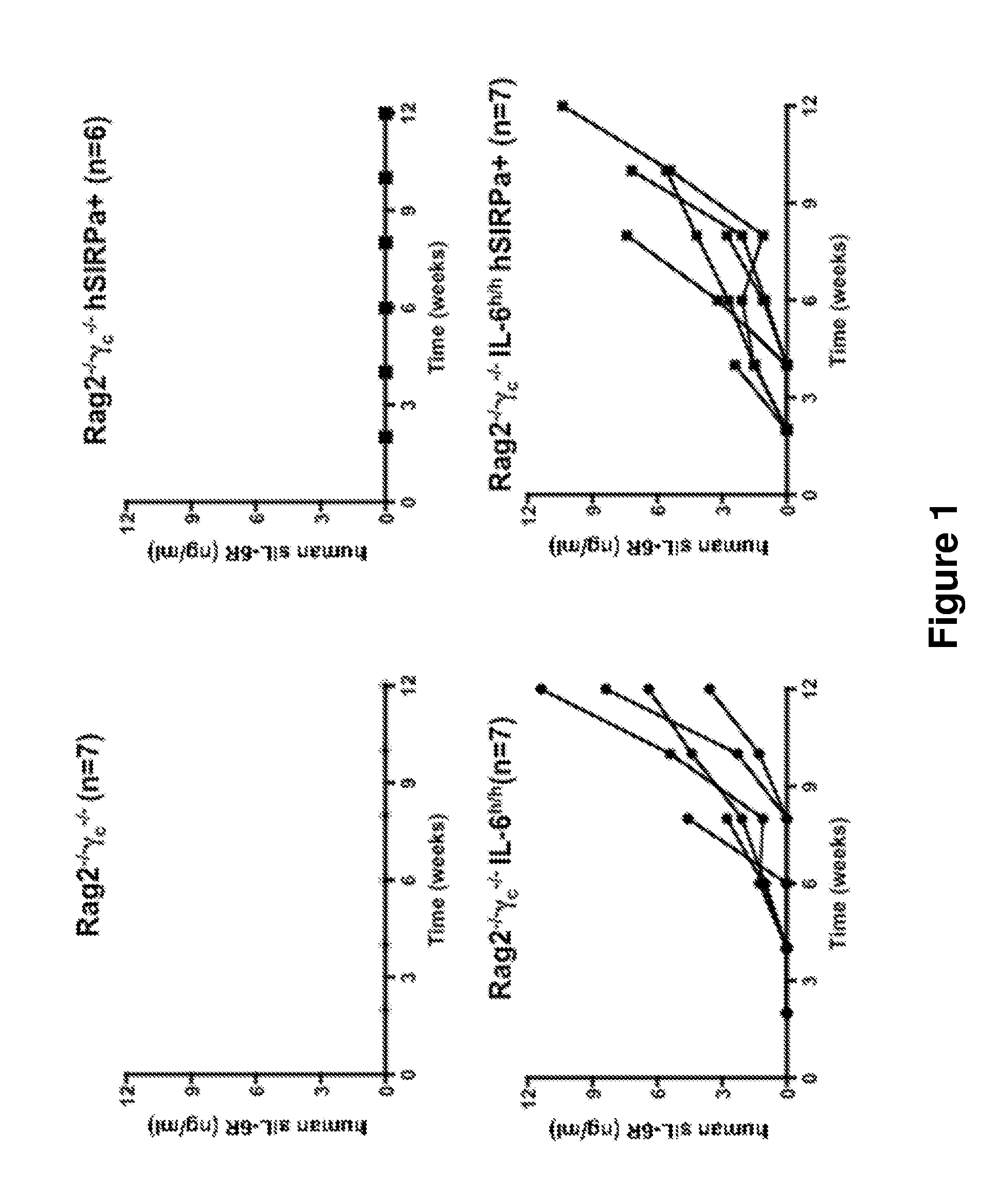
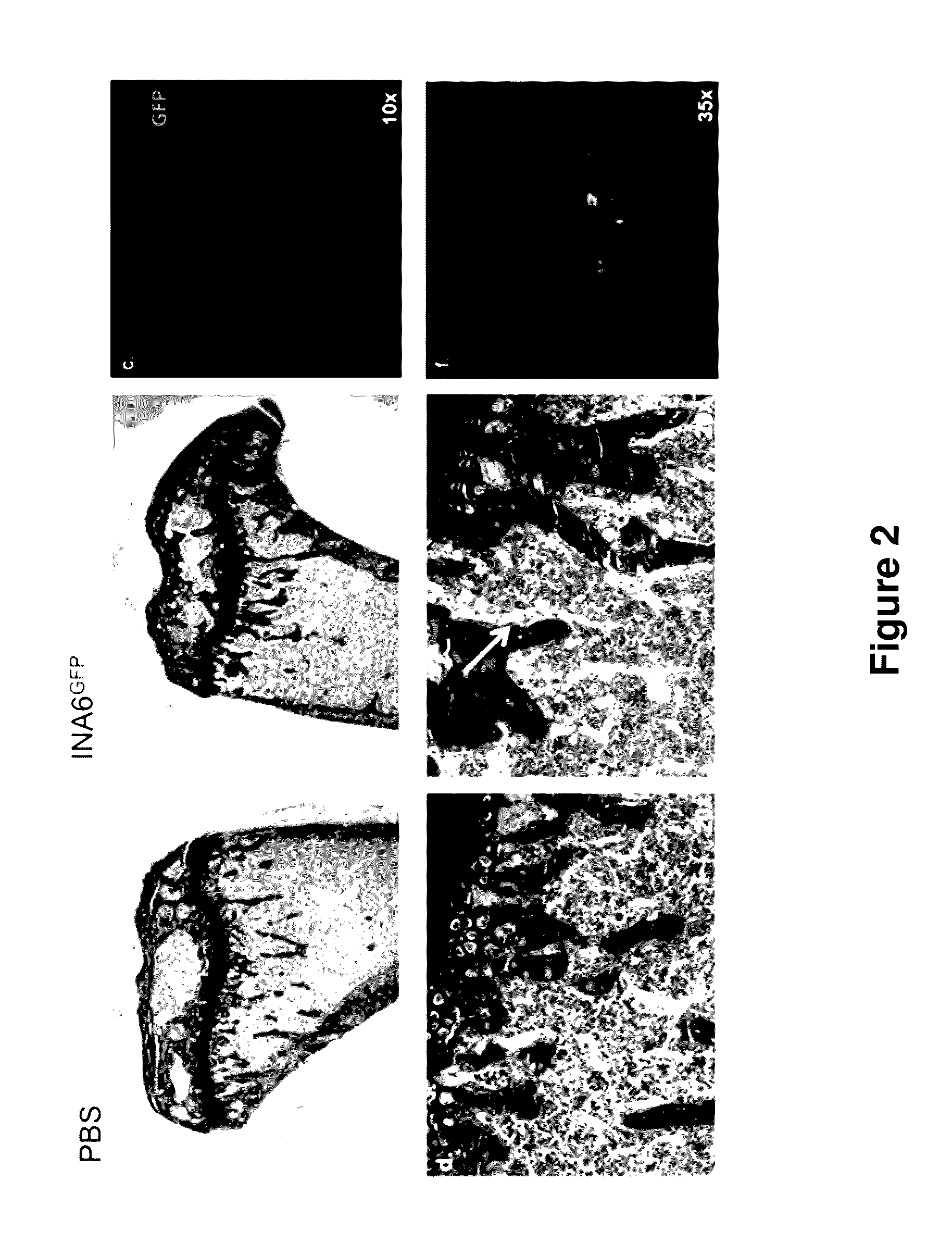
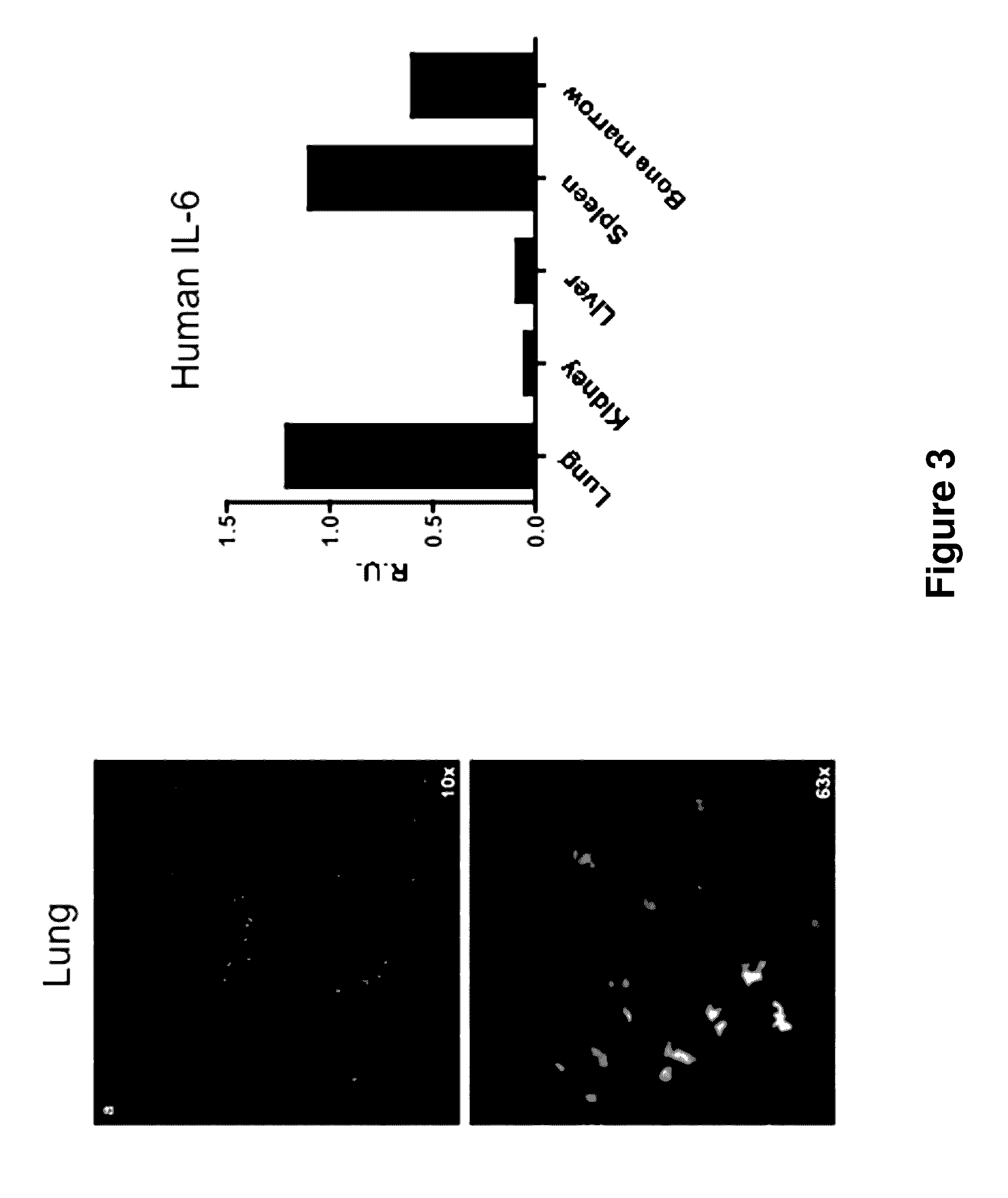
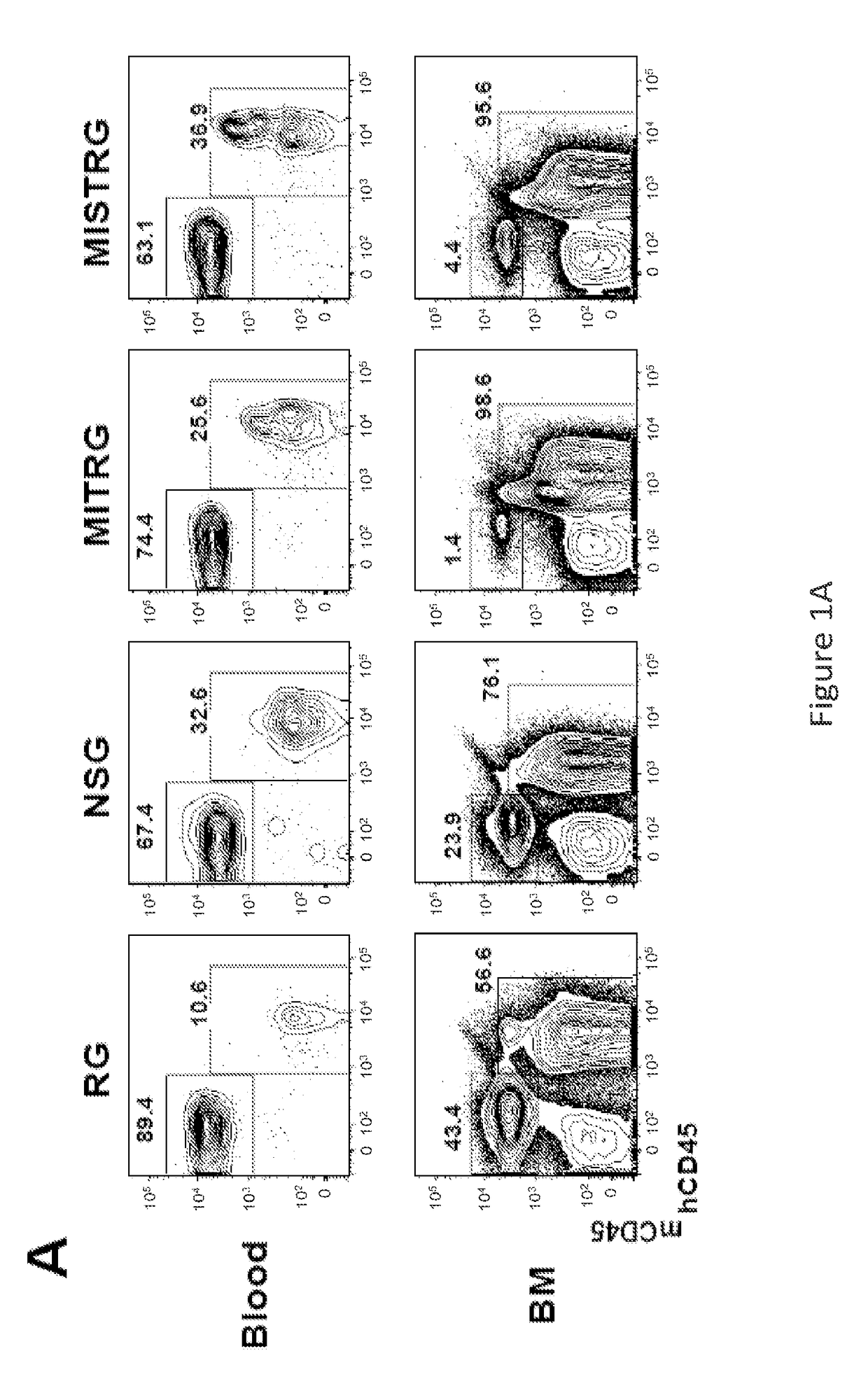
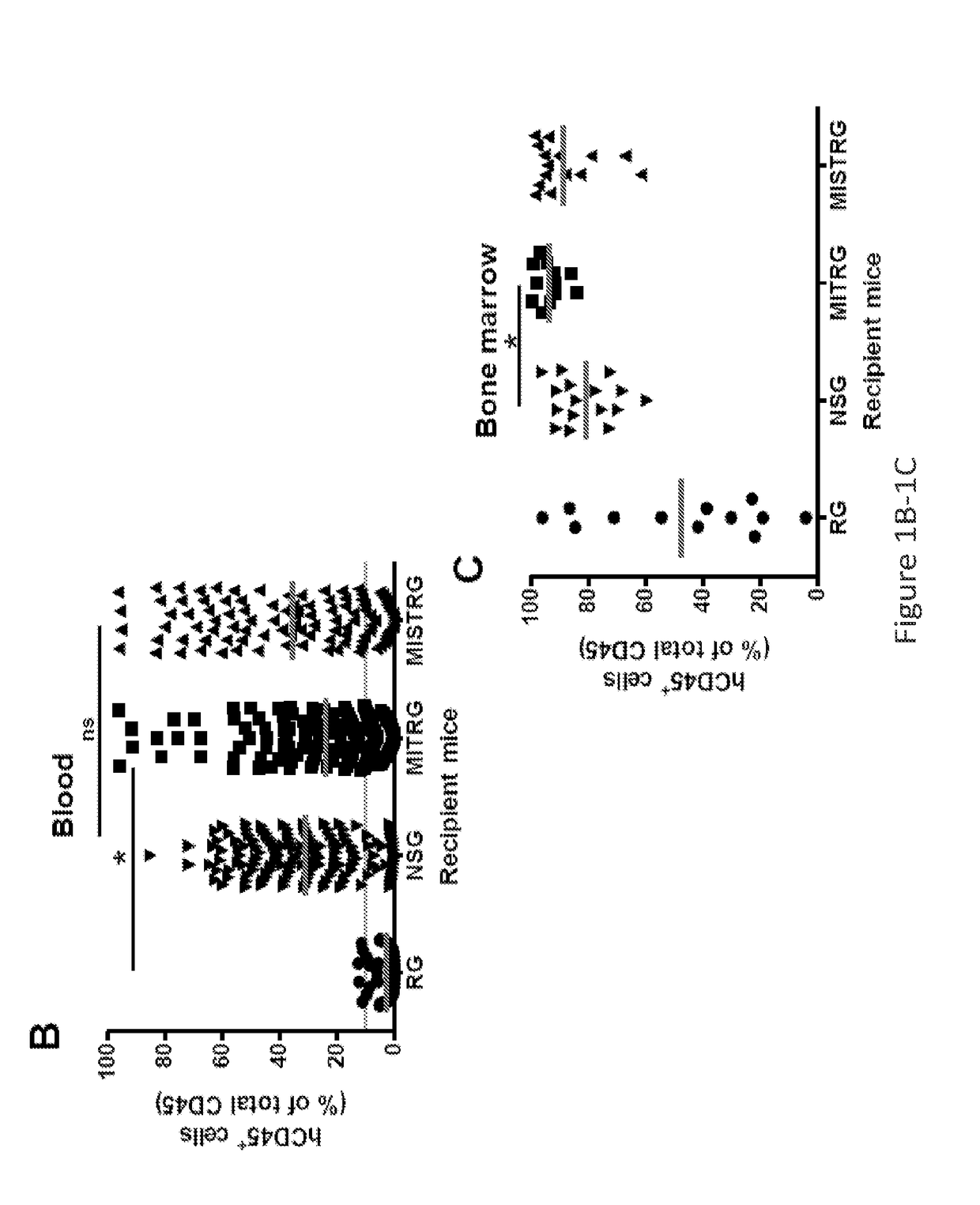
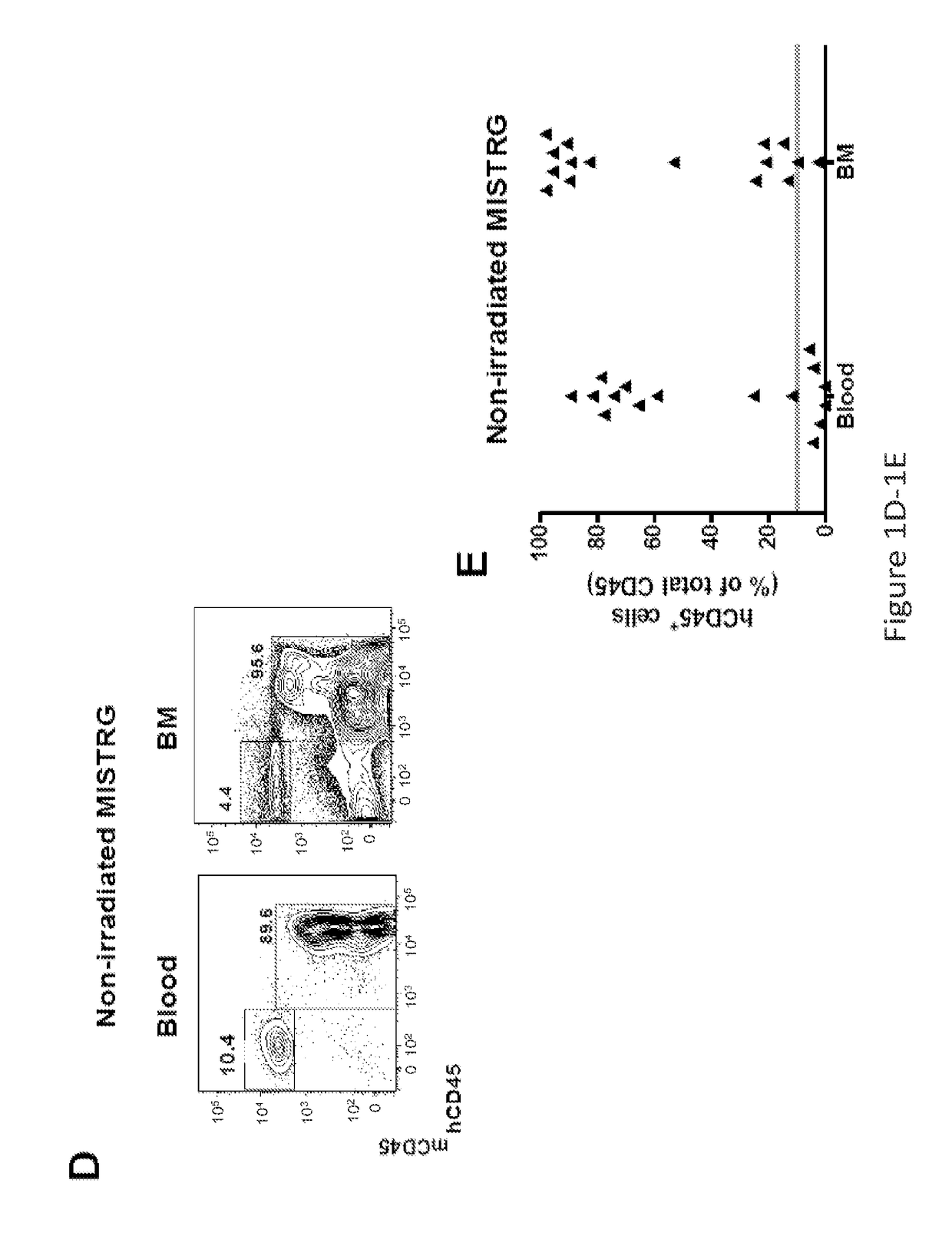
Genetically modified non-human animals are provided that may be used to model human hematopoietic cell development, function, or disease. The genetically modified non-human animals comprise a nucleic acid encoding human IL-6 operably linked to an IL-6 promoter. In some instances, the genetically modified non-human animal expressing human IL-6 also expresses at least one of human M-CSF, human IL-3, human GM-CSF, human SIRPa or human TPO. In some instances, the genetically modified non-human animal is immunodeficient. In some such instances, the genetically modified non-human animal is engrafted with healthy or diseased human hematopoietic cells. Also provided are methods for using the subject genetically modified non-human animals in modeling human hematopoietic cell development, function, and / or disease, as well as reagents and kits thereof that find use in making the subject genetically modified non-human animals and / or practicing the subject methods.
Owner:REGENERON PHARM INC +3
Rationally designed antibodies
InactiveUS7396917B2Highly specificImprove biological activityAntibacterial agentsBacteriaDiagnostic modalitiesBioactive peptide
Antibodies or fragments thereof having at least two CDR regions replaced or fused with biologically active peptides are described. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
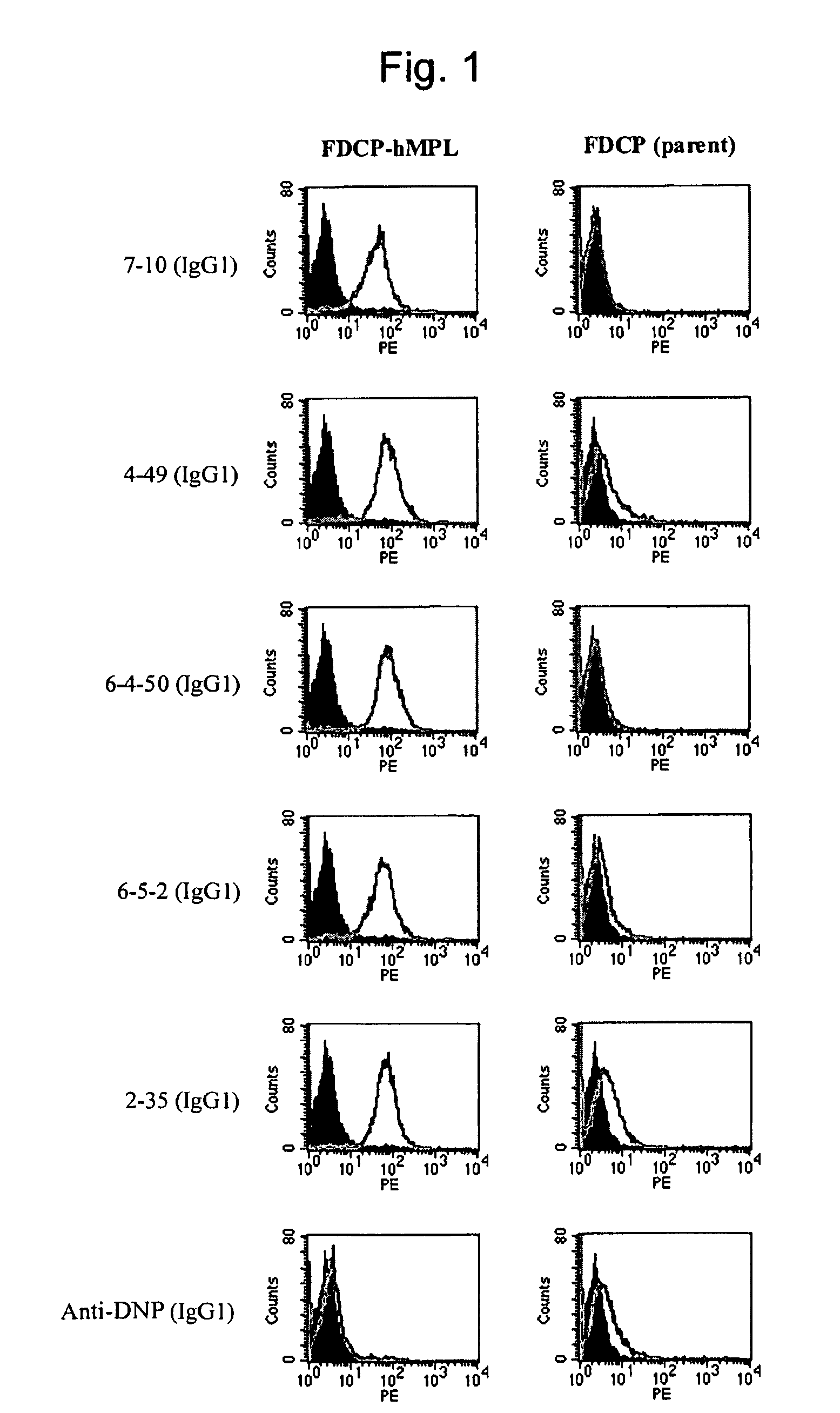
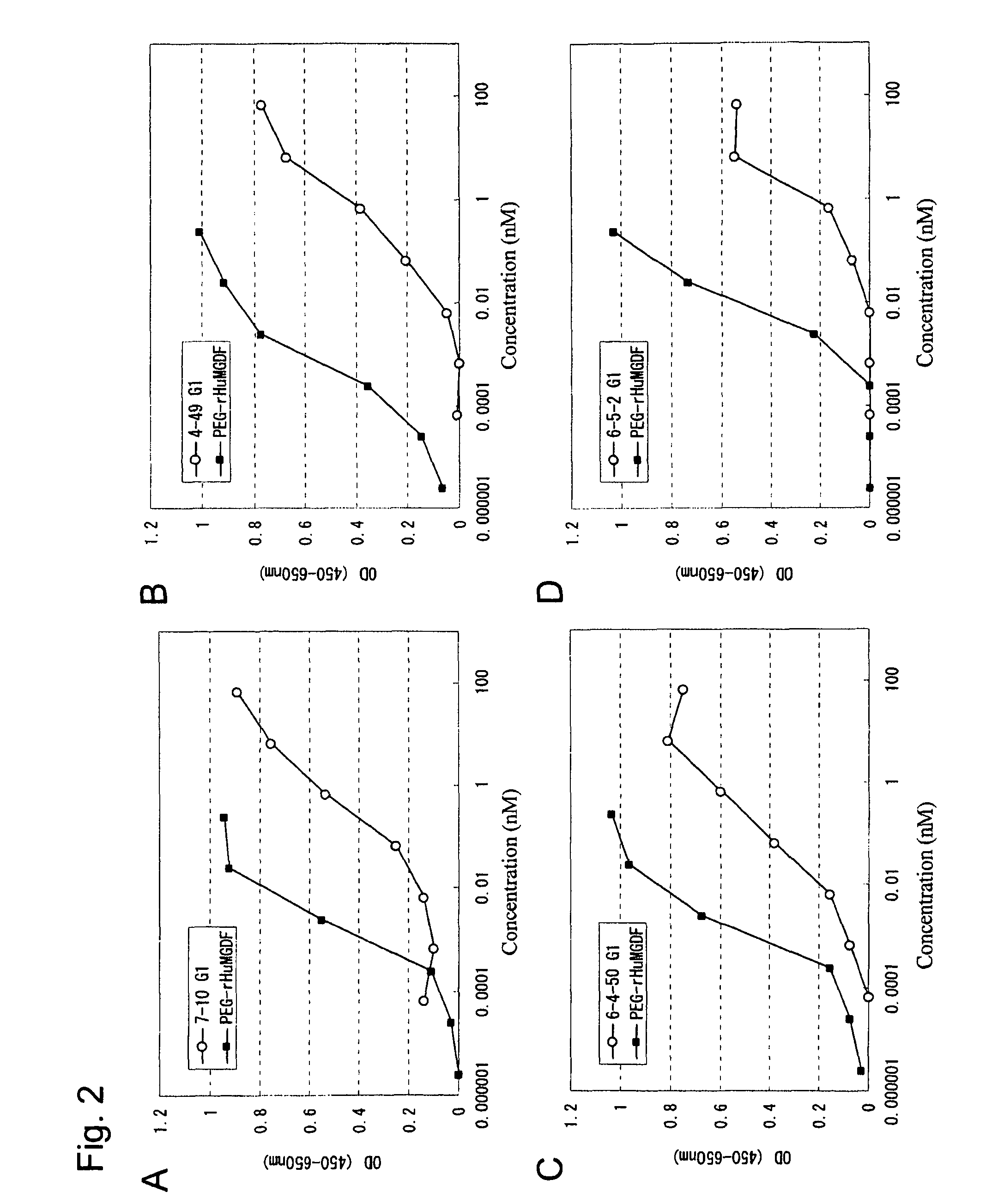
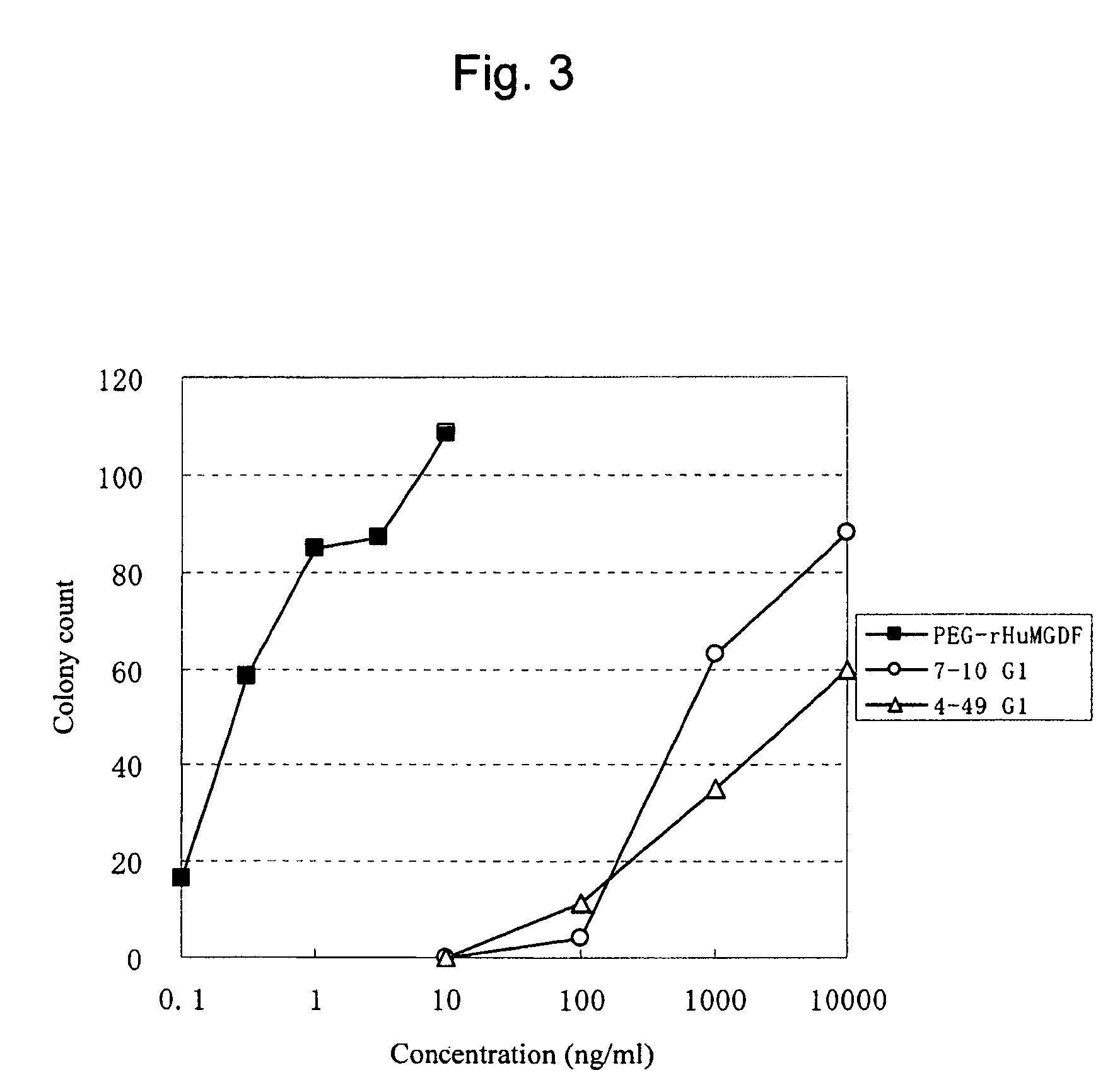
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
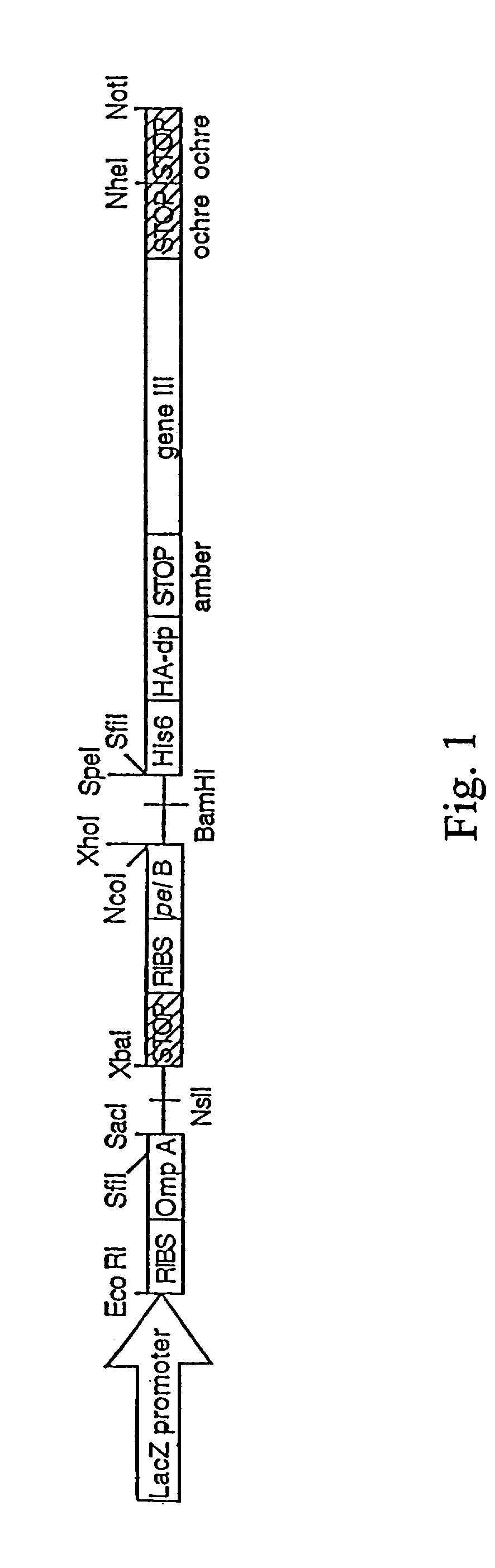
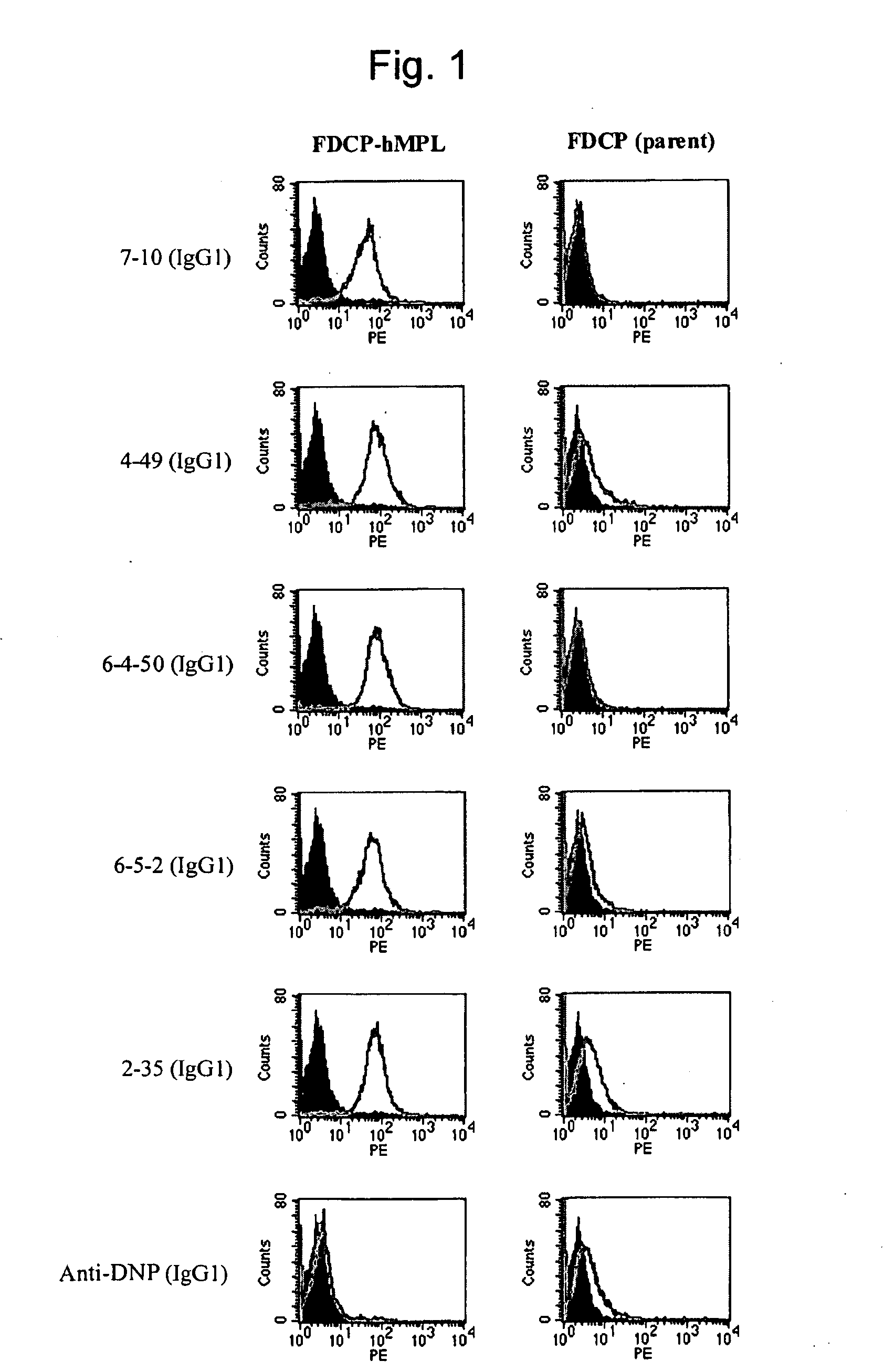
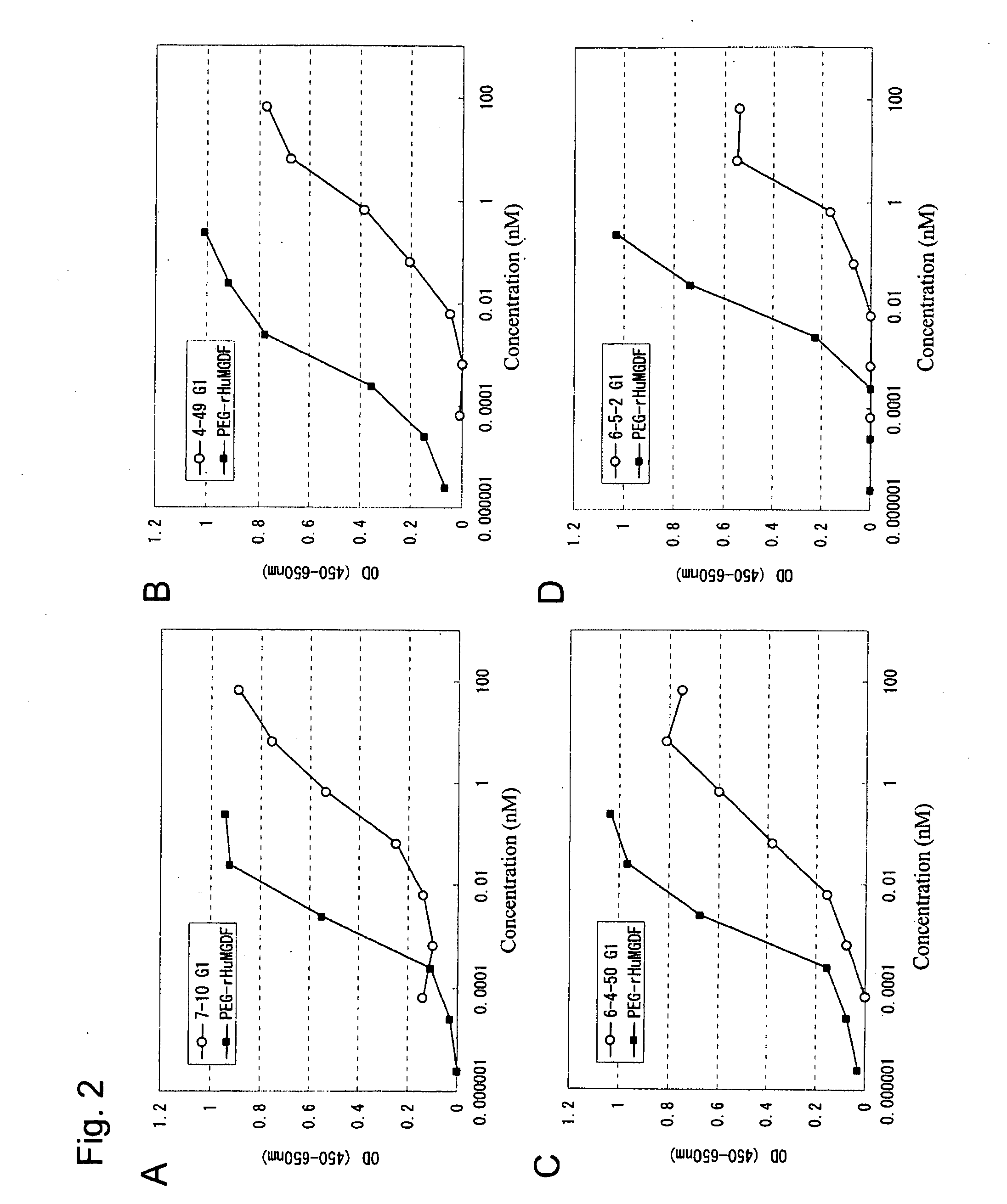
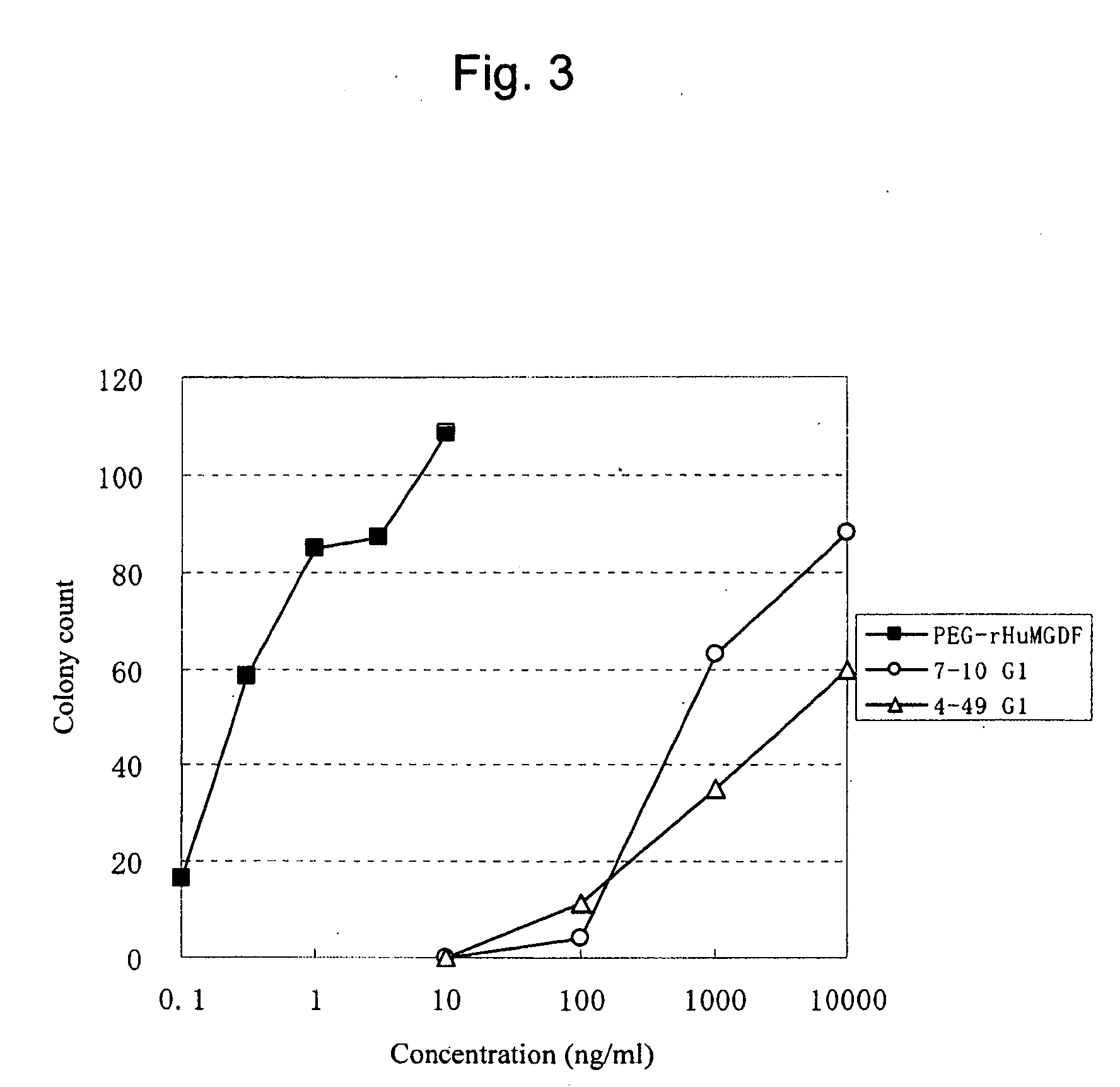
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Rationally designed antibodies
Antibodies or fragments thereof having CDR regions replaced or fused with biologically active peptides are described. Flanking sequences may optionally be attached at one or both the carboxy-terminal and amino-terminal ends of the peptide in covalent association with adjacent framework regions. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
Novel spacer moiety for poly(ethylene glycol)-modified peptide based compounds
The present invention relates to a compound comprising a peptide moiety, a spacer moiety and a water-soluble polymer moiety such as a poly(ethylene glycol) moiety. The spacer moiety is between the peptide moiety and the water-soluble polymer moiety. The spacer moiety has the structure: —NH—(CH2)α—[O—(CH2)β]γ—Oδ—(CH2)ε—Y—wherein α, β, γ, δ, and ε are each integers whose values are independently selected.
Owner:AFFYMAX
Method of improving efficacy of biological response-modifying proteins and the exemplary muteins
ActiveUS20060008872A1Enhance pharmacological effectsMaximizing biological response modifying effectNervous disorderPeptide/protein ingredientsBinding domainA-DNA
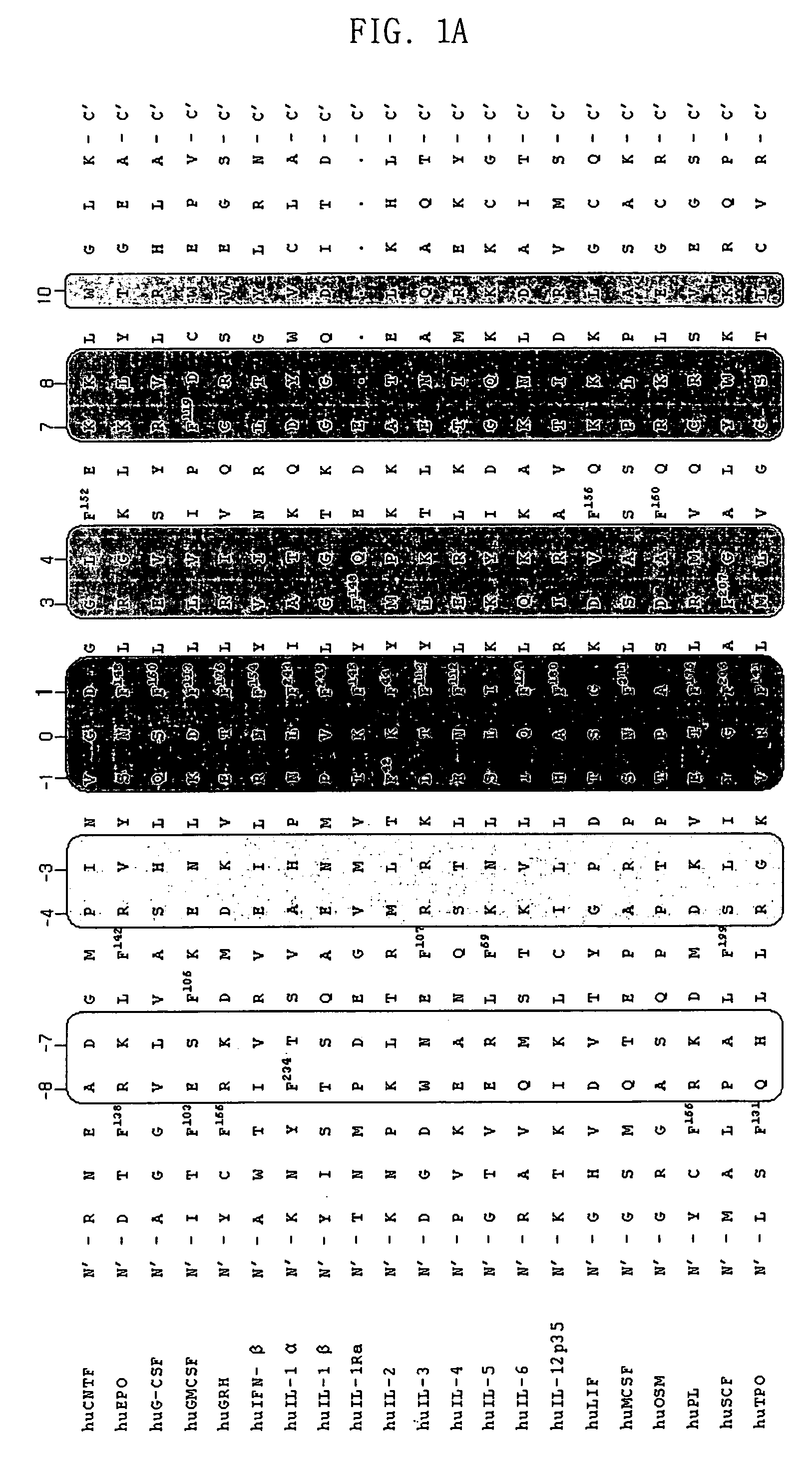
Disclosed is a protein variant which substitutes valine for phenylalanine residue in a binding domain having a biological response-modifying function by binding to a receptor, ligand or substrate. Also, the present invention discloses a DNA encoding the protein variant, a recombinant expression vector to which the DNA is operably linked, a host cell transformed or transfected with the recombinant expression vector, and a method of preparing the protein variant comprising cultivating the host cell and isolating the protein variant from the resulting culture. Further, the present invention discloses a pharmaceutical composition comprising the protein variant and a pharmaceutically acceptable carrier.
Owner:MEDEXGEN
Use of TPO peptide compounds and pharmaceutical compostions in the treatment of anemia
ActiveUS20080119384A1Improve bindingEliminate side effectsThrombopoietinPeptide/protein ingredientsAgonistPeptide
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV
Genetically modified non-human animals expressing human epo
ActiveUS20150327524A1Reduce the amount requiredConducive to survivalCompounds screening/testingHydrolasesDiseaseProgenitor
Genetically modified non-human animals expressing human EPO from the animal genome are provided. Also provided are methods for making non-human animals expressing human EPO from the non-human animal genome, and methods for using non-human animals expressing human EPO from the non-human animal genome. These animals and methods find many uses in the art, including, for example, in modeling human erythropoiesis and erythrocyte function; in modeling human pathogen infection of erythrocytes; in in vivo screens for agents that modulate erythropoiesis and / or erythrocyte function, e.g. in a healthy or a diseased state; in in vivo screens for agents that are toxic to erythrocytes or erythrocyte progenitors; in in vivo screens for agents that prevent against, mitigate, or reverse the toxic effects of toxic agents on erythrocytes or erythrocyte progenitors; in in vivo screens of erythrocytes or erythrocyte progenitors from an individual to predict the responsiveness of an individual to a disease therapy.
Owner:REGENERON PHARM INC +2
Genetically modified non-human animals and methods of use thereof
Owner:REGENERON PHARM INC +2
Rationally designed antibodies
InactiveUS7482435B2Highly specificImprove biological activityBacteriaAntibody mimetics/scaffoldsDiagnostic modalitiesBioactive peptide
Antibodies or fragments thereof having CDR regions replaced or fused with biologically active peptides are described. Flanking sequences may optionally be attached at one or both the carboxy-terminal and amino-terminal ends of the peptide in covalent association with adjacent framework regions. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
Rationally designed antibodies
Antibodies or fragments thereof having at least two CDR regions replaced or fused with biologically active peptides are described. Compositions containing such antibodies or fragments thereof are useful in therapeutic and diagnostic modalities.
Owner:ALEXION PHARMA INC
Peptides and compounds that bind to a receptor
ActiveUS7576056B2Eliminate side effectsImprove bindingThrombopoietinPeptide/protein ingredientsAgonistEphA Receptors
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV +1
Agonist antibody to human thrombopoietin receptor
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Compositions and methods for producing bioactive fusion proteins
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
Genetically modified non-human animals and methods of use thereof
ActiveUS20160366862A1Reduce rateReduced viabilityCompounds screening/testingThrombopoietinDiseaseHematopoietic cell
Genetically modified non-human animals are provided that may be used to model human hematopoietic cell development, function, or disease. The genetically modified non-human animals comprise a nucleic acid encoding human IL-6 operably linked to an IL-6 promoter. In some instances, the genetically modified non-human animal expressing human IL-6 also expresses at least one of human M-CSF, human IL-3, human GM-CSF, human SIRPa or human TPO. In some instances, the genetically modified non-human animal is immunodeficient. In some such instances, the genetically modified non-human animal is engrafted with healthy or diseased human hematopoietic cells. Also provided are methods for using the subject genetically modified non-human animals in modeling human hematopoietic cell development, function, and / or disease, as well as reagents and kits thereof that find use in making the subject genetically modified non-human animals and / or practicing the subject methods.
Owner:REGENERON PHARM INC +2
Peptides and compounds that bind to a receptor
Described are peptides and peptide mimetics that bind to and activate the thrombopoietin receptor. Such peptides and peptide mimetics are useful in methods for treating hematological disorders and particularly, thrombocytopenia resulting from chemotherapy, radiation therapy, or bone marrow transfusions as well as in diagnostic methods employing labeled peptides and peptide mimetics.
Owner:SMITHKLINE BECKMAN CORP
TPO peptide compounds for treatment of anemia
ActiveUS7615533B2Improve bindingEliminate side effectsThrombopoietinPeptide/protein ingredientsAgonistAnemia
Peptide compounds that bind to and activate the thrombopoietin receptor (c-mpl or TPO-R) or otherwise act as a TPO agonist are disclosed.
Owner:JANSSEN PHARMA NV
Compositions and methods for producing bioactive fusion proteins
InactiveUS20090118181A1Low immunogenic riskReduce riskBacteriaPeptide/protein ingredientsDNA constructActive protein
Disclosed is a composition of matter involving a recombinant fusion protein comprising a a pharmacologically active protein partner, and a small pharmacologically inactive protein domain partner of human origin, such as but not limited to, a 10th fibronectin III domain, a SH3 domain, a SH2 domain, a CH2 domain of IgG1, a PDZ domain, a thrombospondin repeat domain, an ubiquitin domain, a leucine-rich repeat domain, a villin headpiece HP35 domain, a villin headpiece HP76 domain, or a fragment or modification of any of these. Also disclosed are nucleic acids (e.g., DNA constructs) encoding the fusion protein, expression vectors and recombinant host cells for expression of the fusion protein, and pharmaceutical compositions containing the recombinant fusion protein and a pharmaceutically acceptable carrier, and method of producing a pharmacologically active recombinant fusion protein.
Owner:AMGEN INC
Cytokines and cytokine receptors with reduced immunogenicity
InactiveUS20050220800A1Low immunogenicityReduced immunogenic responsePeptide/protein ingredientsTissue cultureTumor necrosis factor receptorBiology
The present invention provides methods for the identification of CD4+ T-cell epitopes in the sequences of various proteins, namely, human cytokines and cytokine receptors, as well as the production of peptides which when incorporated into the protein sequence, are no longer capable of initiating the CD4+ T-cell response. In some embodiments, the present invention provides means and compositions suitable for reducing the immunogenicity of cytokines and cytokines receptors such as interferon-β, soluble tumor necrosis factor receptor-1, erythropoietin, and thrombopoietin.
Owner:SCOTT POWER D +1
Genetically modified non-human animals and methods of use thereof
ActiveUS20180049413A1Reduce rateReduced viabilityCompounds screening/testingThrombopoietinHuman animalDisease
Genetically modified non-human animals are provided that may be used to model human hematopoietic cell development, function, or disease. The genetically modified non-human animals comprise a nucleic acid encoding human IL-6 operably linked to an IL-6 promoter. In some instances, the genetically modified non-human animal expressing human IL-6 also expresses at least one of human M-CSF, human IL-3, human GM-CSF, human SIRPa or human TPO. In some instances, the genetically modified non-human animal is immunodeficient. In some such instances, the genetically modified non-human animal is engrafted with healthy or diseased human hematopoietic cells. Also provided are methods for using the subject genetically modified non-human animals in modeling human hematopoietic cell development, function, and / or disease, as well as reagents and kits thereof that find use in making the subject genetically modified non-human animals and / or practicing the subject methods.
Owner:YALE UNIV +2
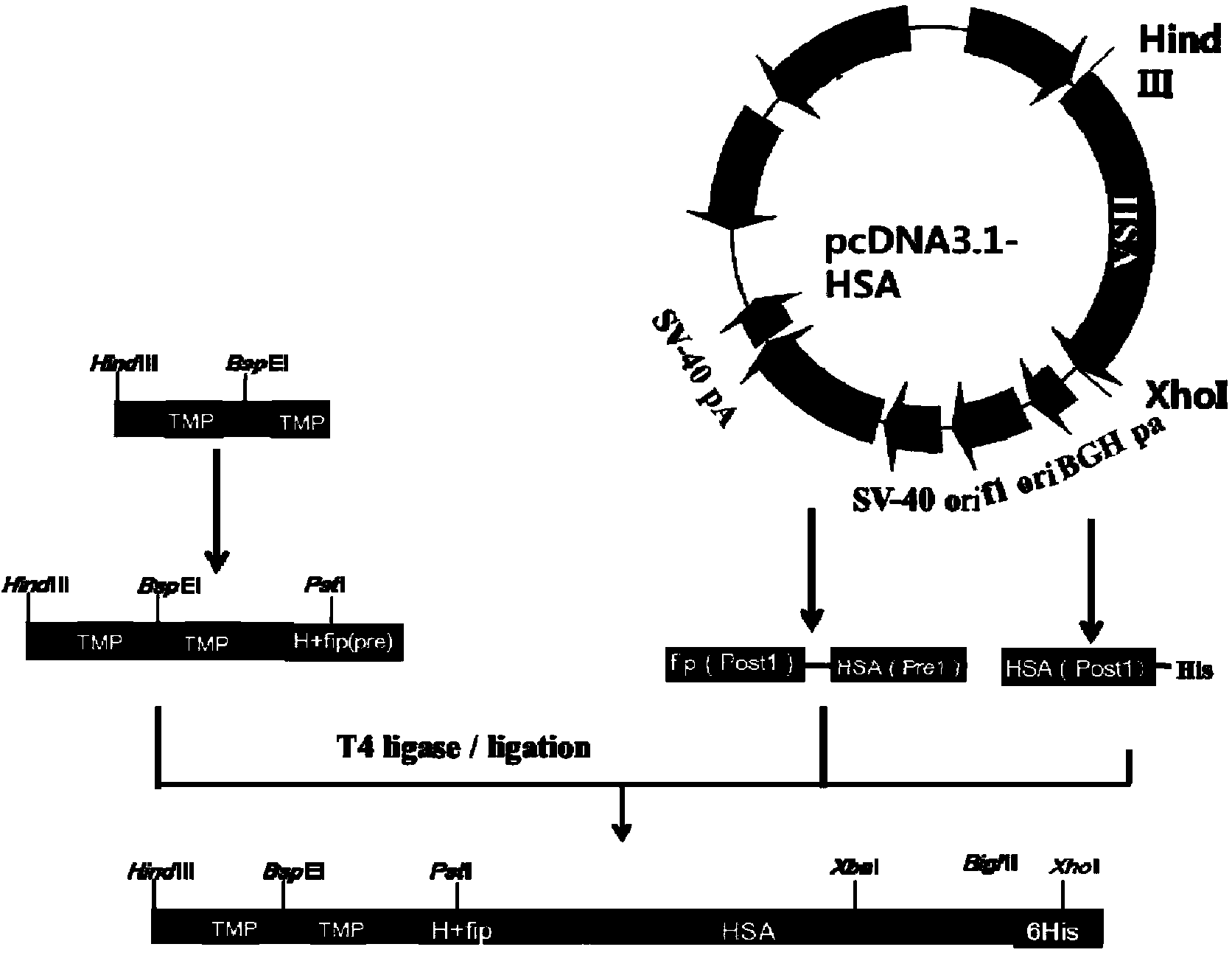
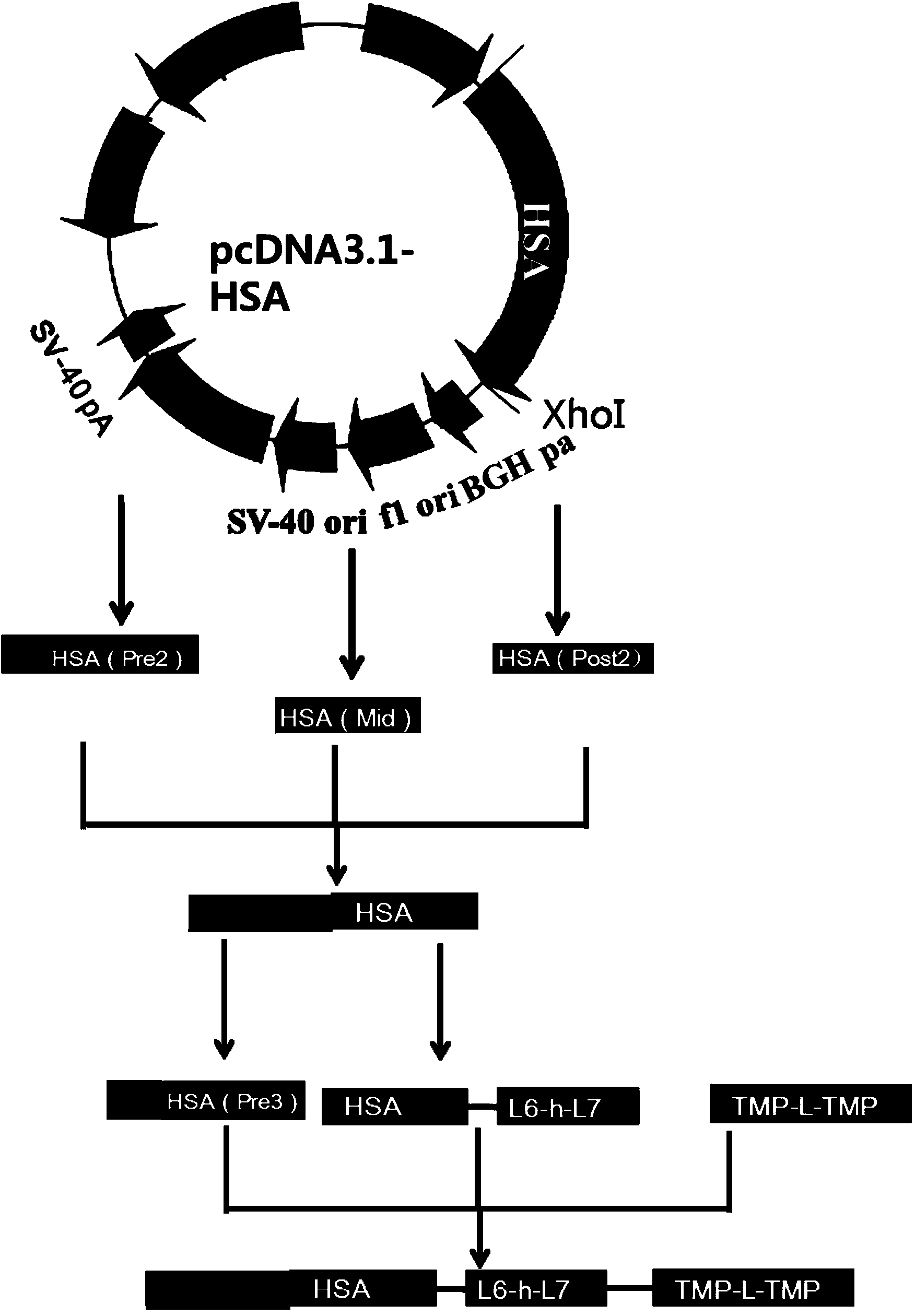
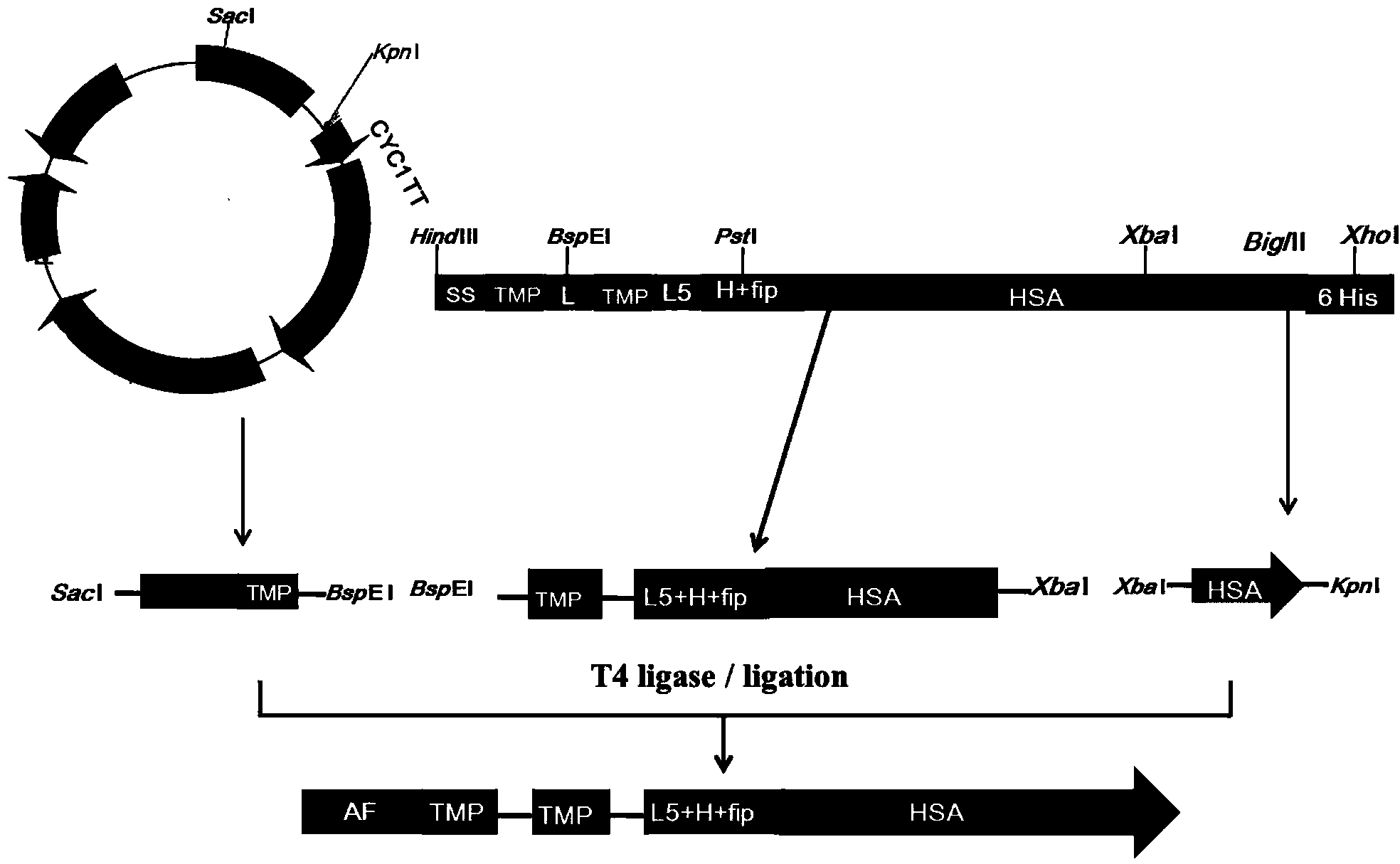
Preparation and application of dimerized fusion protein
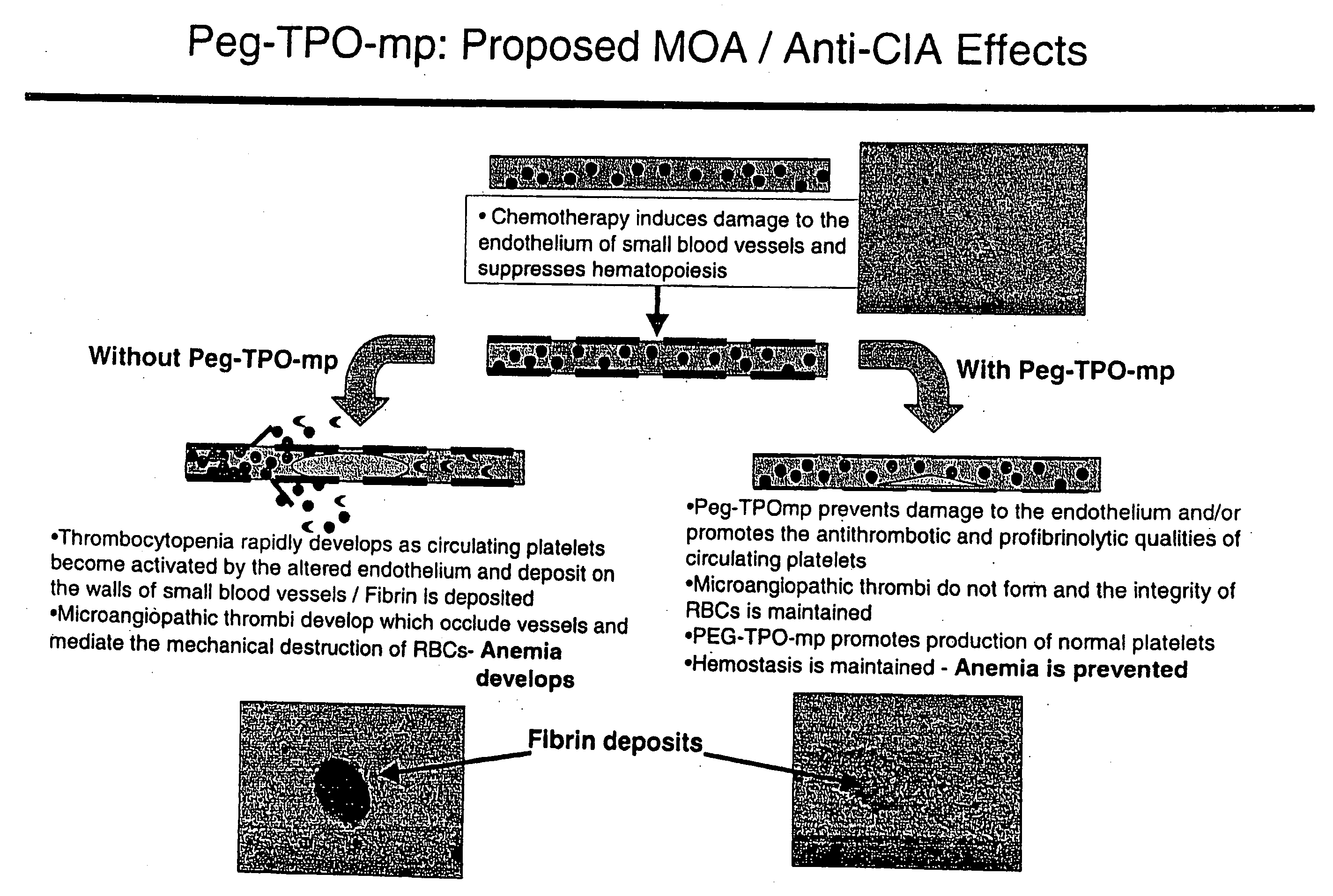
The invention discloses preparation and application of a dimerized thrombopoietin (TPO) mimic peptide TMP diad-human serum albumin fusion protein. The fusion protein contains an HAS and two TPO mimic peptides which are connected through a linkage oligopeptide and a dimerization domain so as to ensure the fusion protein is dimerized correctly when expression. The two TPO mimic peptides can be connected with N terminal or C terminal of HAS. The dimerized HAS protein carrier and the TPO mimic peptide fusion protein provided by the invention have a significant activity of promoting thrombopoiesis in vivo and can be applied in preparation of drugs for treating primary and secondary thrombocytopenia diseases.
Owner:LANZHOU UNIVERSITY
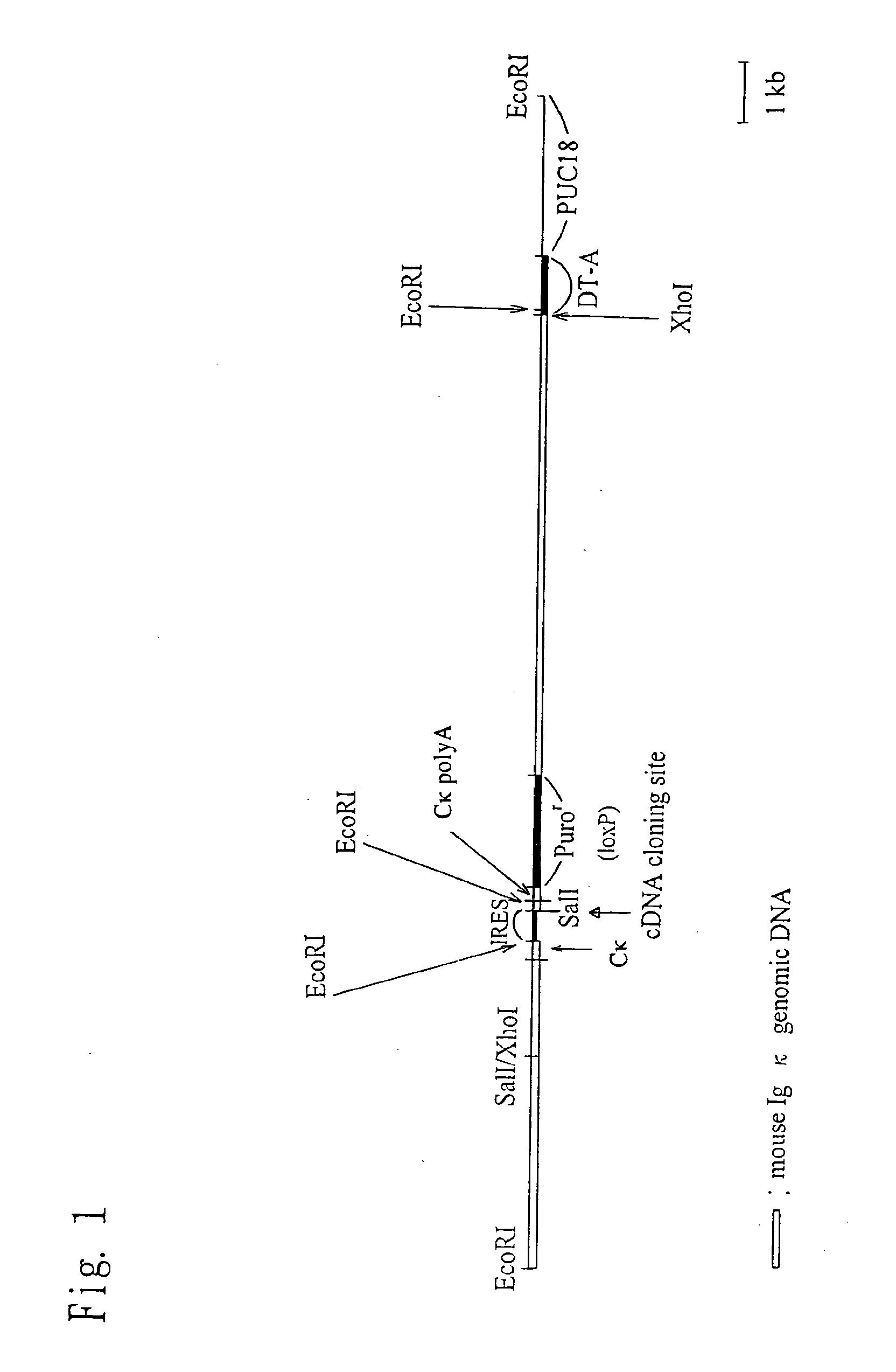
Chimeric nonhuman animal
InactiveUS20050177884A1Improve import efficiencyProvide conformational changeThrombopoietinAnimal cellsHuman animalWild type
The present invention relates to a method for producing a chimeric non-human animal expressing a desired protein, and a chimeric non-human animal or an offspring thereof expressing a desired protein. The present invention also relates to a method for analyzing the functions of a desired protein or a gene encoding the protein by comparing the phenotype of the above chimeric non-human animal with that of a corresponding wild-type animal.
Owner:KIRIN BREWERY CO LTD
Dimeric thrombopoietin peptide mimetics binding to MP1 receptor and having thrombopoietic activity
Owner:AMGEN K A INC
Modified thrombopoietin with reduced immunogenicity
InactiveUS20040071688A1Improve accuracyIncrease the number ofPeptide/protein ingredientsHydrolasesHuman plateletThrombopoietin
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human thrombopoietin (TPO) to result in TPO proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Genetically modified non-human animals and methods of use thereof
Owner:REGENERON PHARM INC +2
Chimeric non-human animal
InactiveUS20100011452A1Simple and highly reproducible methodThrombopoietinCell receptors/surface-antigens/surface-determinantsHuman animalWild type
The present invention relates to a method for producing a chimeric non-human animal expressing a desired protein, and a chimeric non-human animal or an offspring thereof expressing a desired protein.The present invention also relates to a method for analyzing the functions of a desired protein or a gene encoding the protein by comparing the phenotype of the above chimeric non-human animal with that of a corresponding wild-type animal.
Owner:KYOWA HAKKO KIRIN CO LTD
Fusion protein with reinforced erythrocytin activity in vivo
InactiveCN1421461AImprove in vivo activityThrombopoietinPeptide/protein ingredientsIn vivoC-terminus
Owner:CJ HEALTHCARE CORP
hG-CSF fusion polypeptide having c-mpl activity, DNA coding for same and methods of treating anemia using same
InactiveUS6884419B1Efficient purificationEfficient preparationThrombopoietinPeptide/protein ingredientsDna encodingBULK ACTIVE INGREDIENT
The present invention relates to a fusion polypeptide which comprises a polypeptide having G-CSF activity and a polypeptide having TPO activity and DNA which codes for the fusion polypeptide, to a fusion polypeptide in which a polypeptide having G-CSF activity and a polypeptide having TPO activity are fused via a spacer peptide and DNA which codes for the fusion polypeptide and to a polypeptide in which the fusion polypeptide comprising a polypeptide having G-CSF activity and a polypeptide having TPO activity is chemically modified with a polyalkylene glycol derivative. It also relates to an anemia-treating composition containing the fusion polypeptide as an active ingredient.
Owner:KYOWA HAKKO KOGYO CO LTD
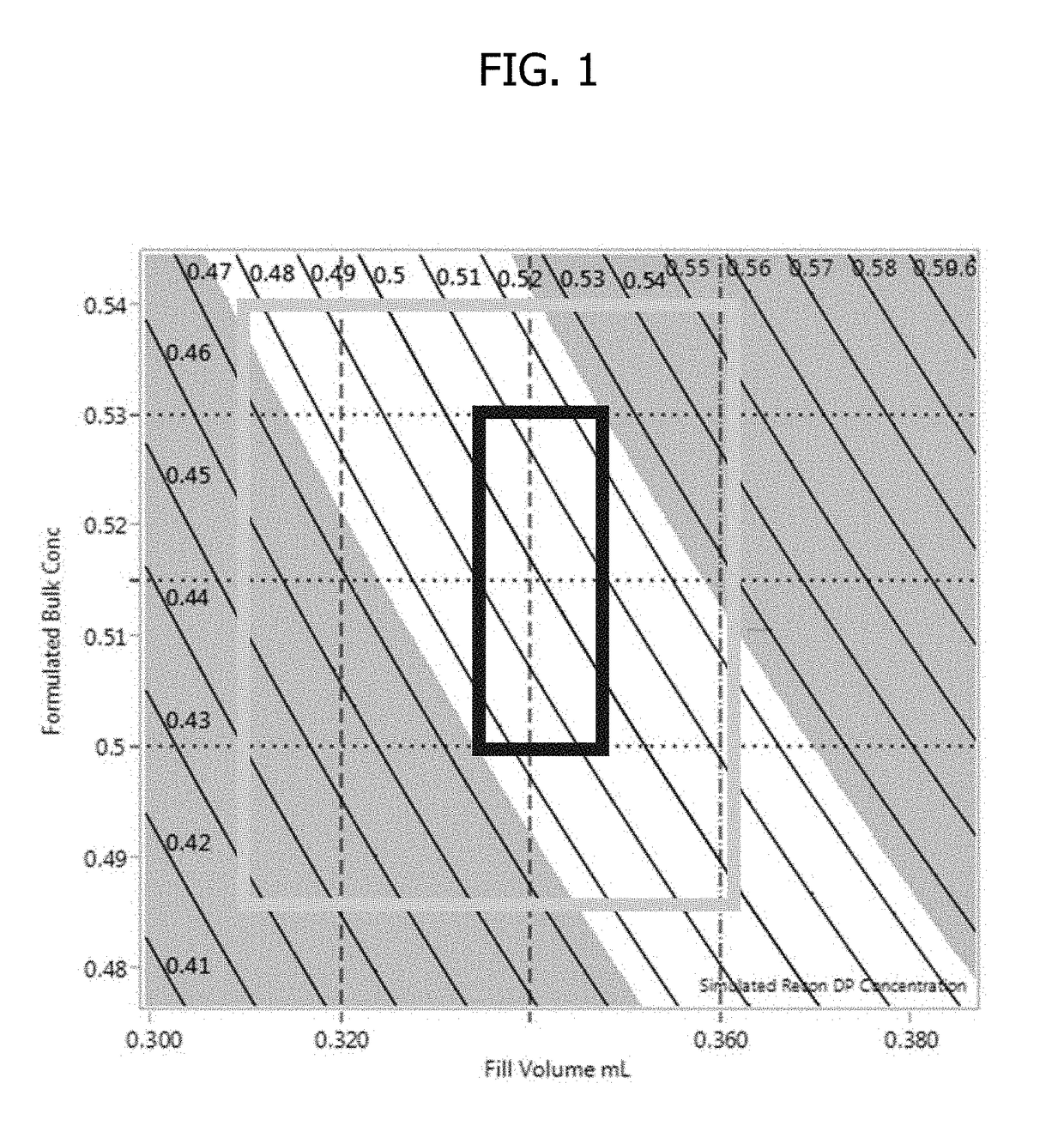
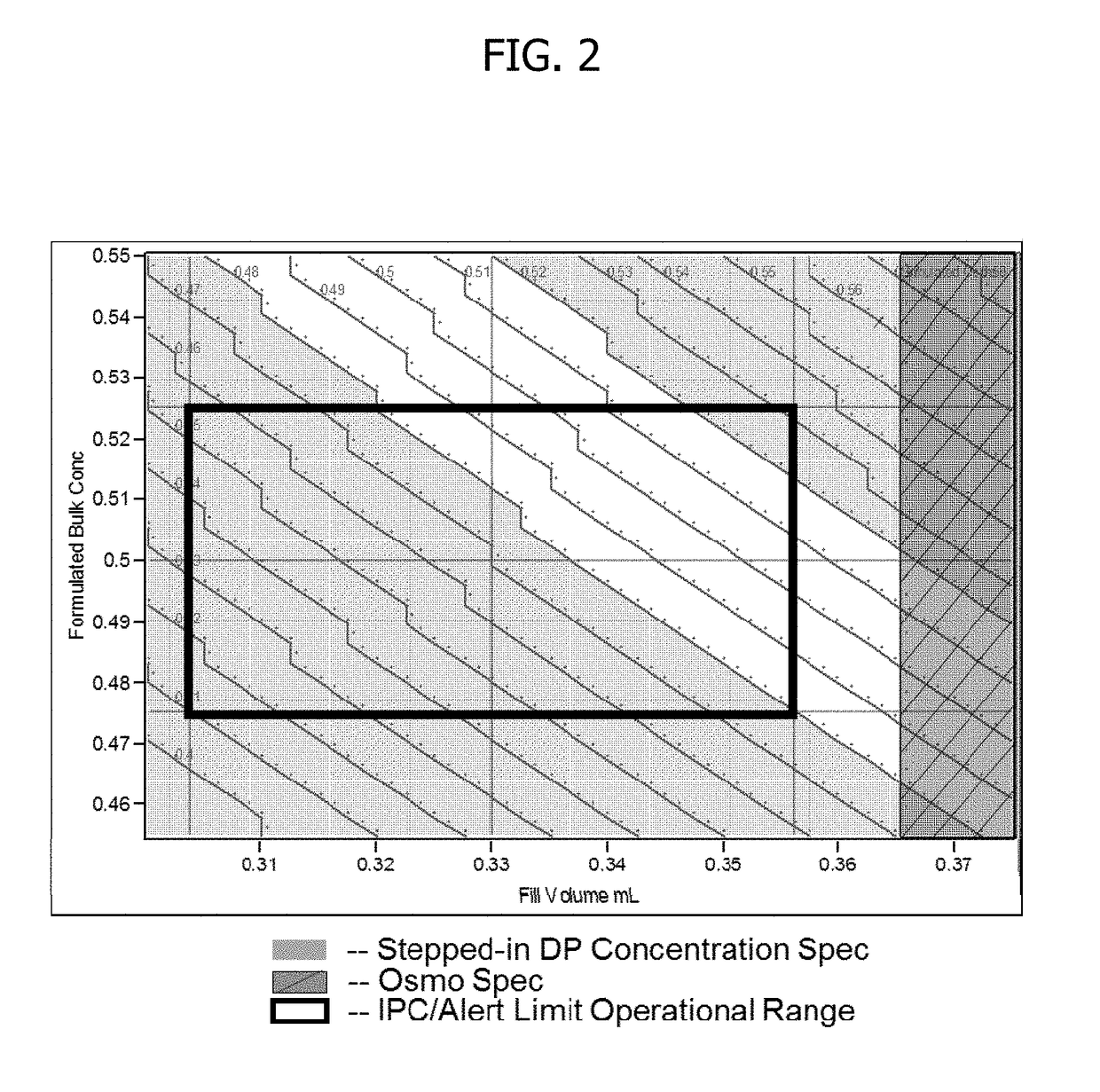
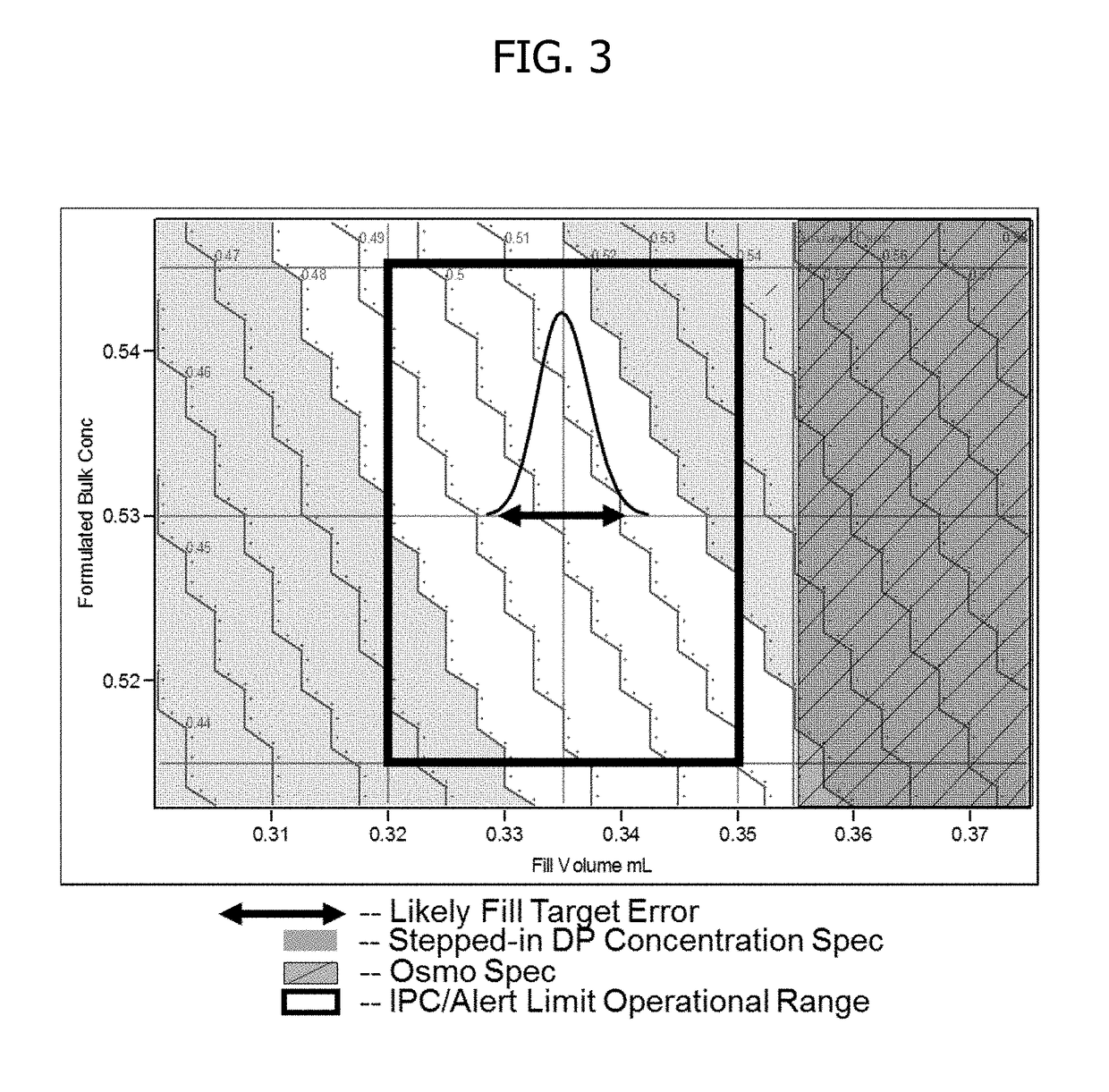
Process for lyophilized pharmaceutical formulation of a therapeutic protein
This invention concerns a process for making a lyophilized pharmaceutical formulation of a therapeutic protein, which comprises (a) providing a formulation of a bulk amount of the therapeutic protein, (b) measuring the concentration of the therapeutic protein in said bulk formulation, (c) adjusting the fill weight of the protein in said bulk formulation to achieve a fixed dose of the protein, and (d) lyophilizing the protein fill weight-adjusted formulation to achieve a final formulation in a container, wherein the product concentration post reconstitution with a fixed volume is within a predetermined acceptance range. The process is particularly suitable for formulations with low protein concentrations (e.g., 0.05 to 20 mg / mL).
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com