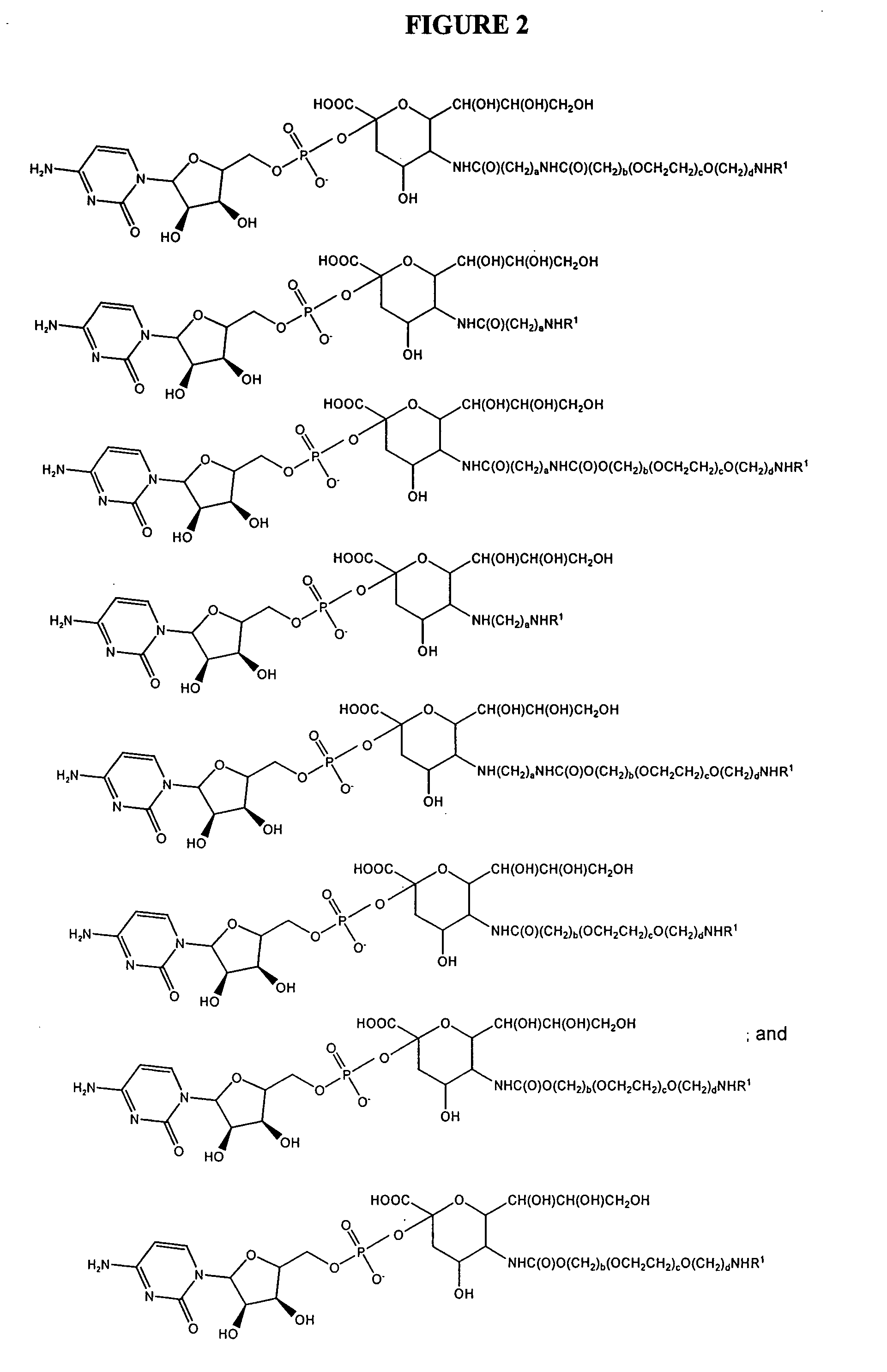
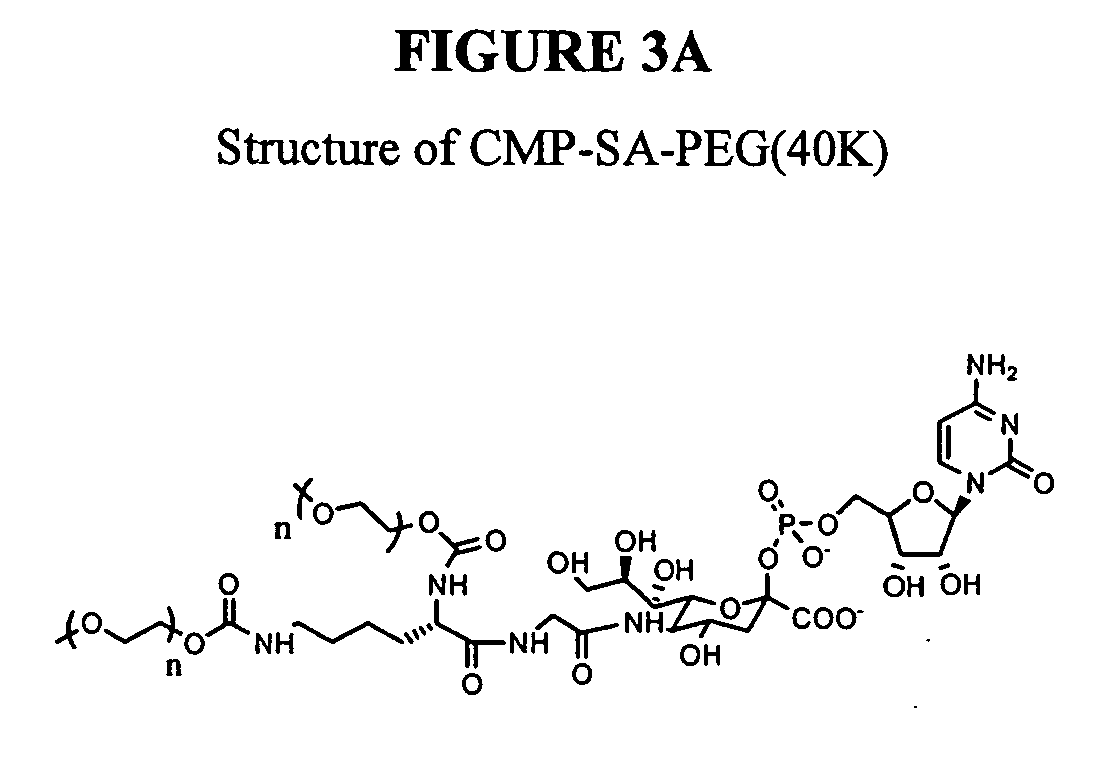
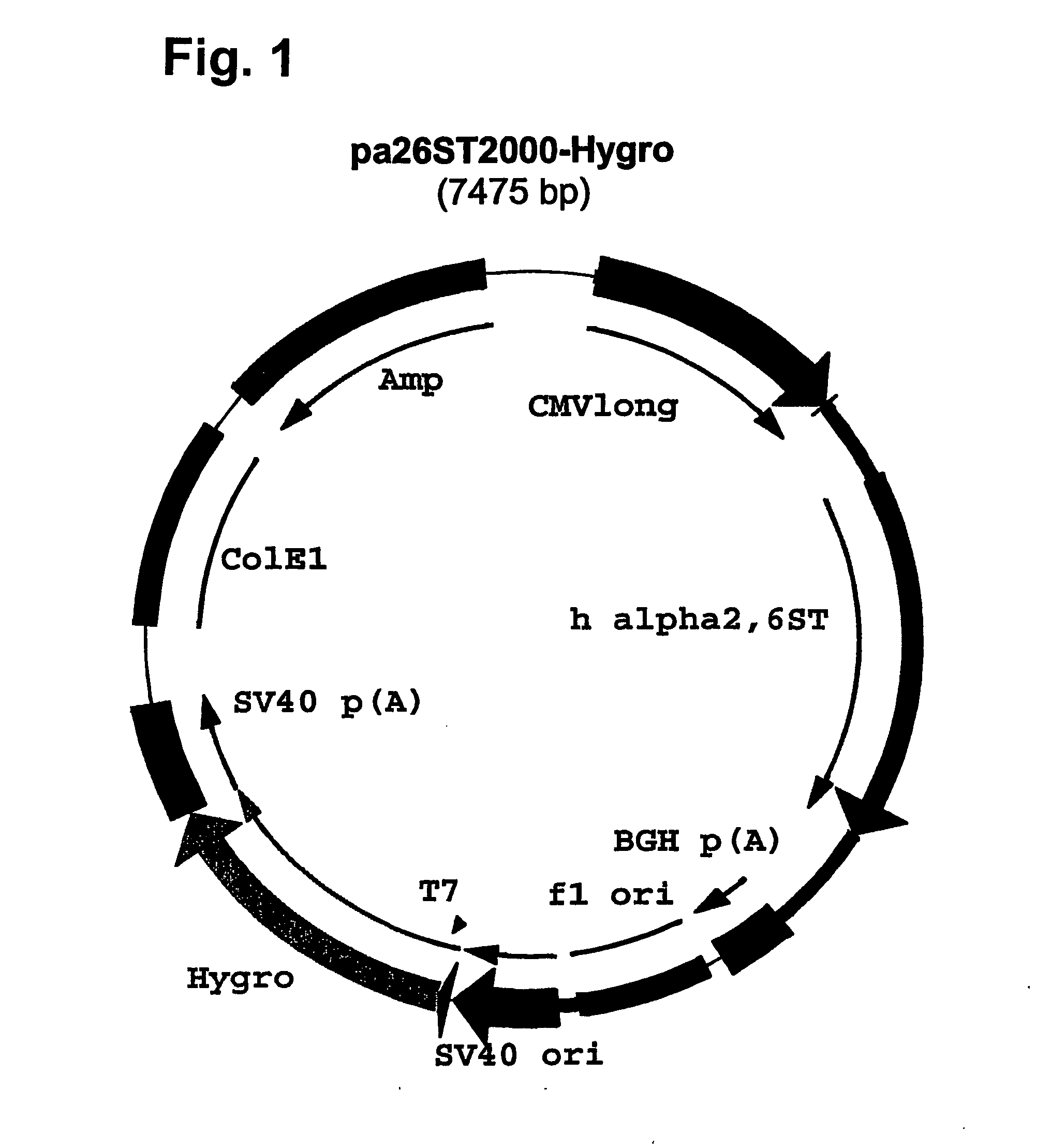
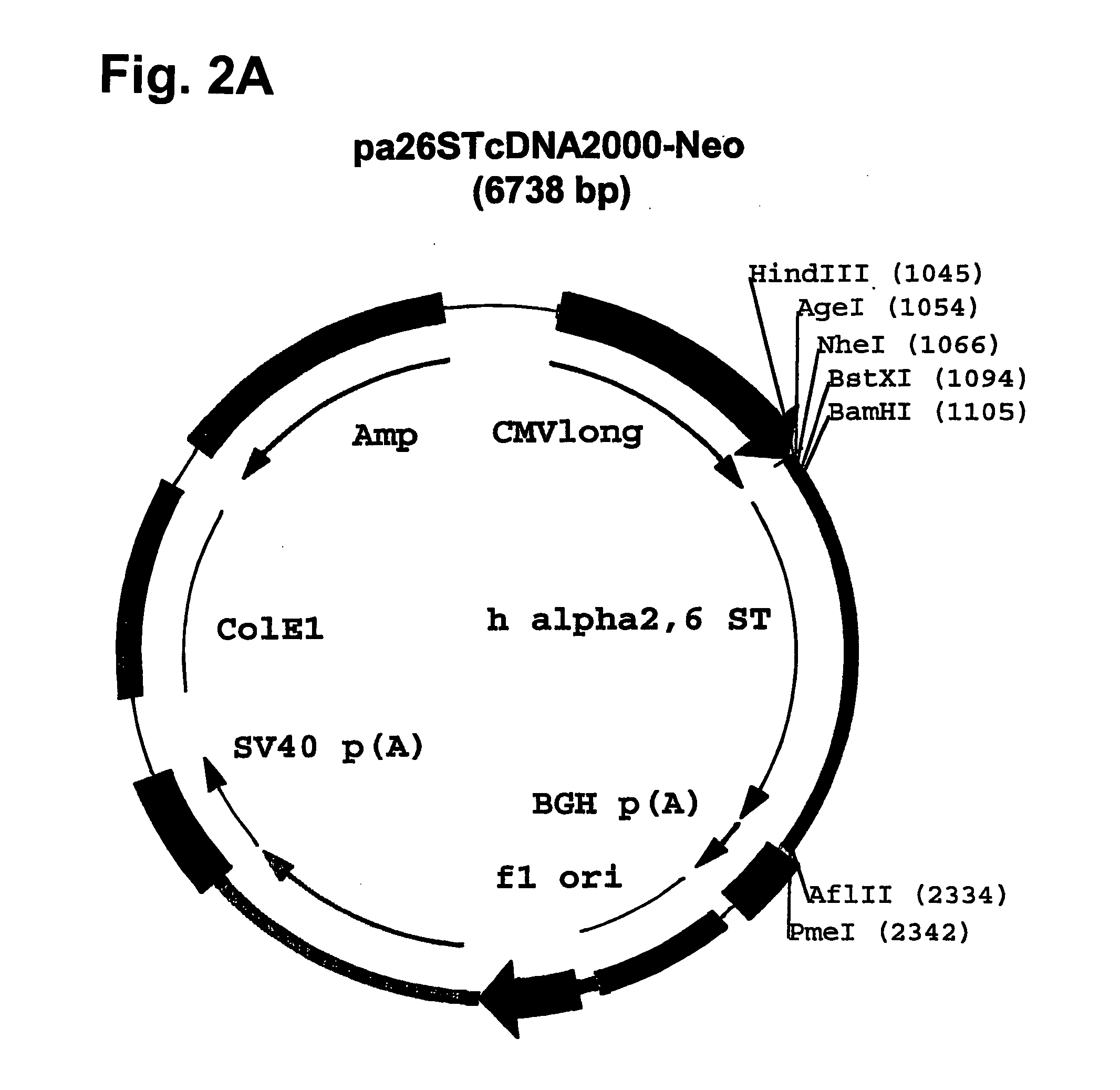
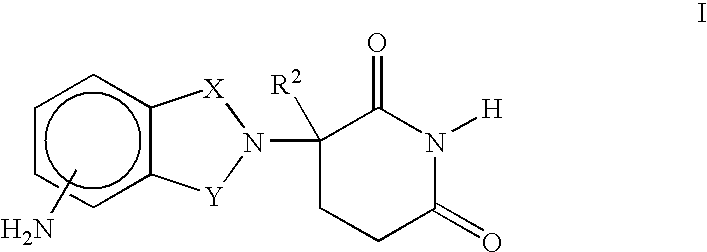
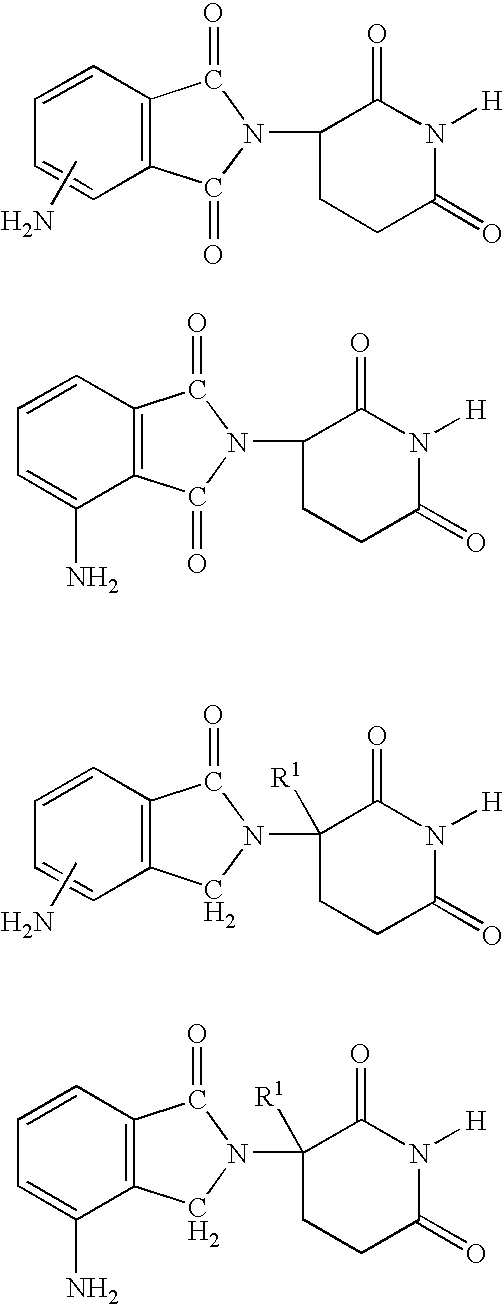
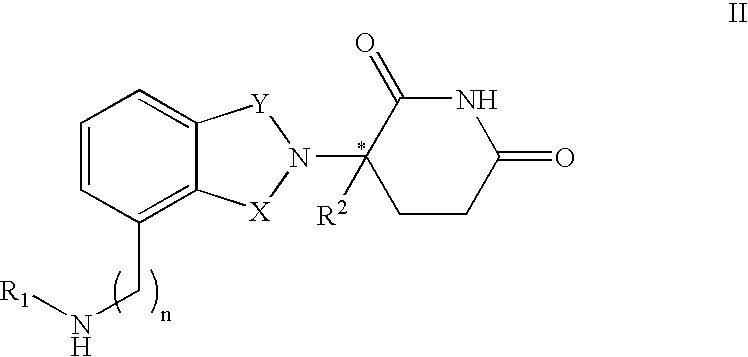
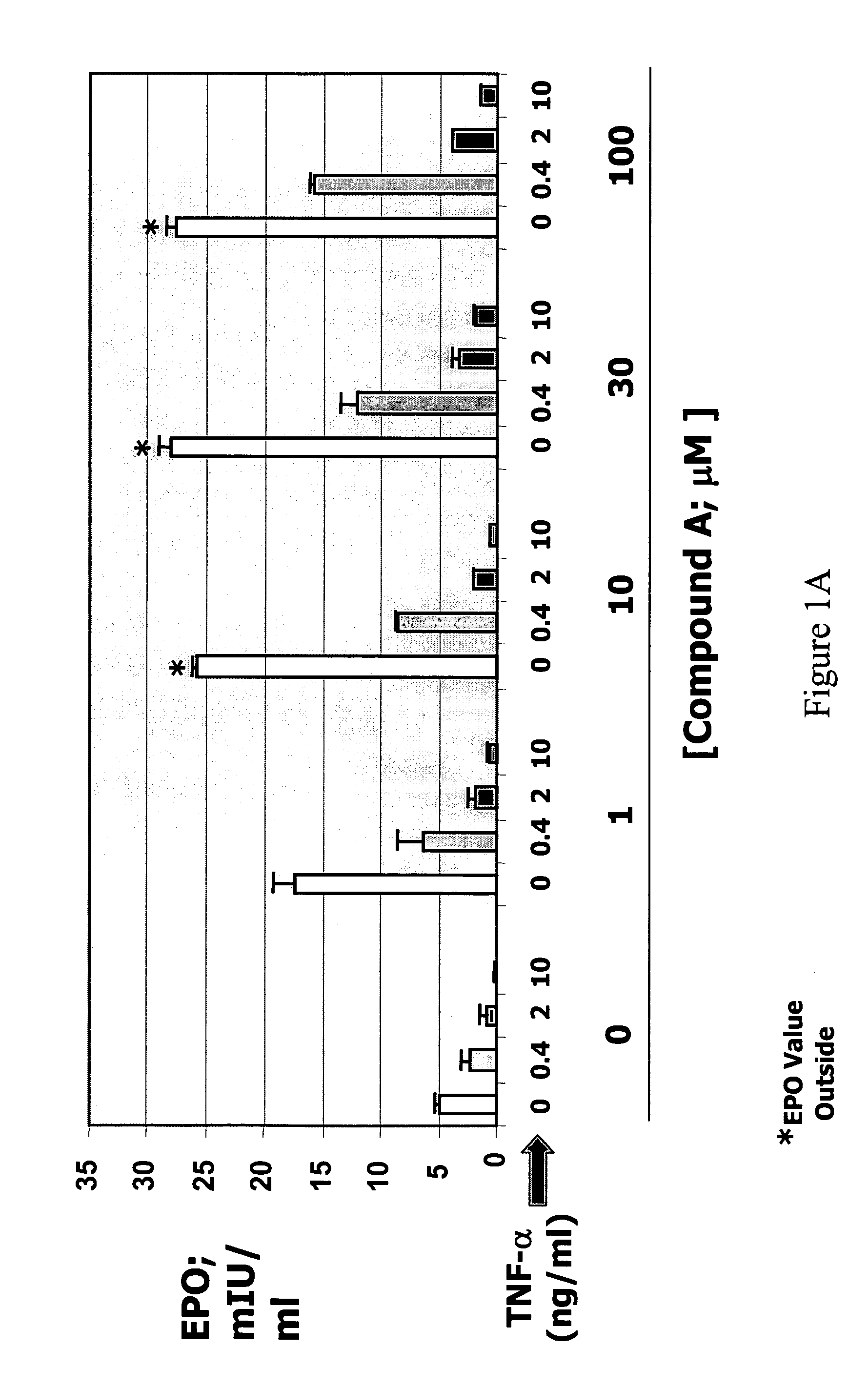
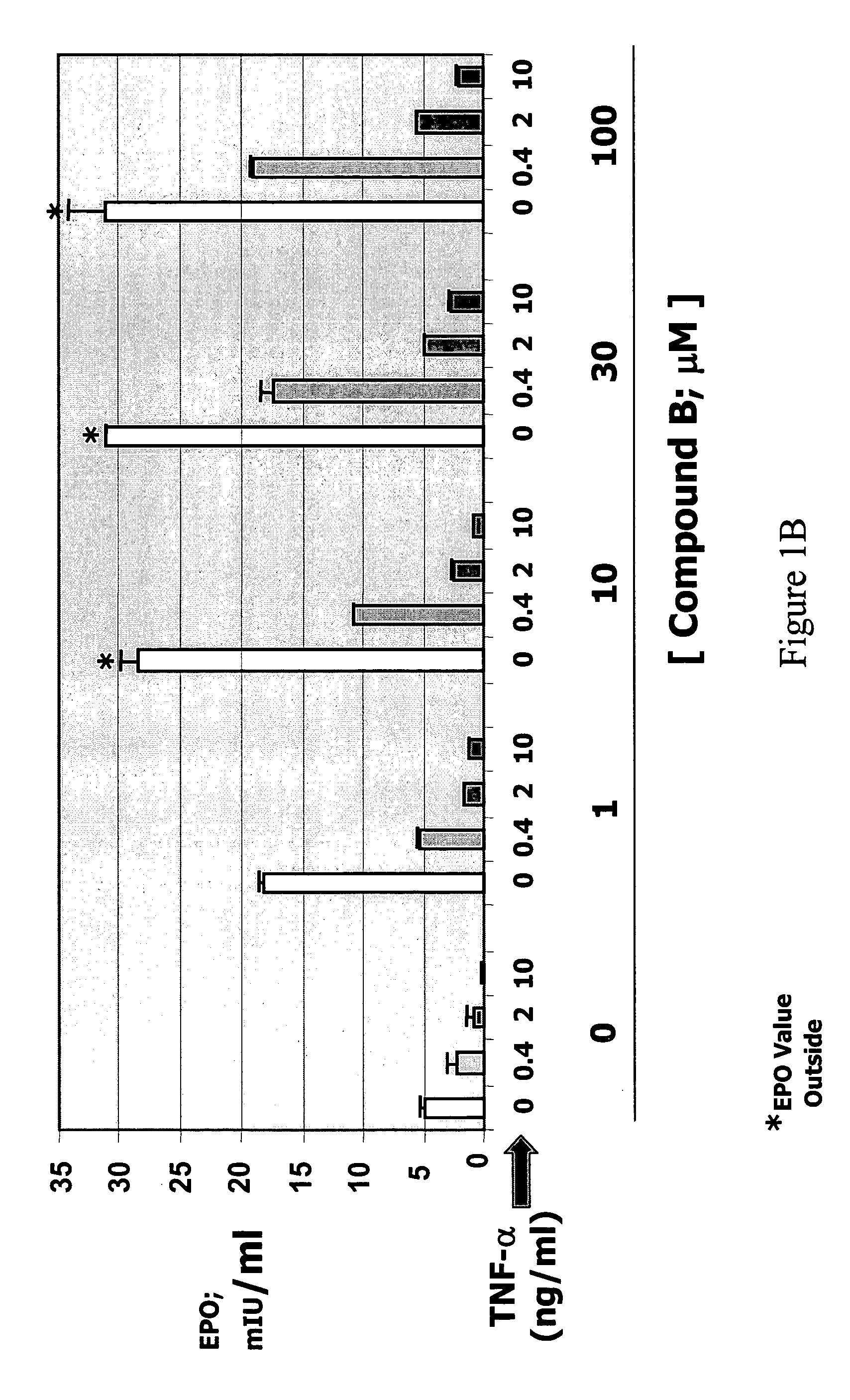
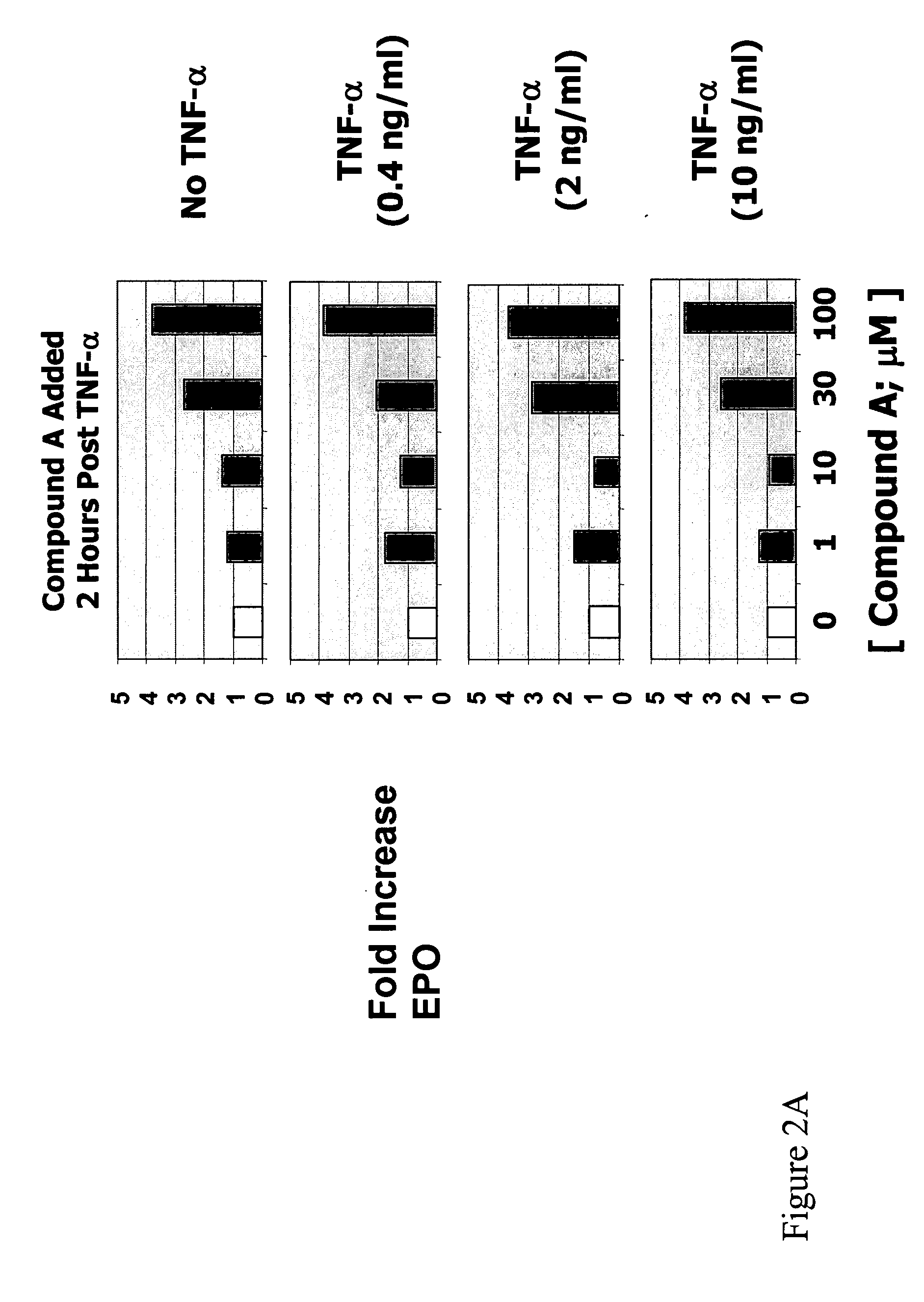
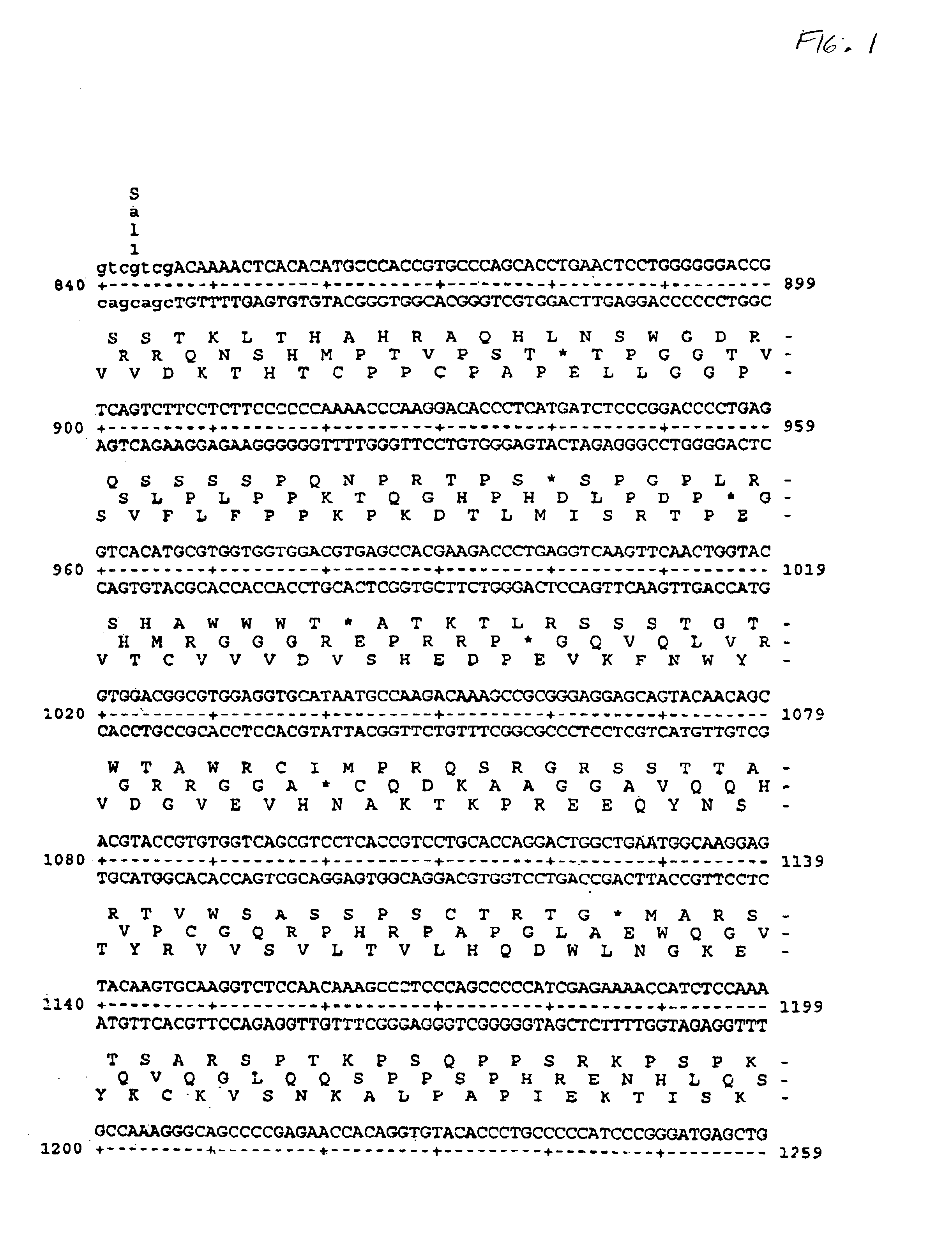
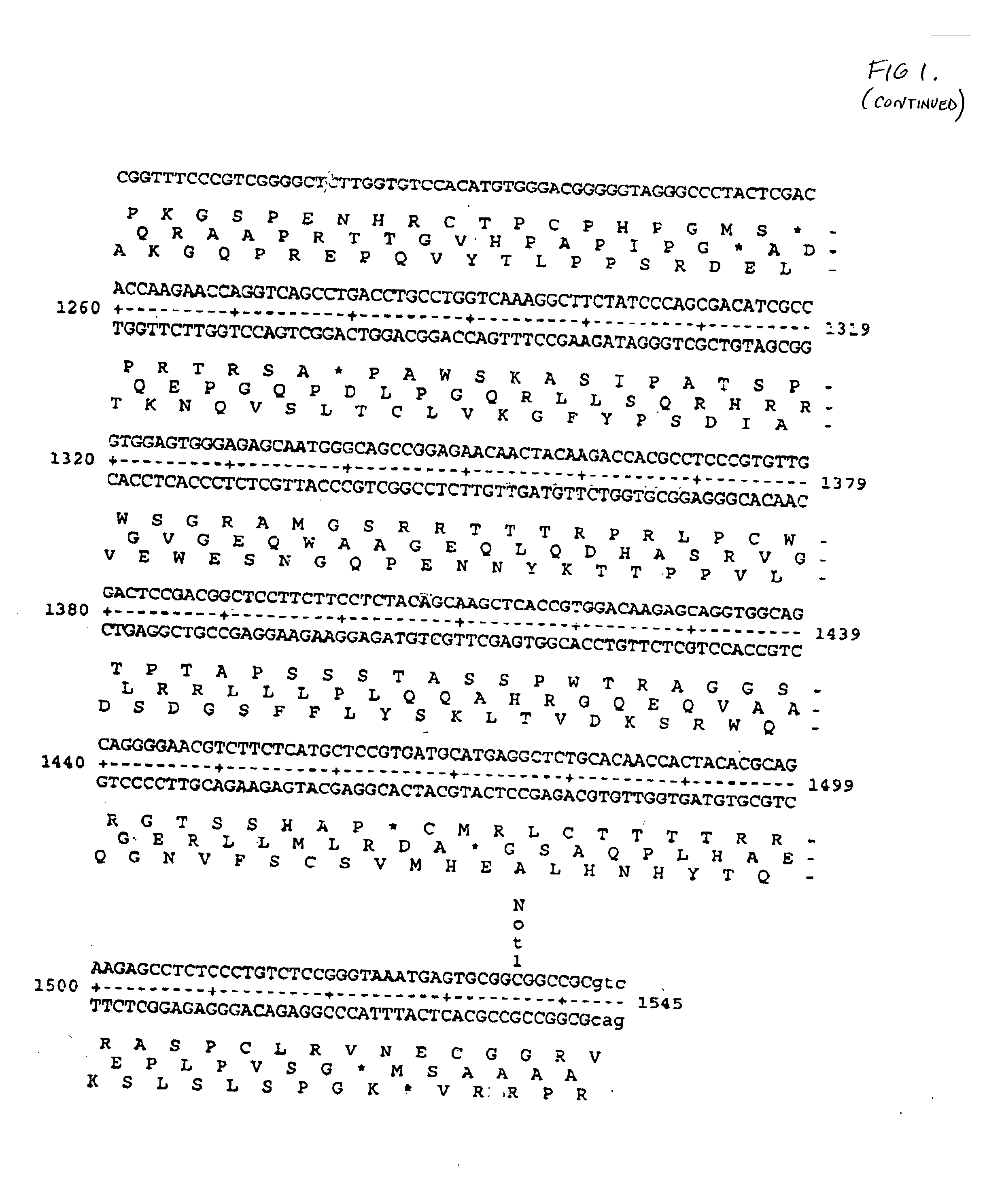
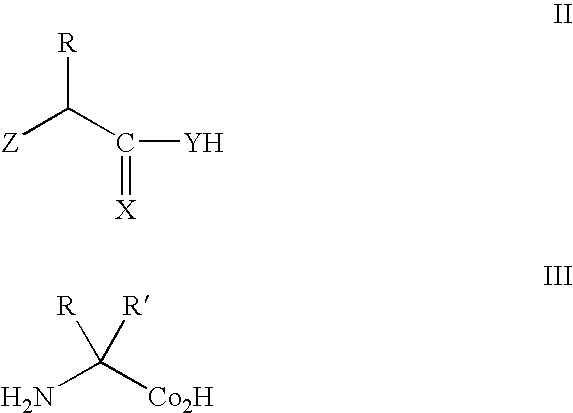
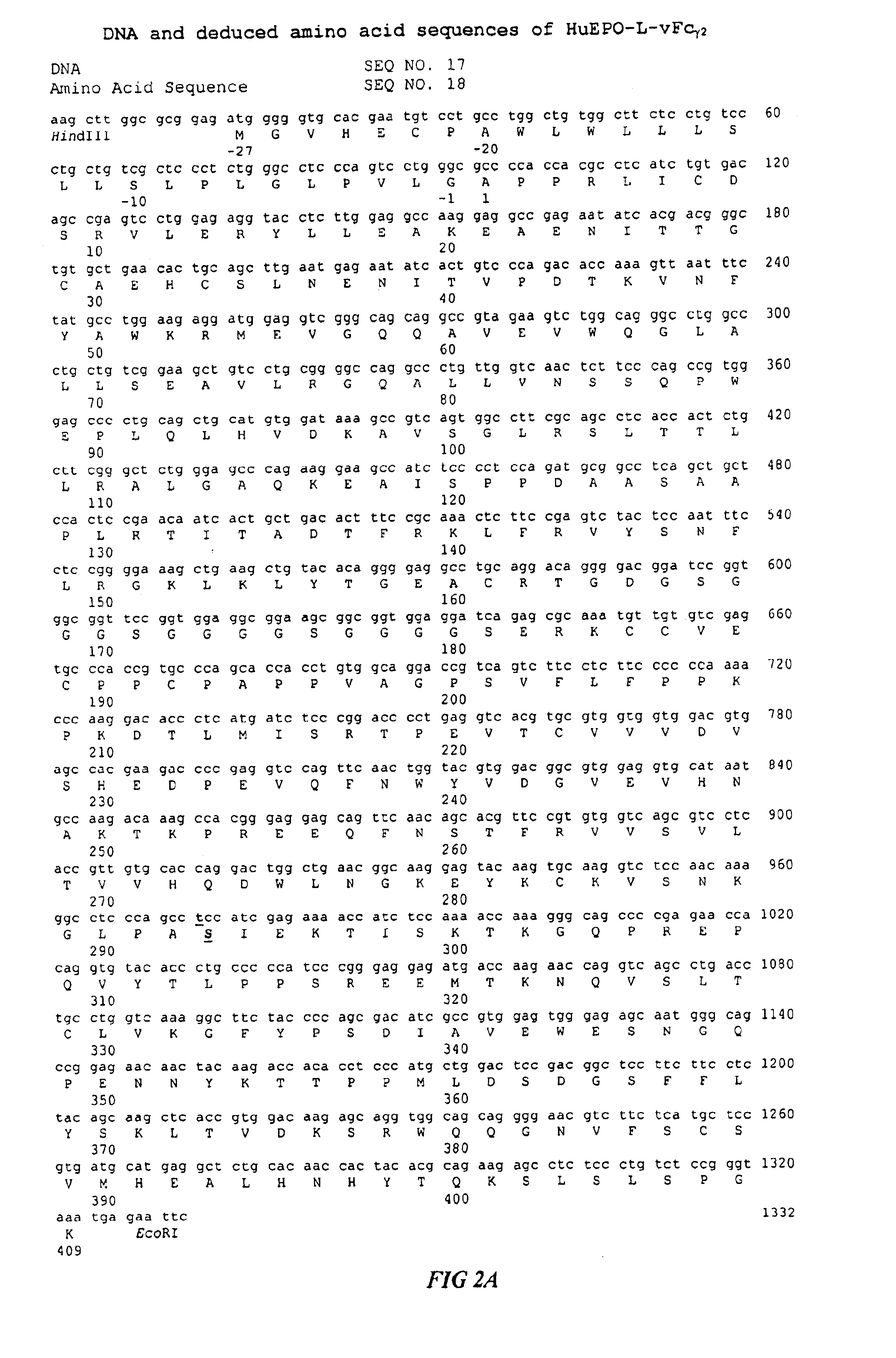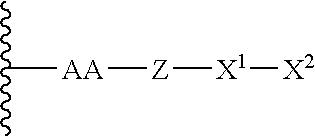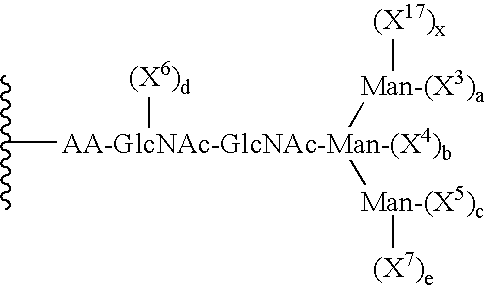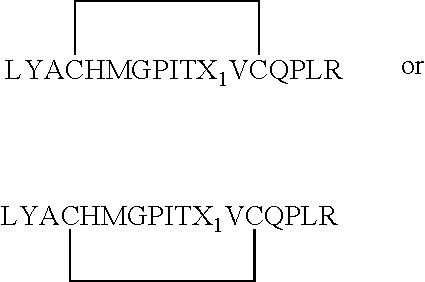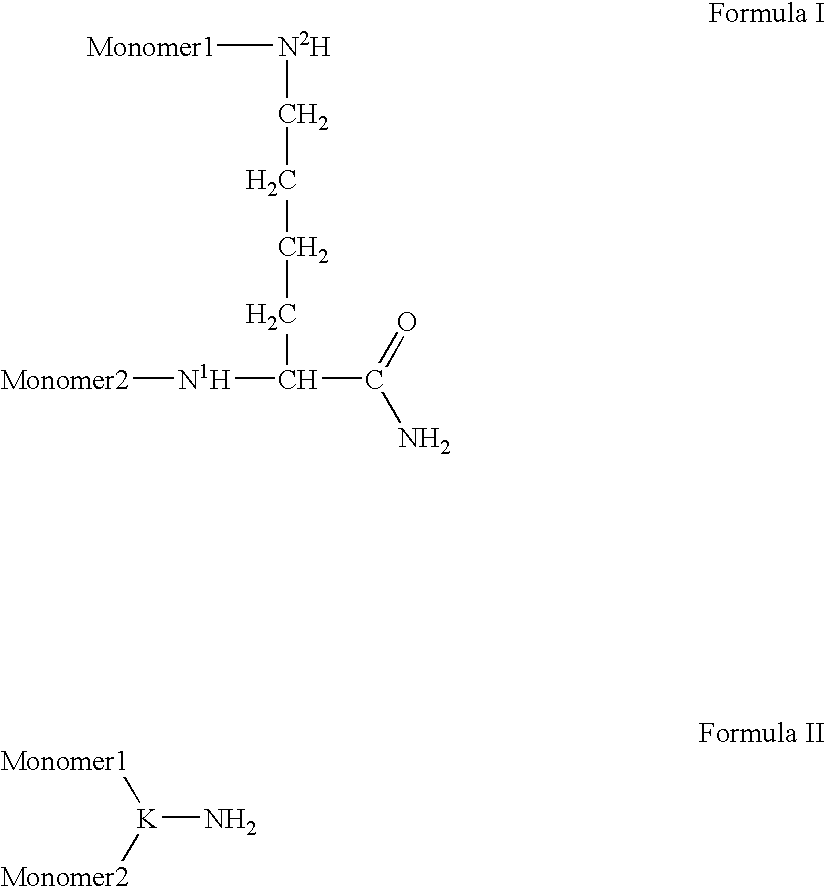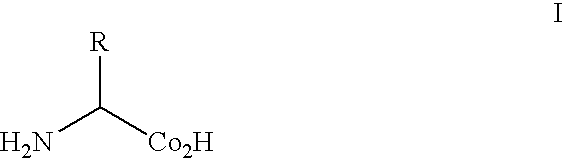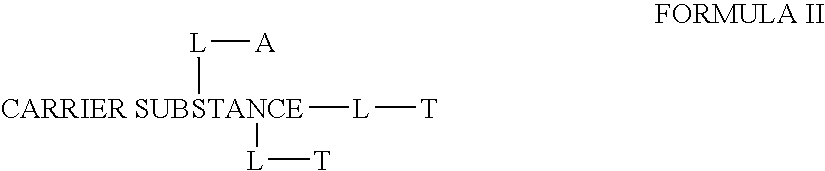Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
824results about "Erythropoietin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
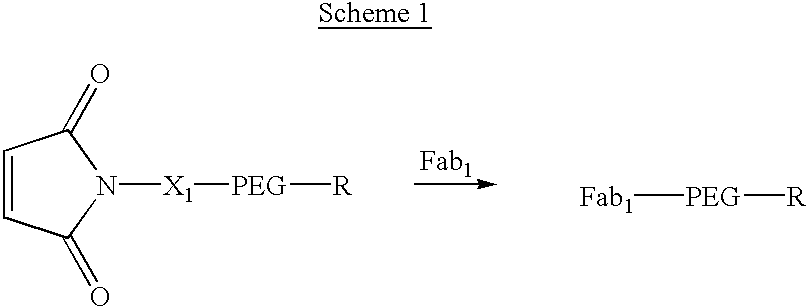
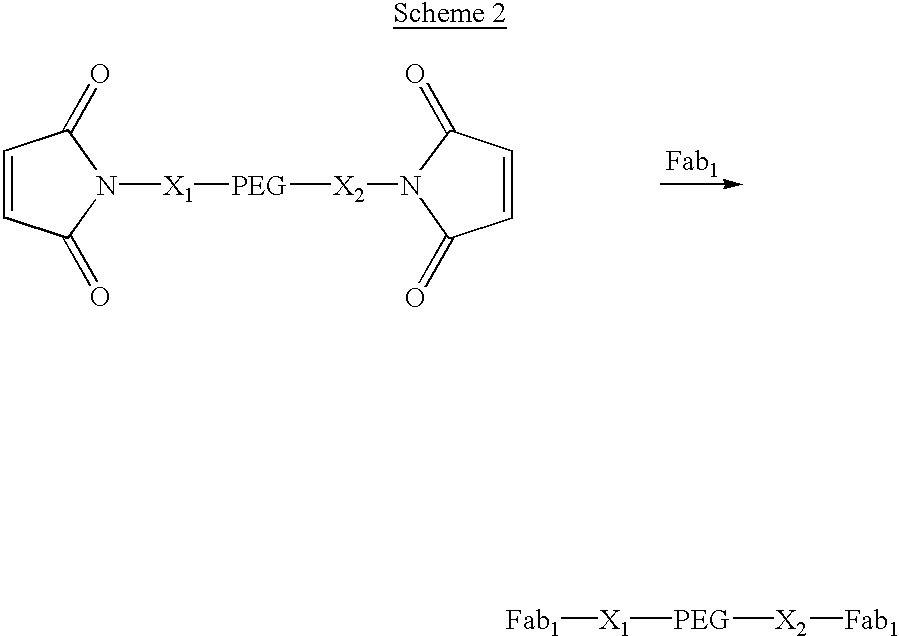
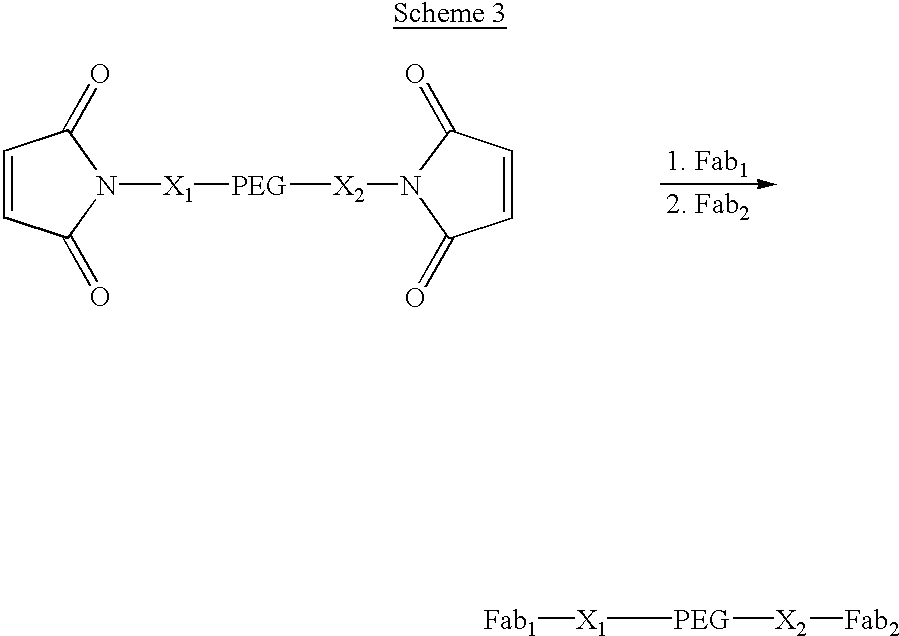
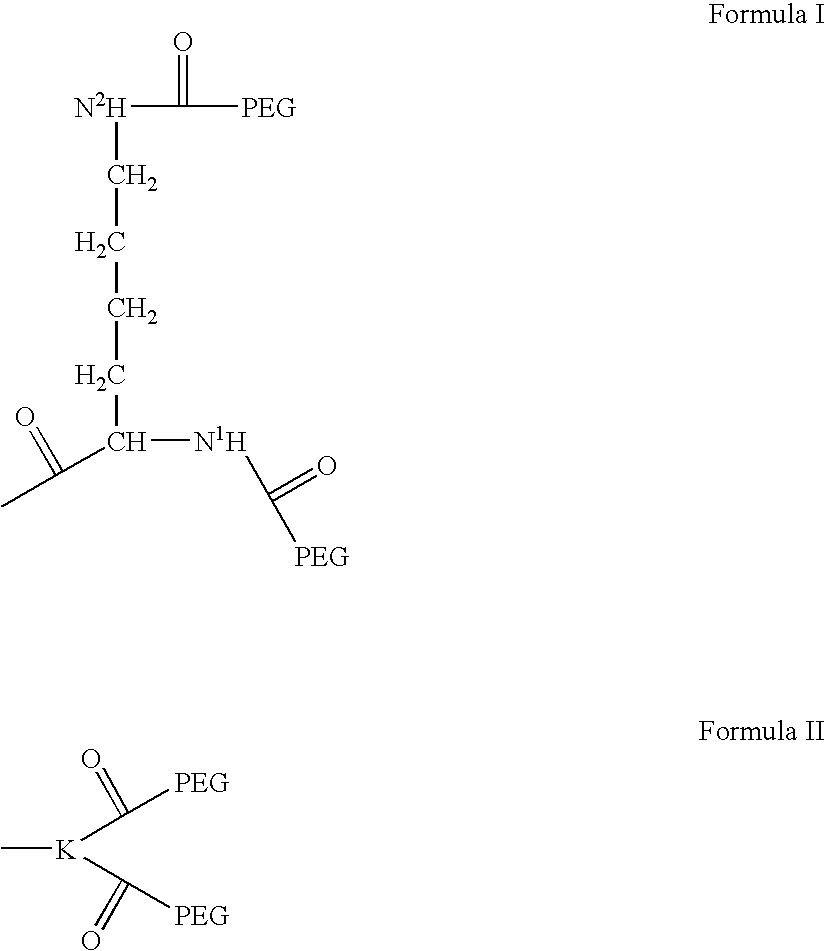
Methods for rational pegylation of proteins
The present invention relates to the use of simulation technology to rationally optimize the locations and sizes of attached polymeric moieties for modification of therapeutic proteins and the proteins generated from this method.
Owner:XENCOR
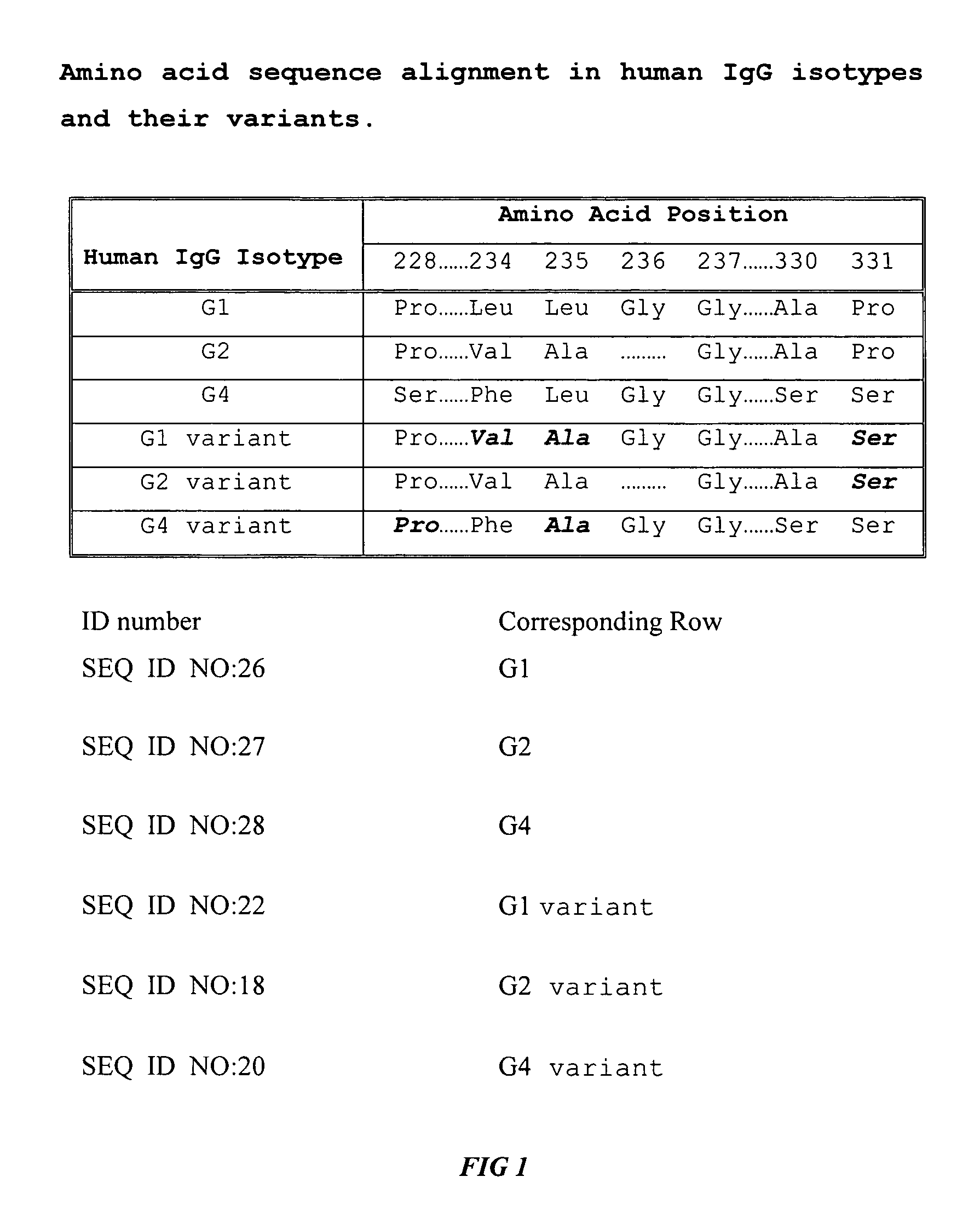
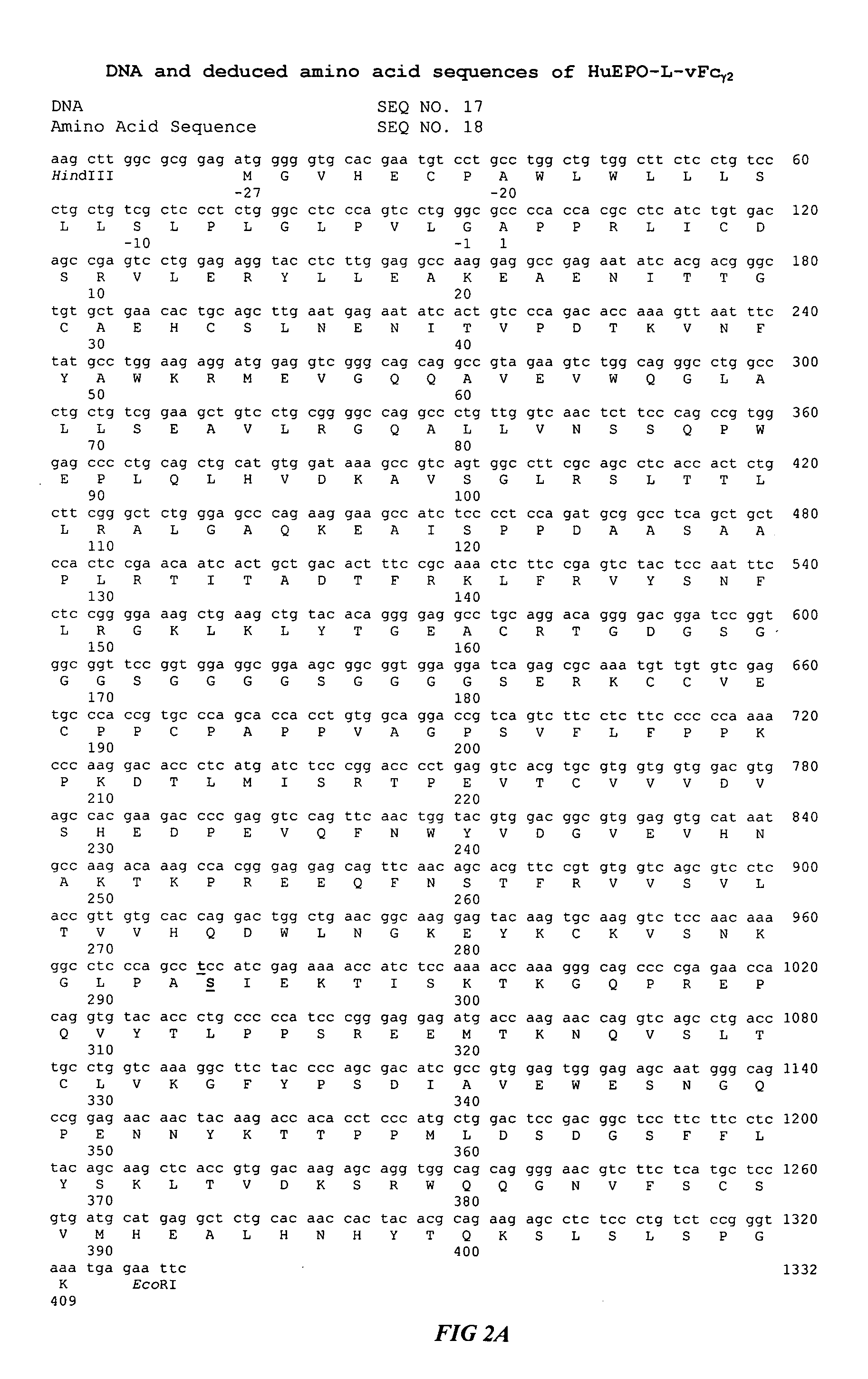
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
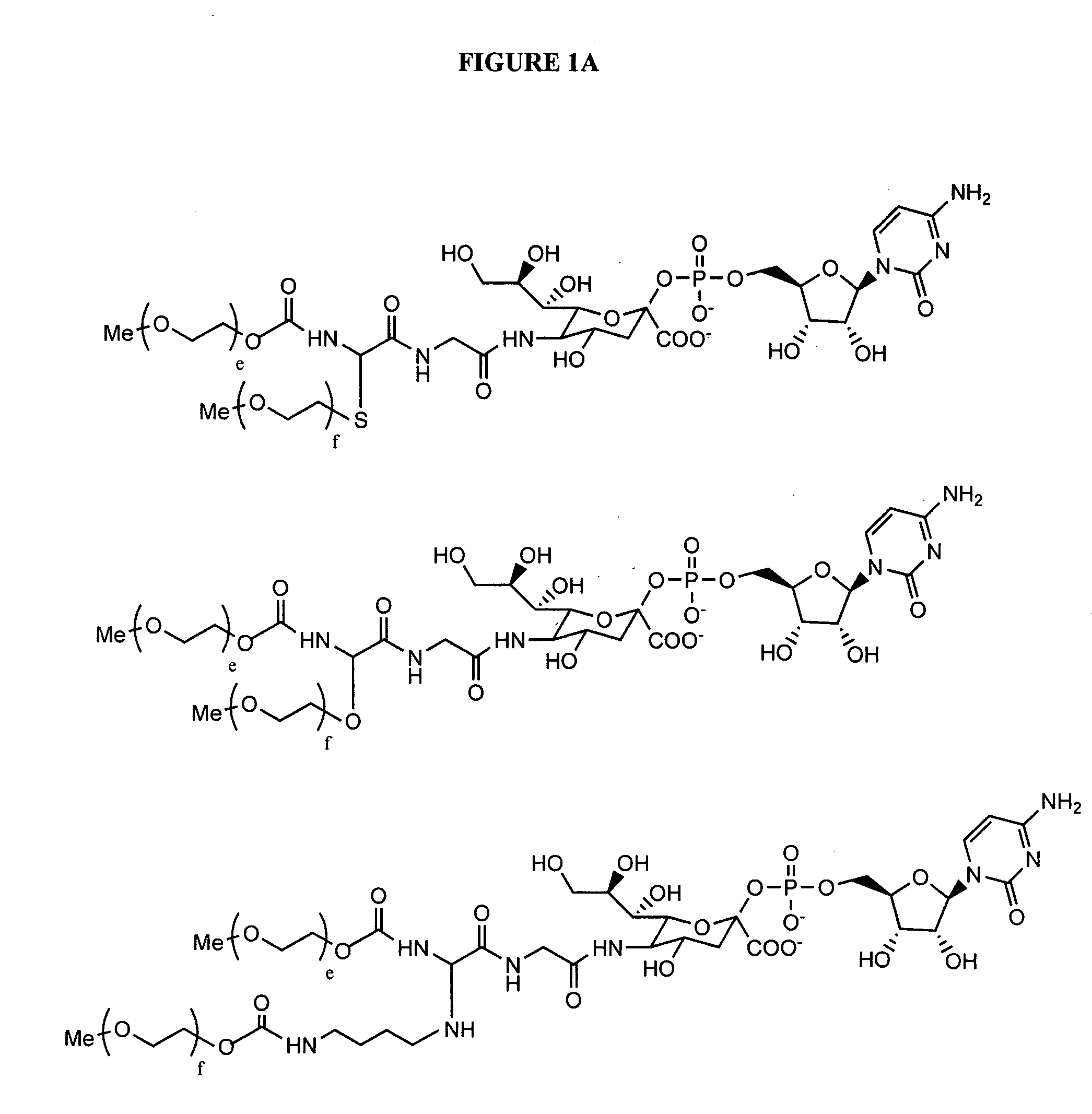
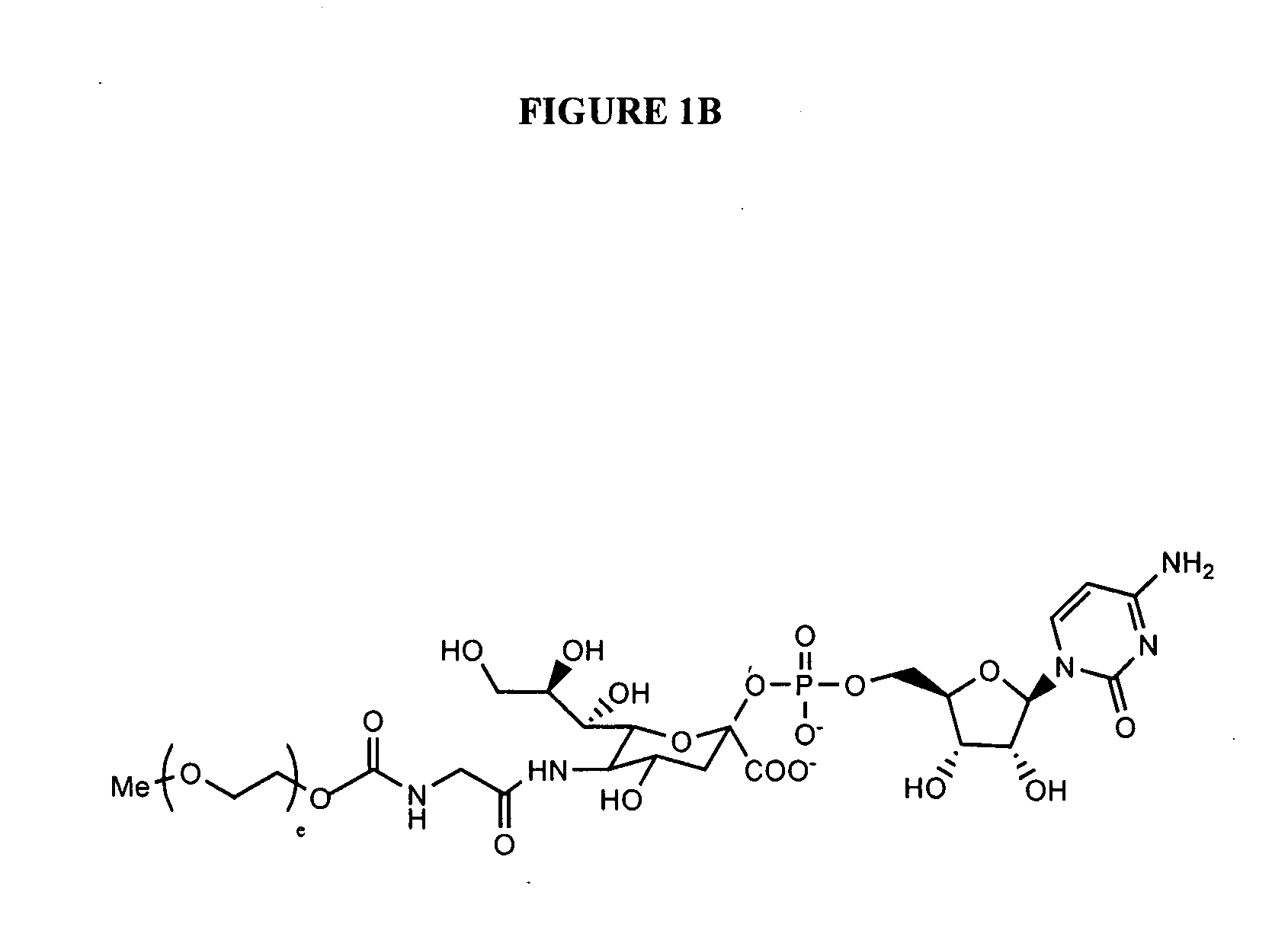
Glycopegylated erythropoietin
InactiveUS20060111279A1Improved pharmacokinetic propertiesCost effectiveSaccharide peptide ingredientsDepsipeptidesDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
RNA preparations comprising purified modified RNA for reprogramming cells
ActiveUS20110143397A1Promote growthArtificial cell constructsCell culture active agentsSingle strandSomatic cell
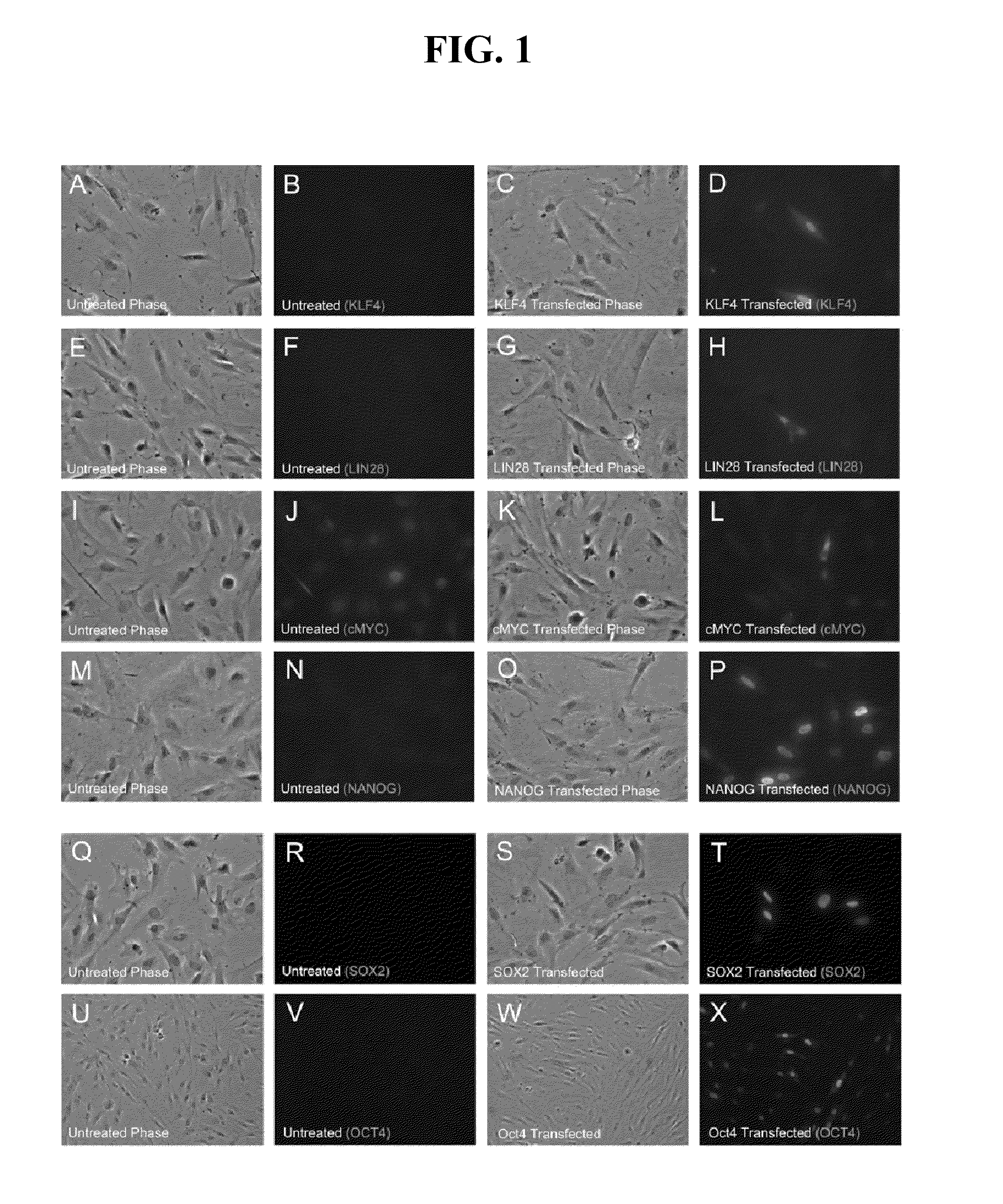
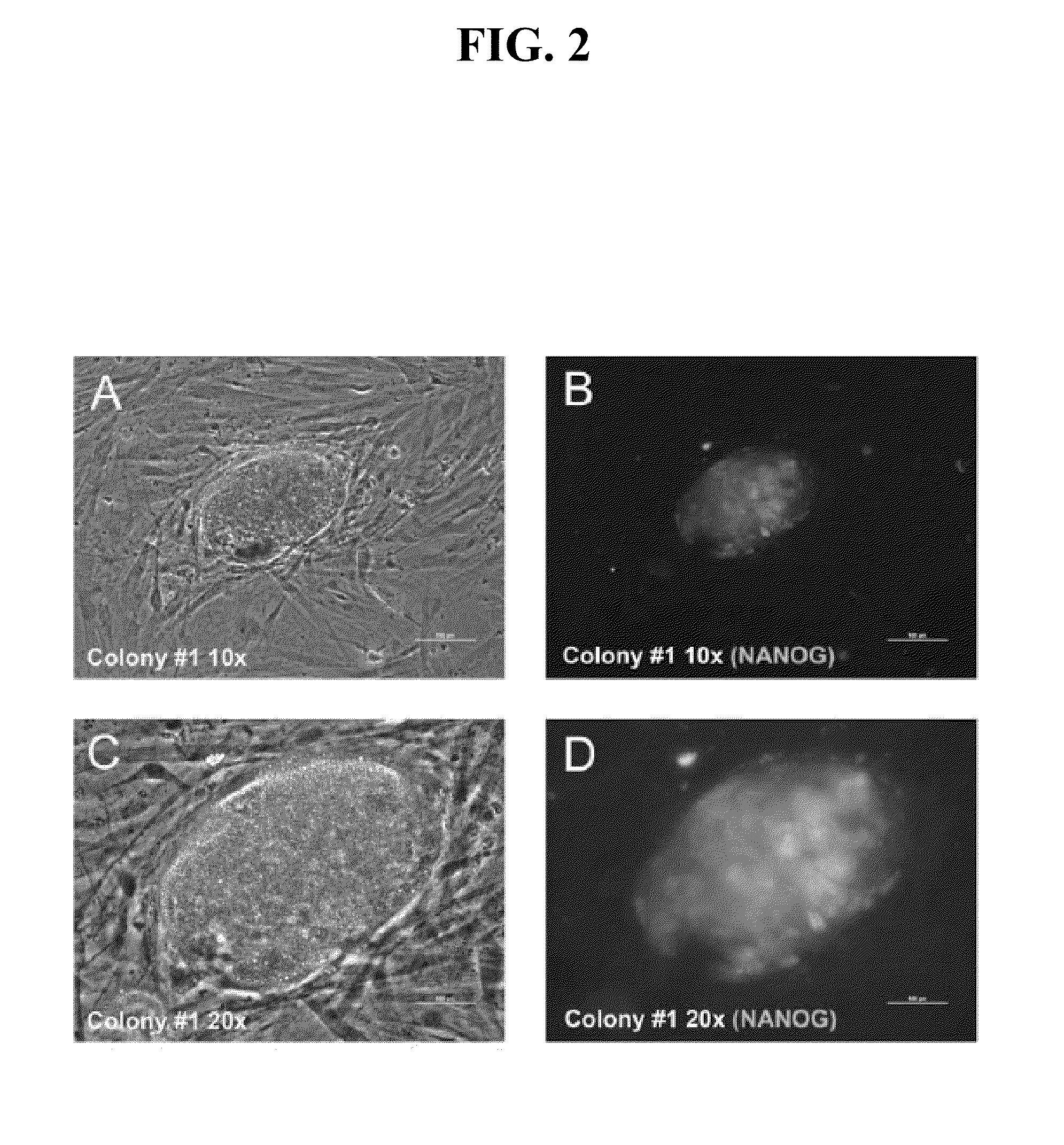
The present invention provides compositions and methods for reprogramming somatic cells using purified RNA preparations comprising single-strand mRNA encoding an iPS cell induction factor. The purified RNA preparations are preferably substantially free of RNA contaminant molecules that: i) would activate an immune response in the somatic cells, ii) would decrease expression of the single-stranded mRNA in the somatic cells, and / or iii) active RNA sensors in the somatic cells. In certain embodiments, the purified RNA preparations are substantially free of partial mRNAs, double-stranded RNAs, un-capped RNA molecules, and / or single-stranded run-on mRNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Erythropoietin: remodeling and glycoconjugation of erythropoietin
InactiveUS20060088906A1Increase hematocrit levelImprove the level ofPeptide/protein ingredientsPeptide preparation methodsErythropoiesisErythropoietin
Owner:NEOSE TECH
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050202538A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
Owner:MERCK PATENT GMBH
Glycopegylated erythropoietin
InactiveUS20050143292A1Improved pharmacokinetic propertiesCost-effectiveSugar derivativesPeptide/protein ingredientsDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Glycosylation analogs of erythropoietin
ActiveUS7217689B1Increase the number ofHigh sialic acid contentPeptide/protein ingredientsTissue culturePlasmidDNA
Erythropoietin analogs having at least one additional site for glycosylation, or a rearrangement of at least one site for glycosylation are disclosed. The invention also relates to DNA sequences encoding said erythropoietin analogs, and recombinant plasmids and host cells for analog expression.
Owner:AMGEN INC
Methods for making proteins containing free cysteine residues
The present invention relates to novel methods of making soluble proteins having free cysteines in which a host cell is exposed to a cysteine blocking agent. The soluble proteins produced by the methods can then be modified to increase their effectiveness. Such modifications include attaching a PEG moiety to form pegylated proteins.
Owner:BOLDER BIOTECH
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050192211A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
The present invention provides Fc-erythropoietin (“Fc-EPO”) fusion proteins with improved pharmacokinetics. Nucleic acids, cells, and methods relating to the production and practice of the invention are also provided.
Owner:MERCK PATENT GMBH
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
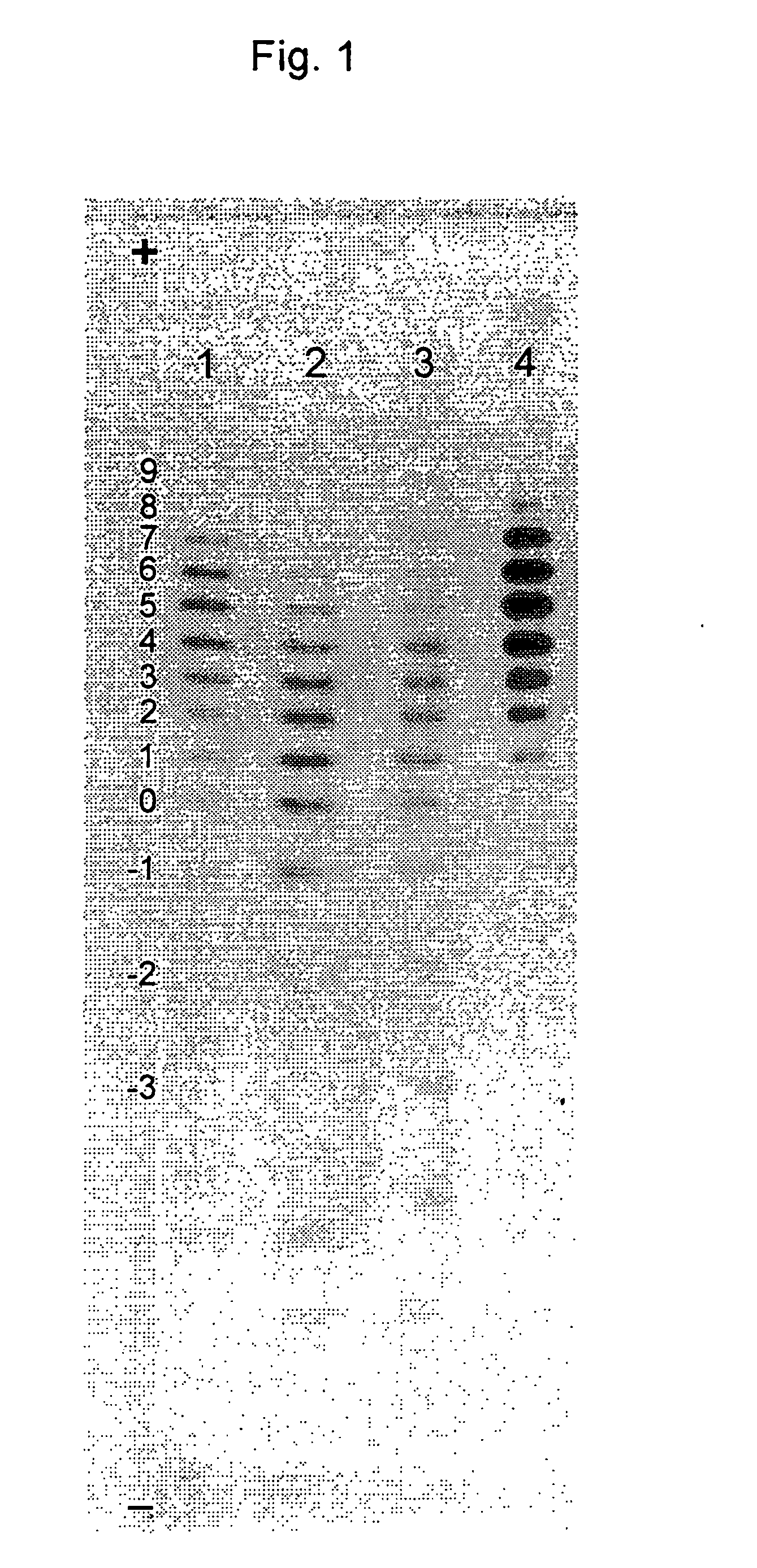
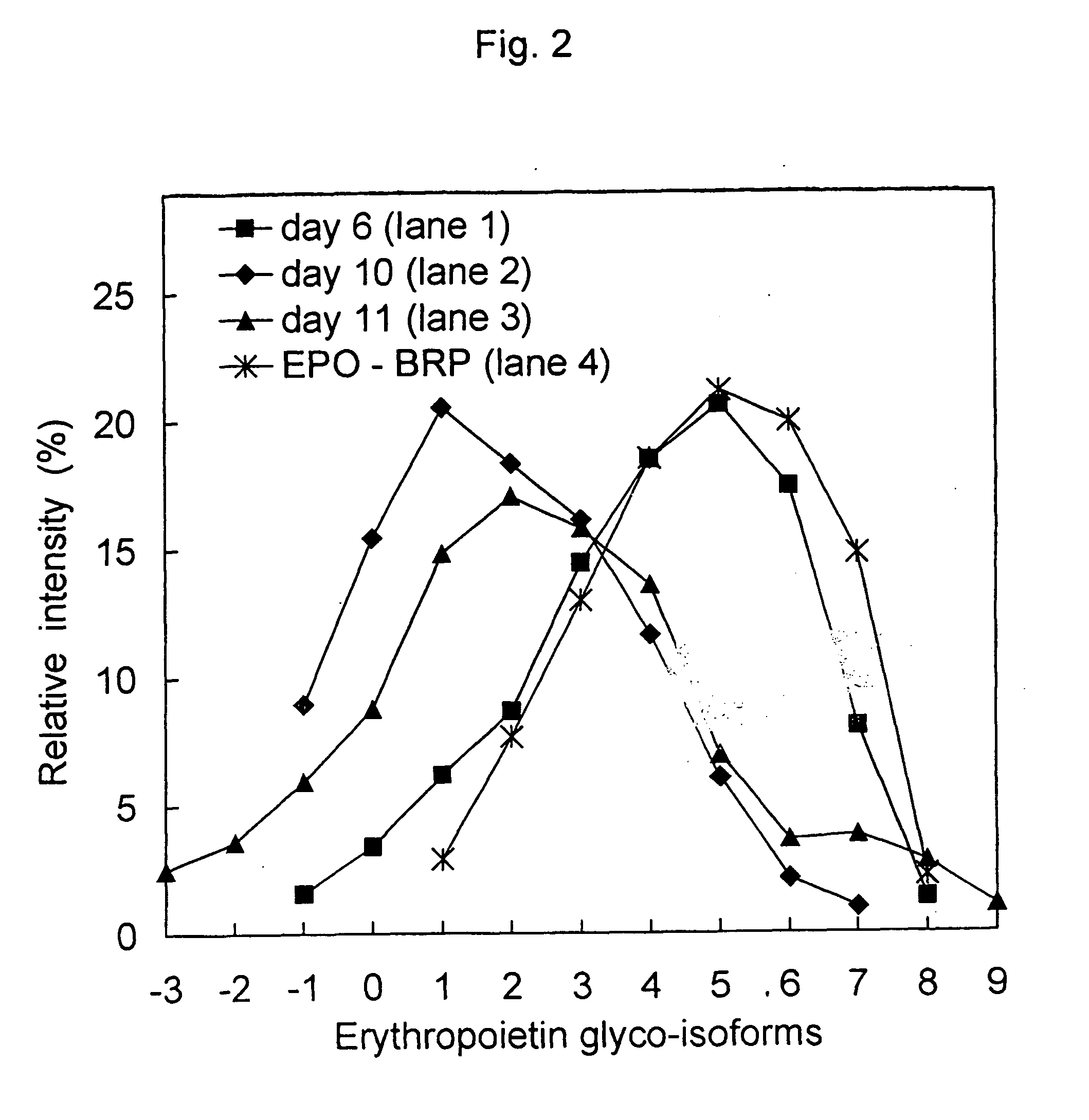
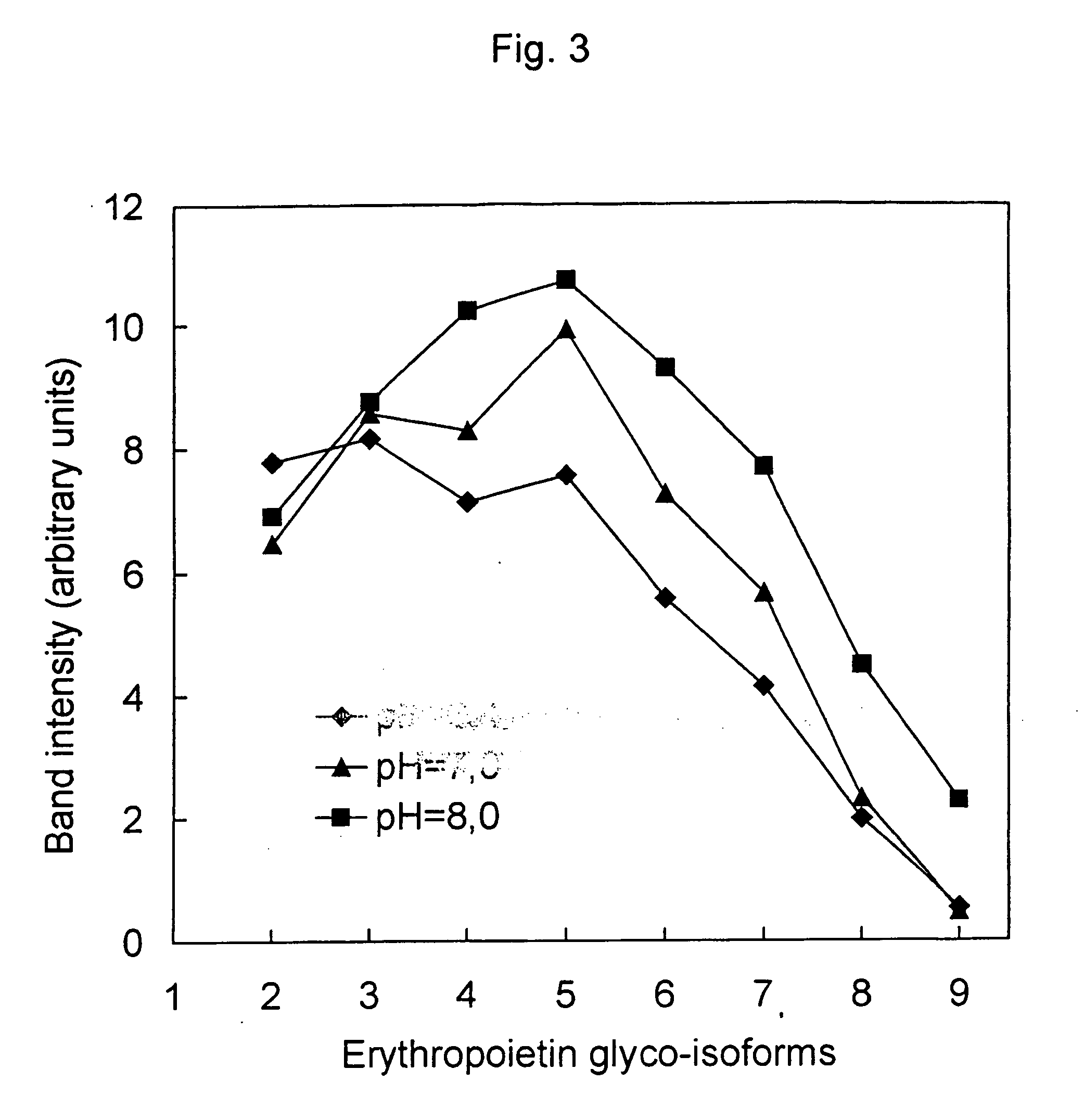
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
Pseudo-antibody constructs
InactiveUS20030211078A1Reduce productionInhibit synthesisOrganic active ingredientsBiocideHalf-lifeIn vivo
This invention relates to novel pharmaceutically useful compositions that bind to a biological molecule, having improved circulatory half-life, increased avidity, increased affinity, or multifunctionality, and methods of use thereof. The present invention provides a pseudo-antibody comprising an organic moiety covalenty coupled to at least two target-binding moieties, wherein the target-binding moieties are selected from the group consisting of a protein, a peptide, a peptidomimetic, and a non-peptide molecule that binds to a specific targeted biological molecule. The pseudo-antibody of the present invention may affect a specific ligand in vitro, in situ and / or in vivo. The pseudo-antibodies of the present invention can be used to measure or effect in an cell, tissue, organ or animal (including humans), to diagnose, monitor, modulate, treat, alleviate, help prevent the incidence of, or reduce the symptoms of, at least one condition.
Owner:CENTOCOR
Novel recombinant proteins with N-terminal free thiol
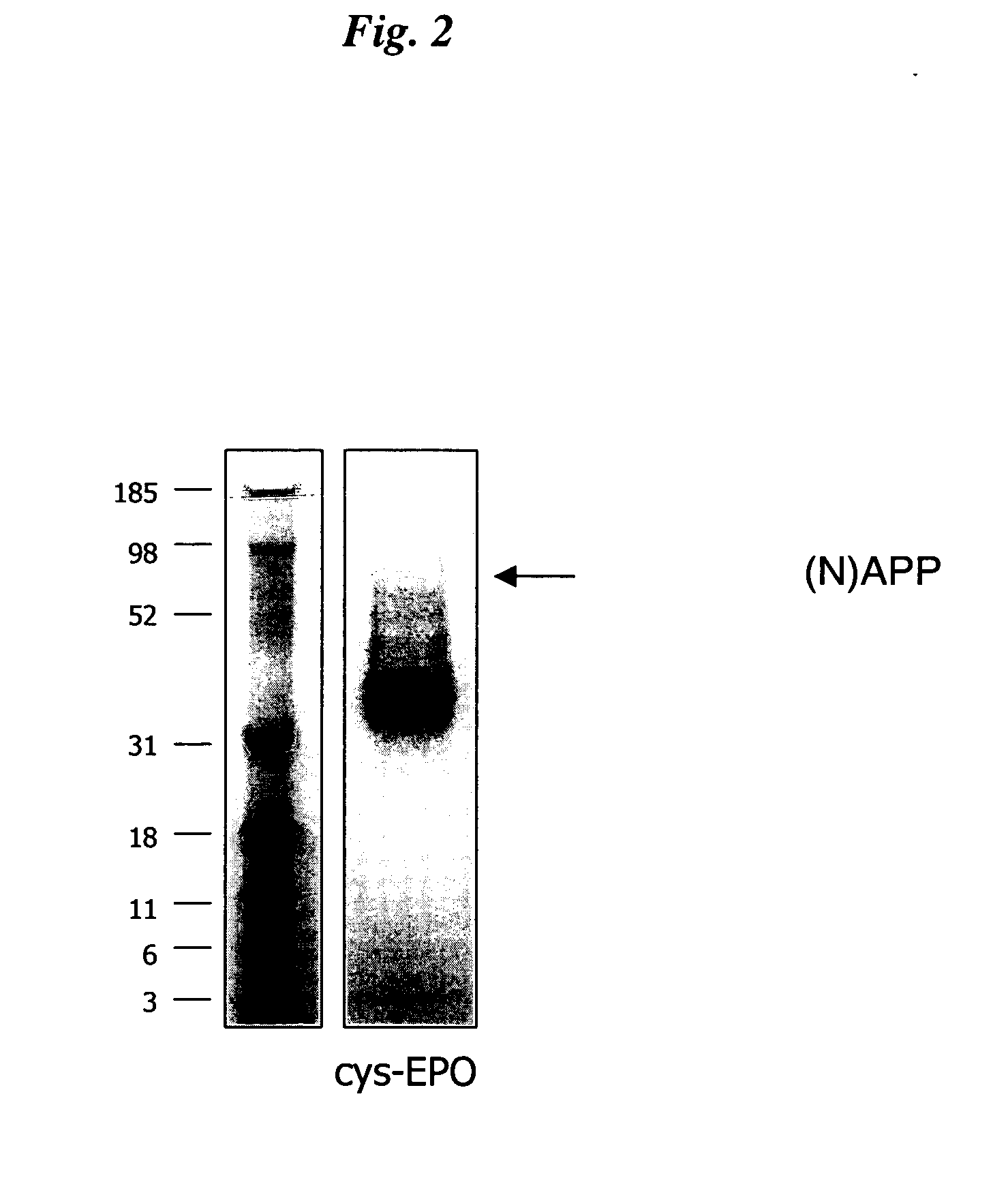
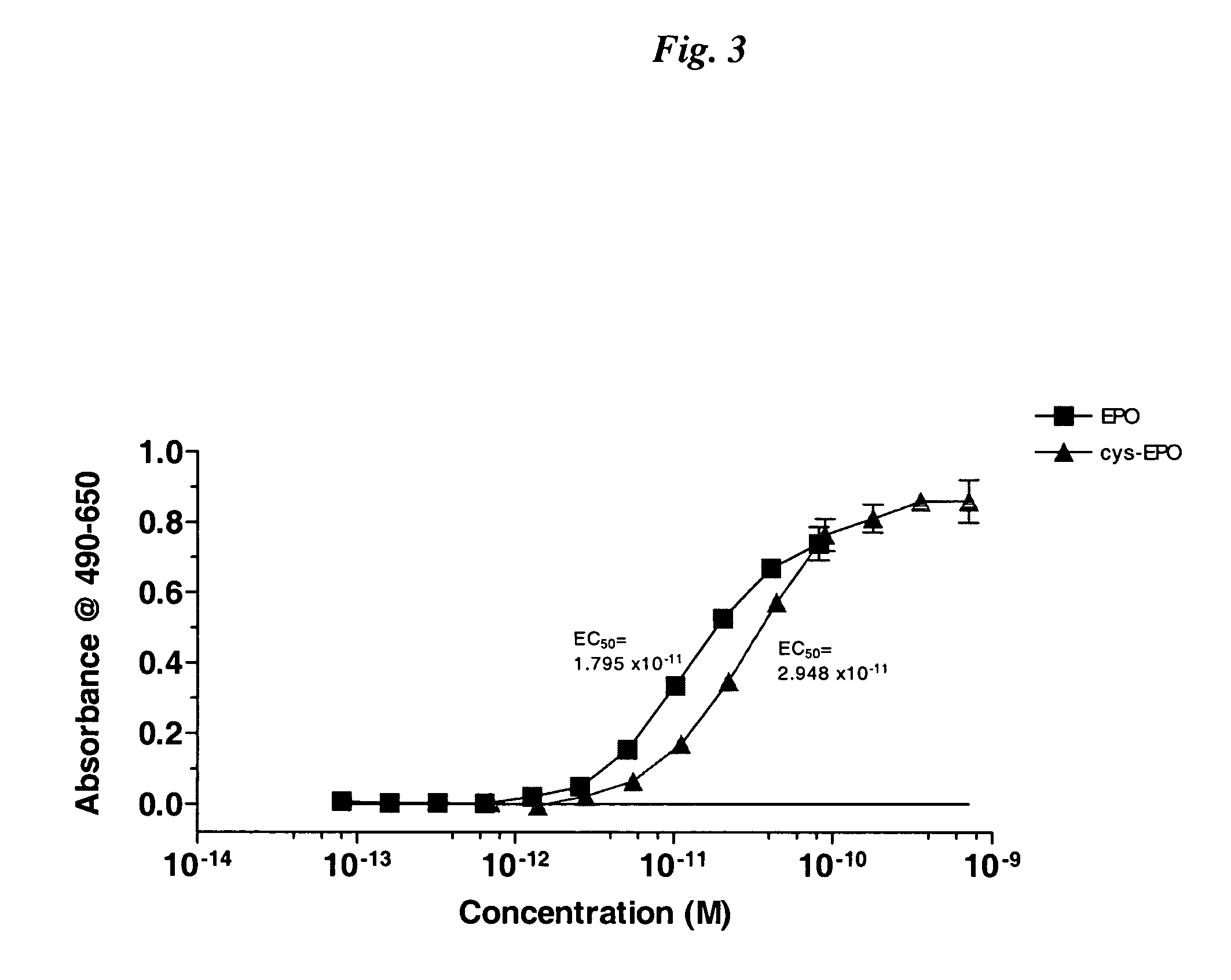
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
Novel poly(ethylene glycol) modified compounds and uses thereof
InactiveUS20050107297A1Good antigenicityIncreased durabilityPeptide/protein ingredientsDepsipeptidesPolyethylene glycolGlycol synthesis
The present invention relates to a peptide-based compound comprising a peptide moiety and a poly(ethylene glycol) moiety wherein the poly(ethylene glycol) moiety (preferably linear) has a molecular weight of more than 20 KDaltons (preferably from 20 to 60 KDaltons). The peptide moiety may be monomeric, dimeric or oligomeric. Such peptide-based compounds may optional include a linker moiety and / or a spacer moiety.
Owner:AFFYMAX
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myelodysplastic syndromes
InactiveUS20040220144A1Extension of timeBiocidePeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
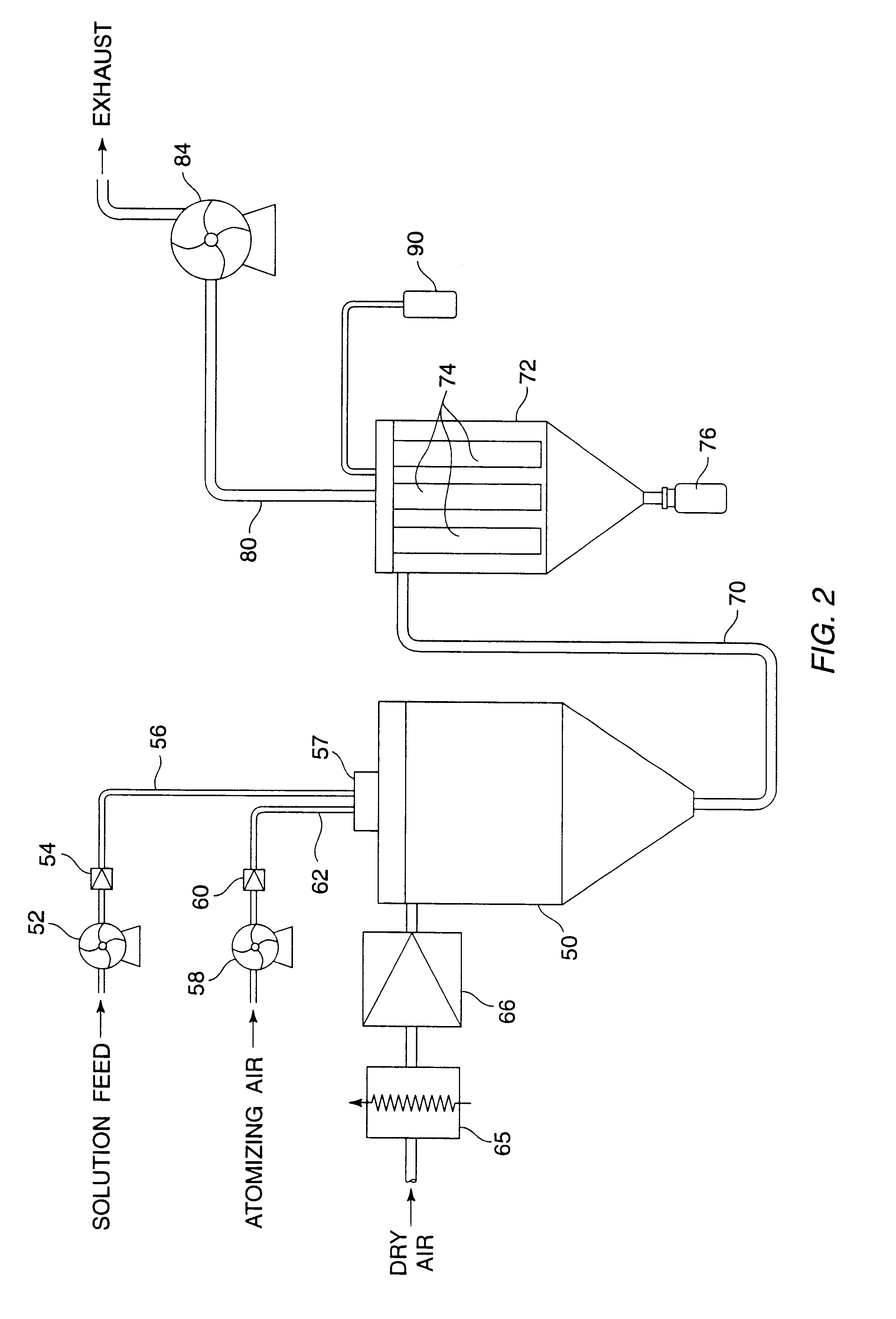
Dispersible macromolecule compositions and methods for their preparation and use
A process for preparing ultrafine powders of biological macromolecules comprises atomizing liquid solutions of the macromolecules, drying the droplets formed in the atomization step, and collecting the particles which result from drying. By properly controlling each of the atomization, drying, and collection steps, ultrafine dry powder compositions having characteristics particularly suitable for pulmonary delivery for therapeutic and other purposes may be prepared.
Owner:NOVARTIS FARMA
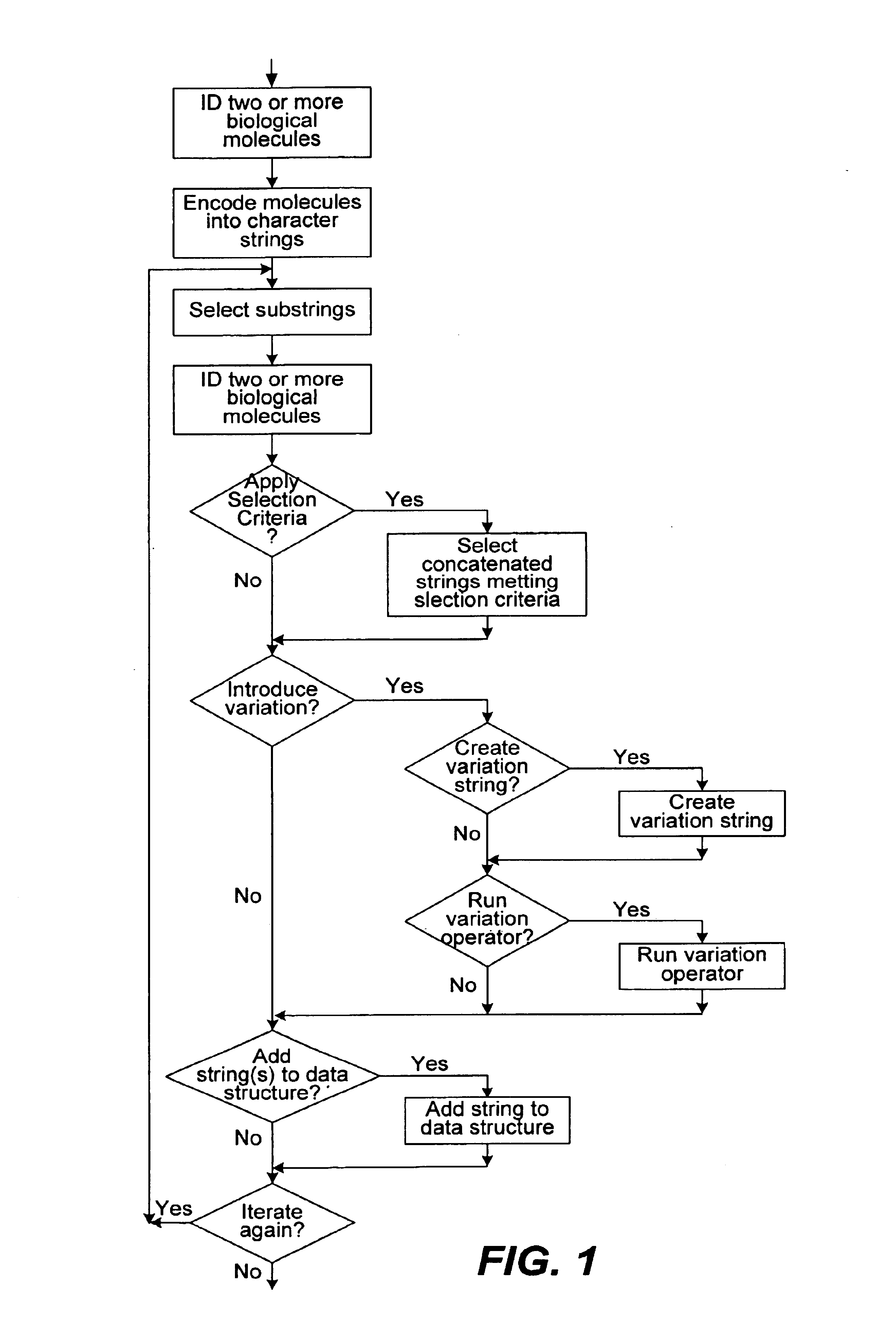
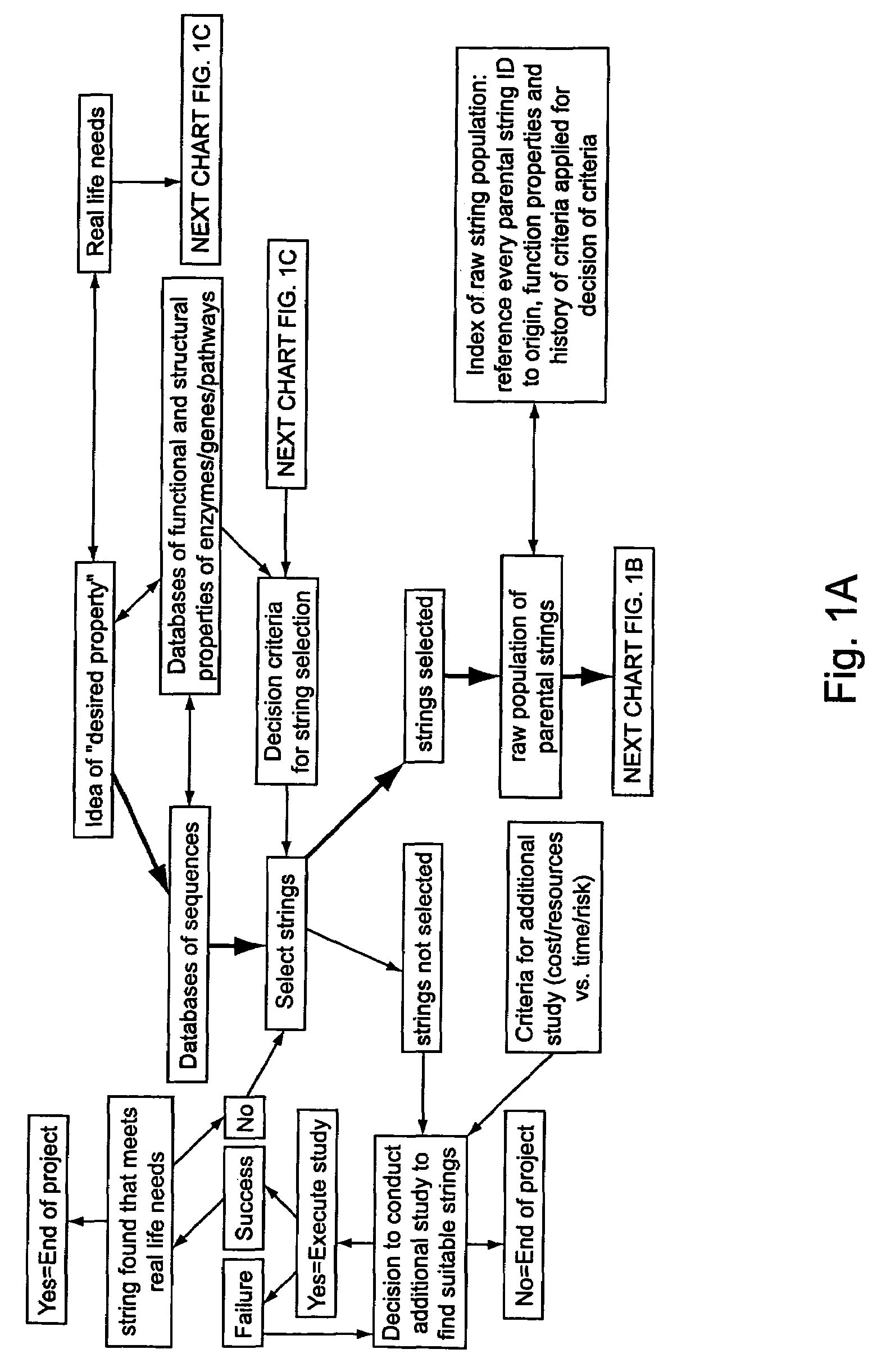
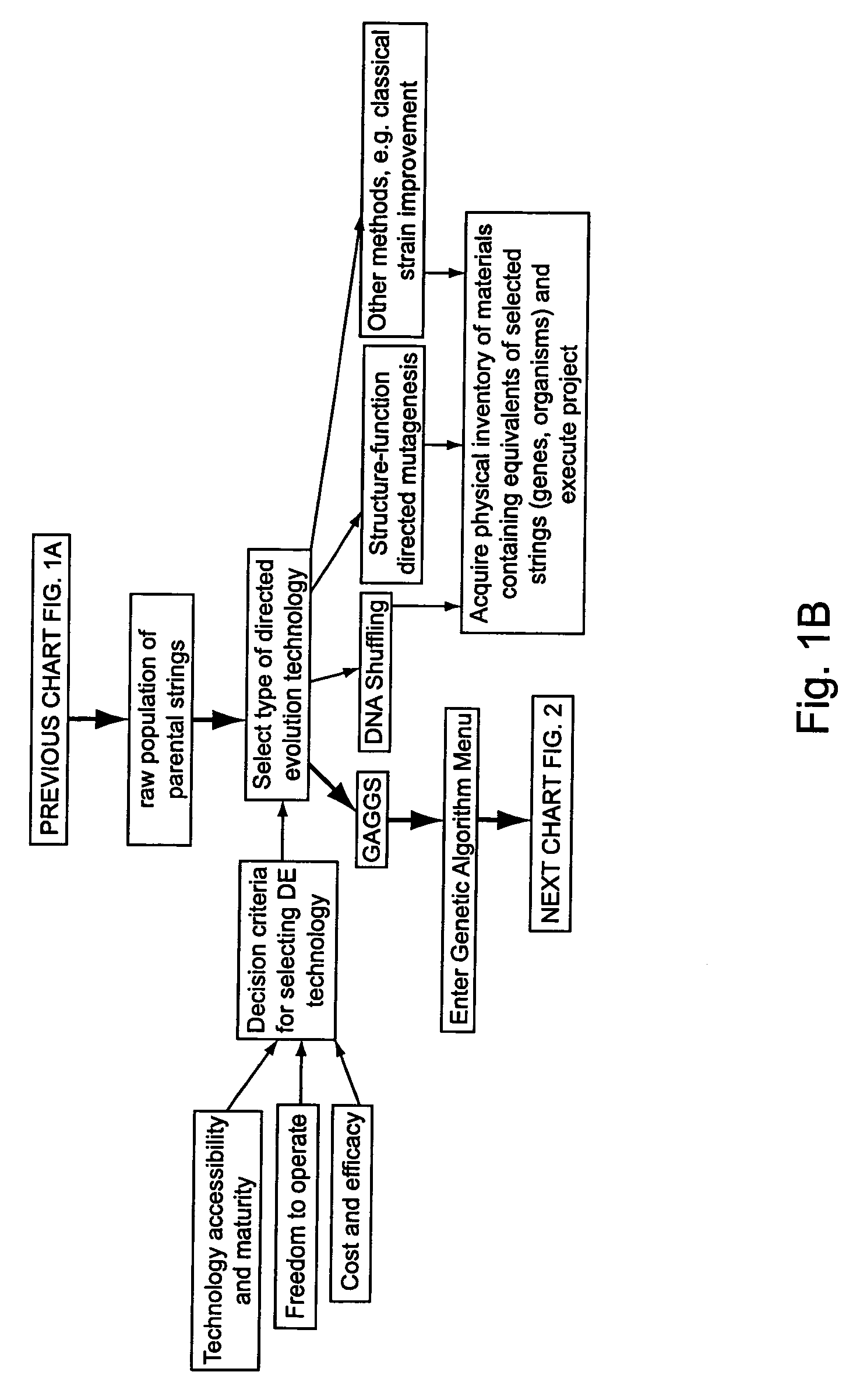
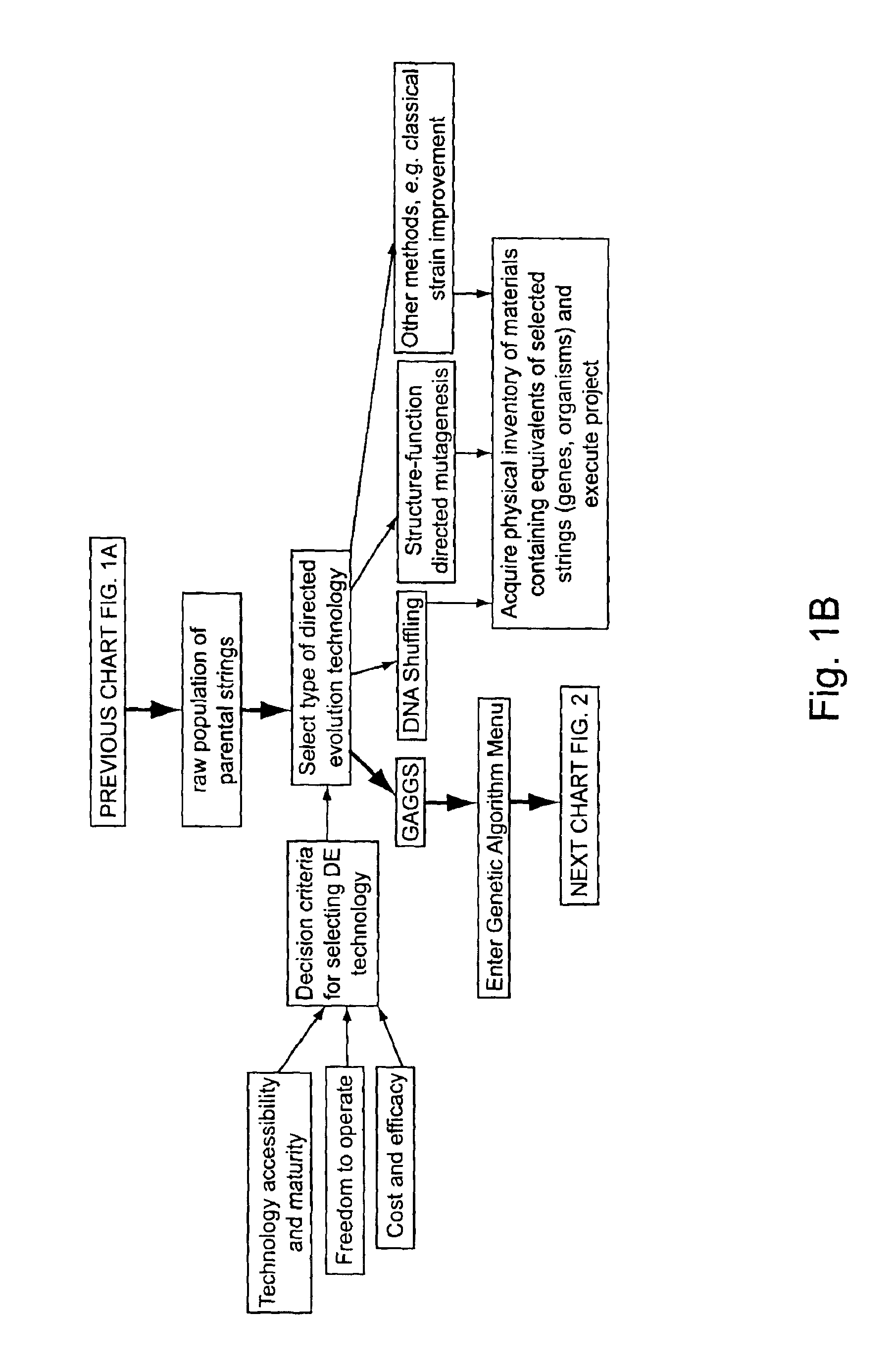
Methods of populating data structures for use in evolutionary simulations
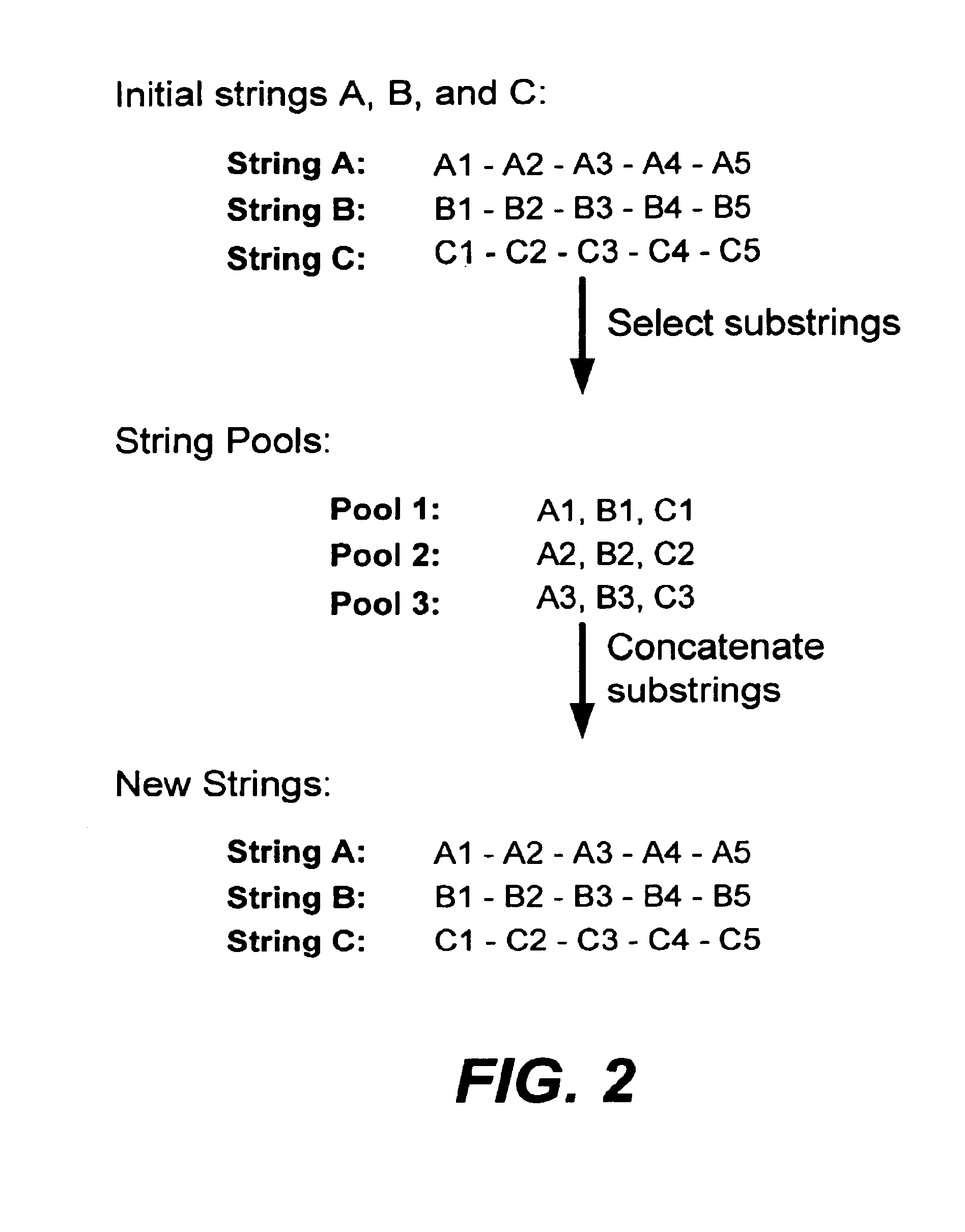
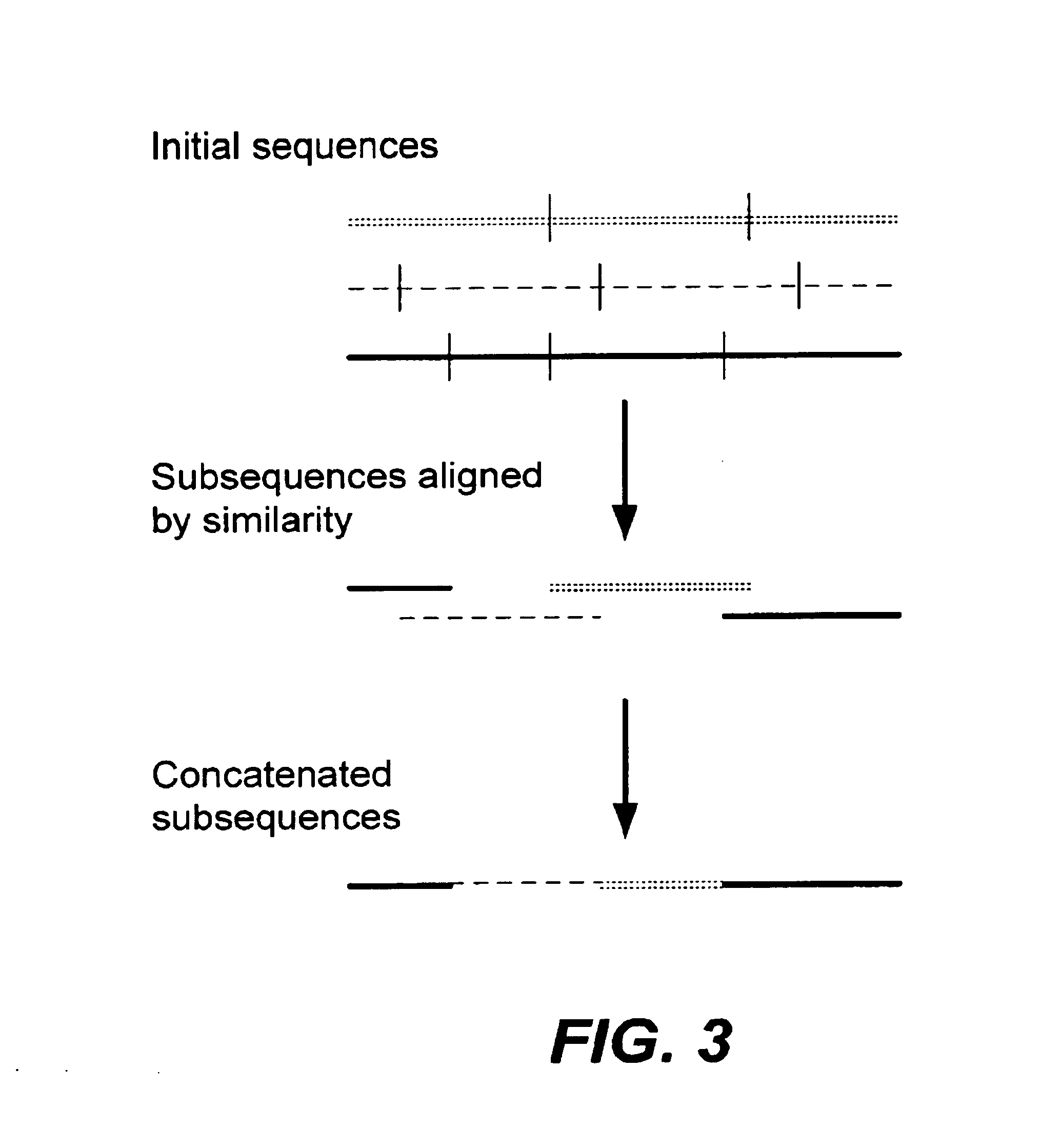
In particular, this invention provides novel methods of populating data structures for use in evolutionary modeling. In particular, this invention provides methods of populating a data structure with a plurality of character strings. The methods involve encoding two or more a biological molecules into character strings to provide a collection of two or more different initial character strings; selecting at least two substrings from the pool of character strings; concatenating the substrings to form one or more product strings about the same length as one or more of the initial character strings; adding the product strings to a collection of strings; and optionally repeating this process using one or more of the product strings as an initial string in the collection of initial character strings.
Owner:CODEXIS MAYFLOWER HLDG LLC
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
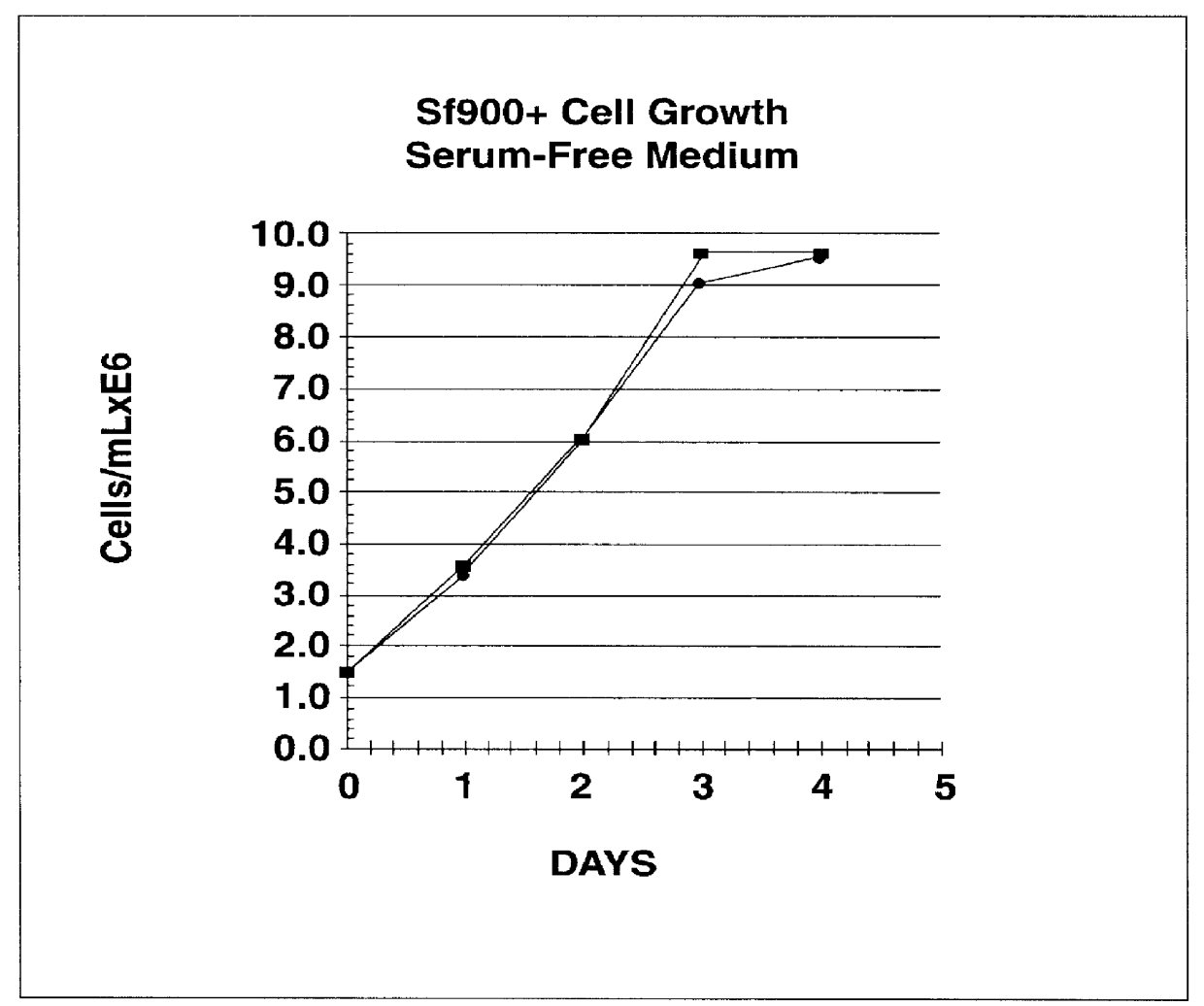
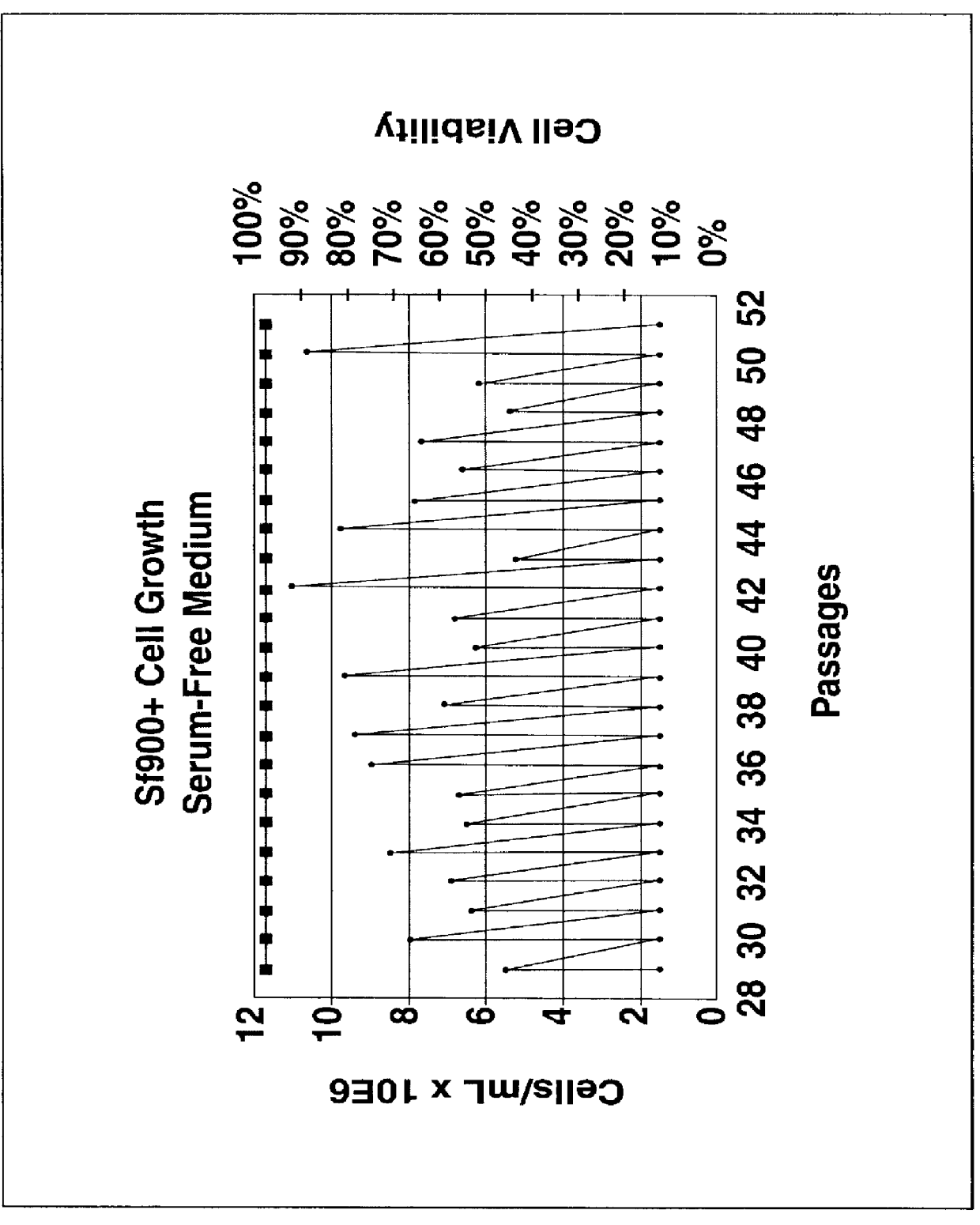
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
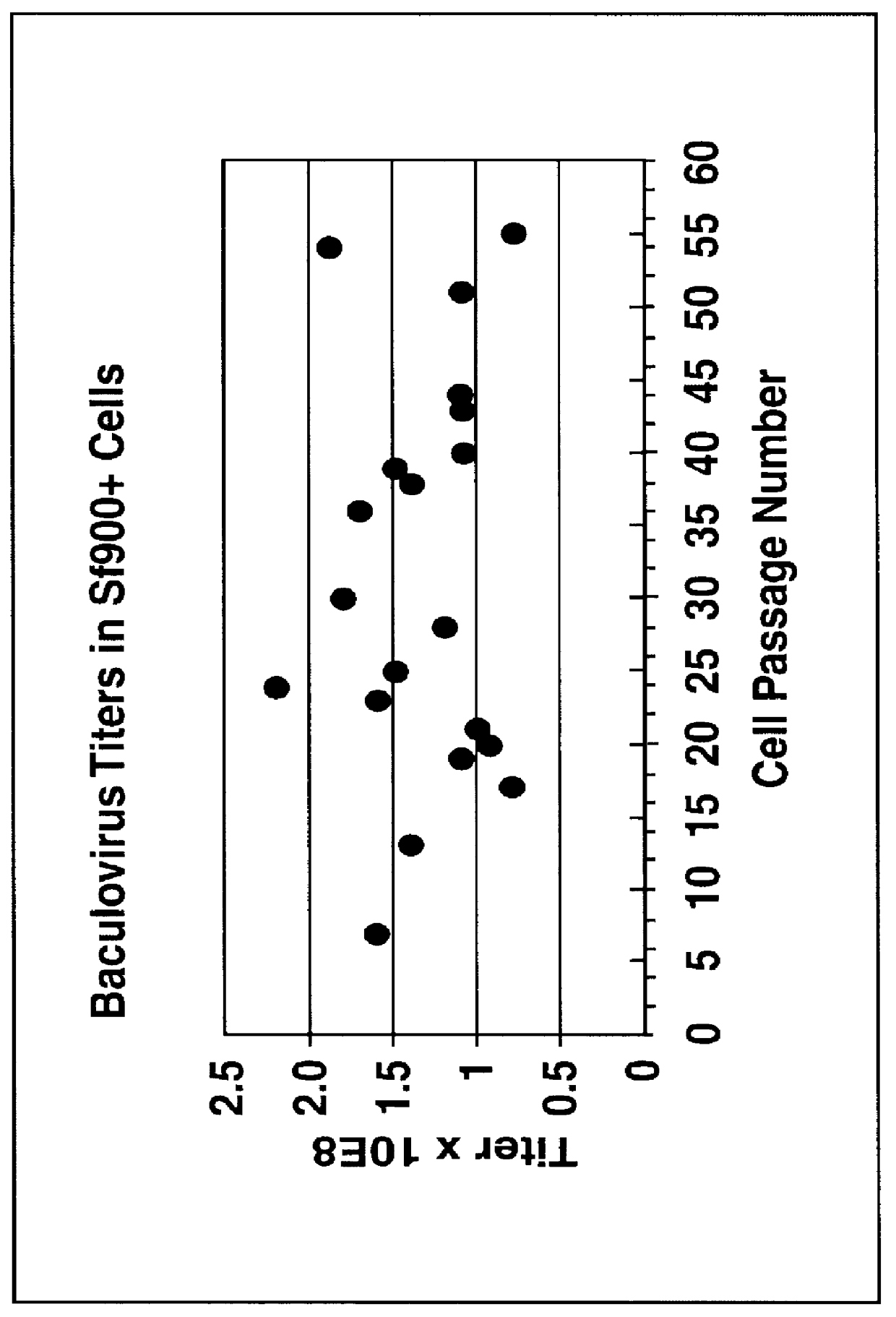
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
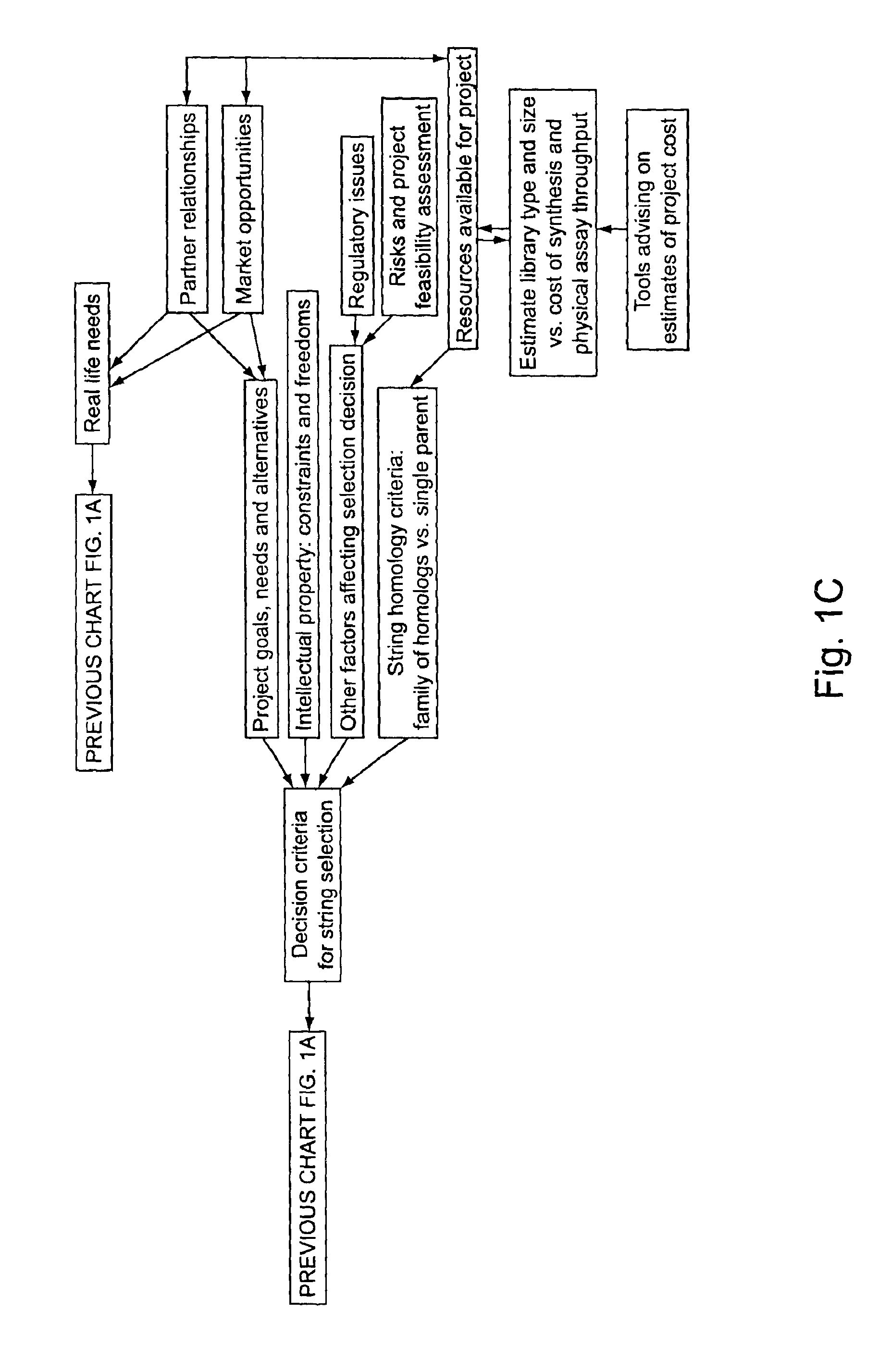
Identifying oligonucleotides for in vitro recombination
“In silico” nucleic acid recombination methods, related integrated systems utilizing genetic operators and libraries made by in silico shuffling methods are provided.
Owner:CODEXIS INC
Enhanced erythropoiesis and iron metabolism
ActiveUS20050020487A1Increase productionReduce microcytosisBiocidePeptide/protein ingredientsDiseaseRed blood cell
The present invention relates to methods and compounds for regulating or enhancing erthropoiesis and iron metabolism, and for treating or preventing iron deficiency and anemia of chronic disease.
Owner:FIBROGEN INC
Receptor specific transepithelial transport of therapeutics
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
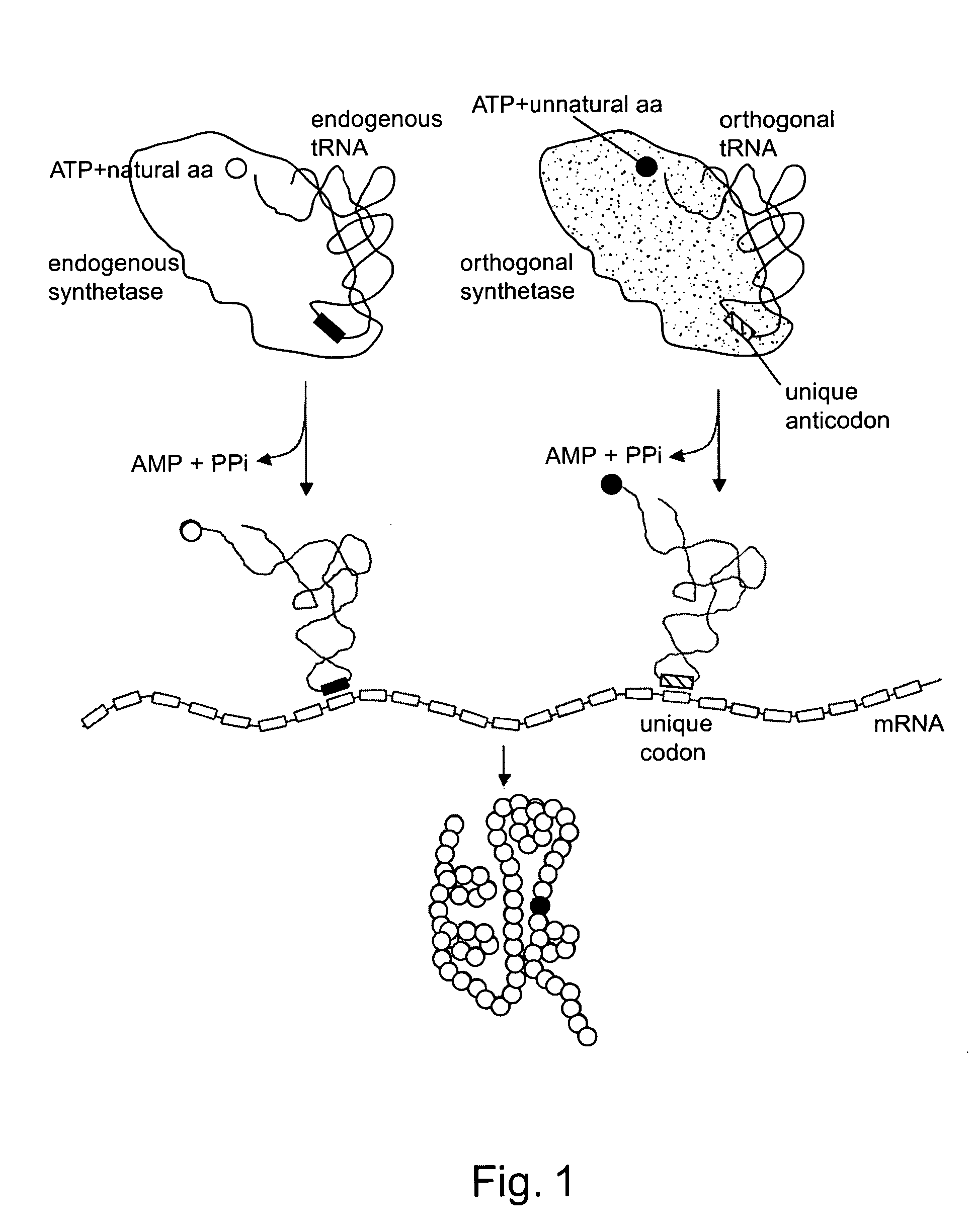
Methods and composition for the production of orthogonal tRNA-aminoacyltRNA synthetase pairs
This invention provides compositions and methods for generating components of protein biosynthetic machinery including orthogonal tRNAs, orthogonal aminoacyl-tRNA synthetases, and orthogonal pairs of tRNAs / synthetases. Methods for identifying orthogonal pairs are also provided. These components can be used to incorporate unnatural amino acids into proteins in vivo.
Owner:THE SCRIPPS RES INST +1
Delivery of highly lipophilic agents via medical devices
InactiveUS20060240070A1Easy to transportIncrease drug retentionBiocideFibrinogenMedicineMedical device
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Methods for making character strings, polynucleotides and polypeptides having desired characteristics
“In silico” nucleic acid recombination methods, related integrated systems utilizing genetic operators and libraries made by in silico shuffling methods are provided.
Owner:CODEXIS MAYFLOWER HLDG LLC
Trans-capsular administration of high specificity cytokine inhibitors into orthopedic joints
ActiveUS20050025765A1Extended half-lifeEliminate side effectsOrganic active ingredientsPeptide/protein ingredientsWhite blood cellOrthopedic department
The present invention relates to trans-capsularly administering into a diseased joint a high specificity antagonist selected from the group consisting of: i) an inhibitor of a pro-inflammatory interleukin; ii) an inhibitor of TNF-α synthesis; iii) an inhibitor of membrane-bound TNF-α; iv) an inhibitor of a natural receptor of TNF-α; v) an inhibitor of NO synthase, vi) an inhibitor of PLA2 enzyme; vii) an anti-proliferative agent; viii) an anti-oxidant; ix) an apoptosis inhibitor selected from the group consisting of EPO mimetic peptides, EPO mimetibodies, IGF-I, IGF-II, and caspase inhibitors, and x) an inhibitor of MMPs; and xi) an inhibitor of p38 kinase.
Owner:DEPUY SYNTHES PROD INC
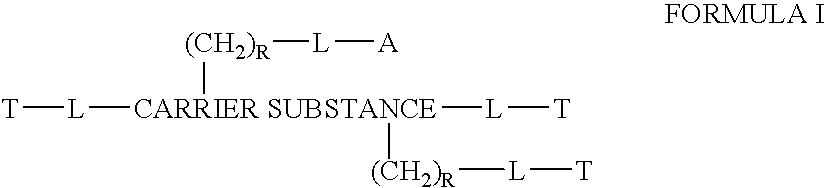
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com