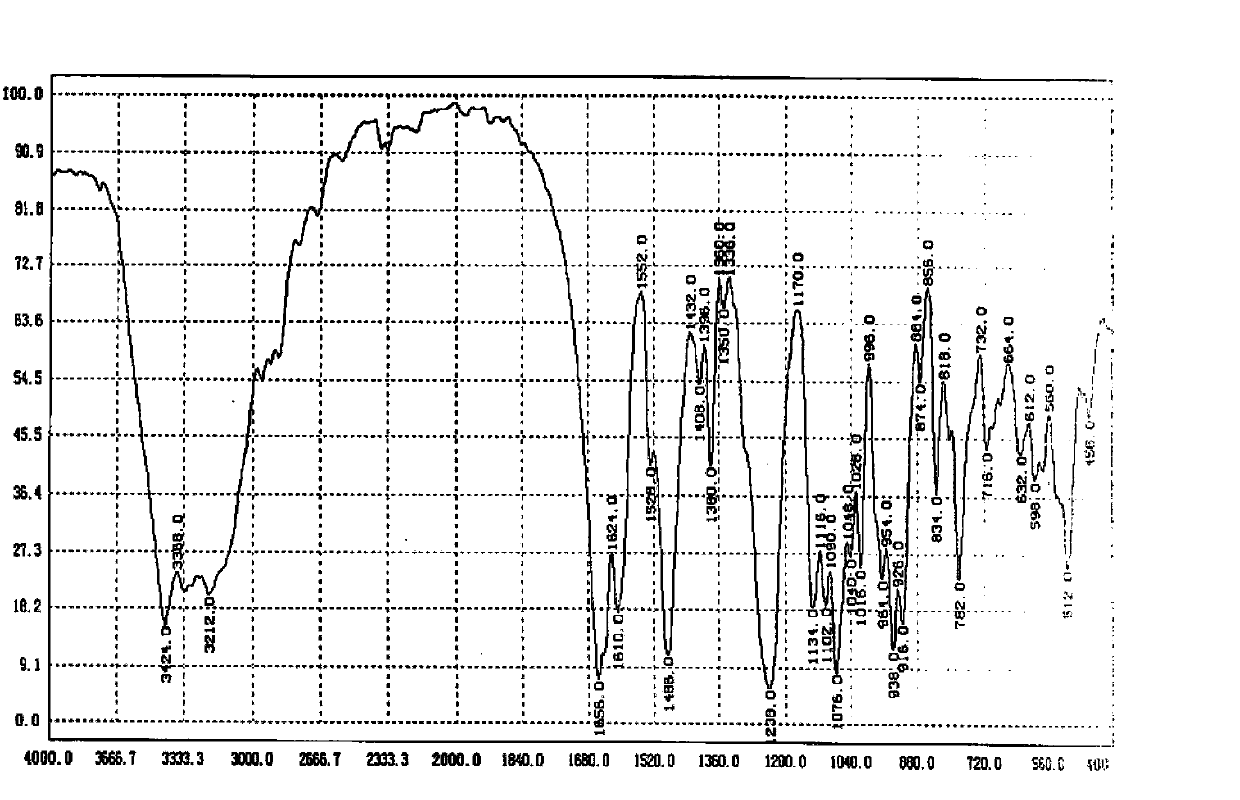
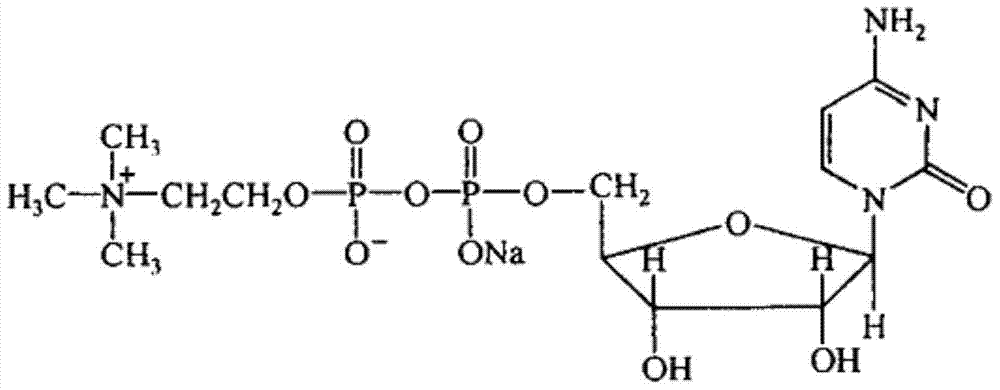
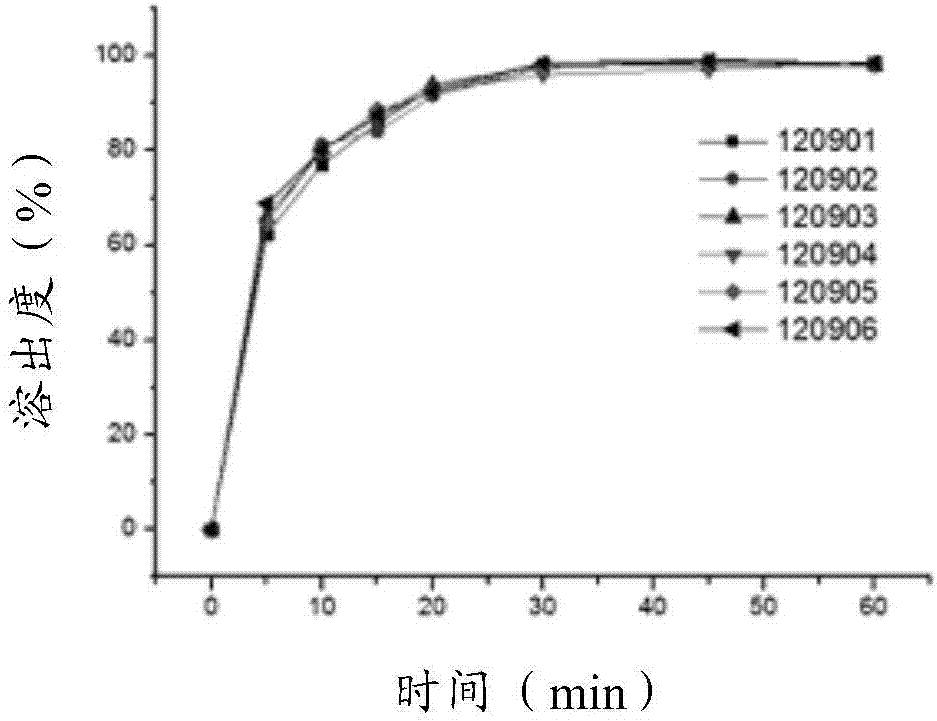
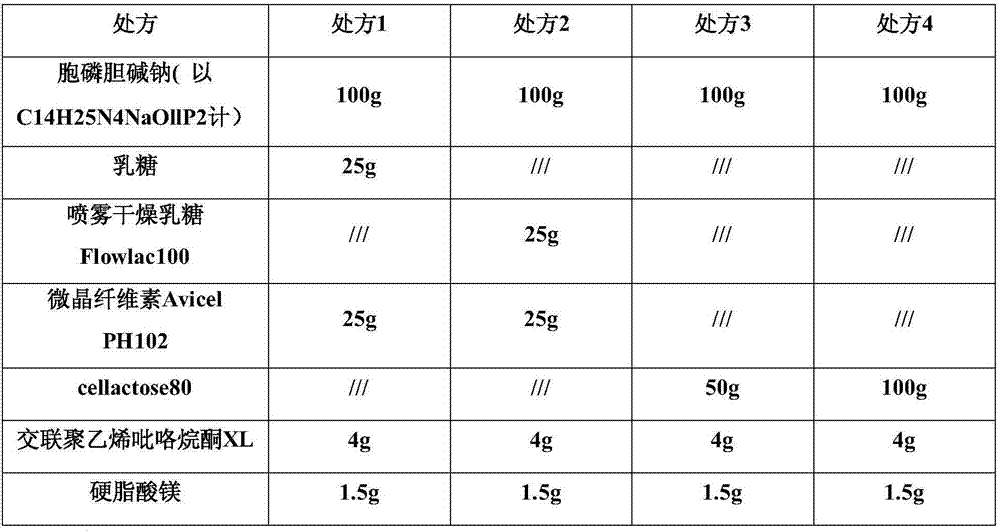
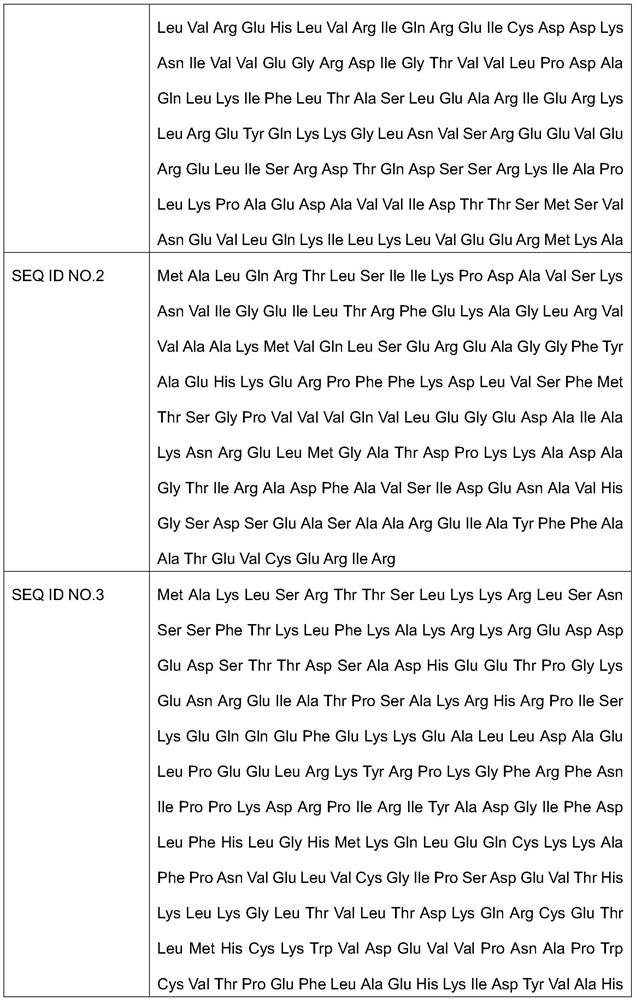
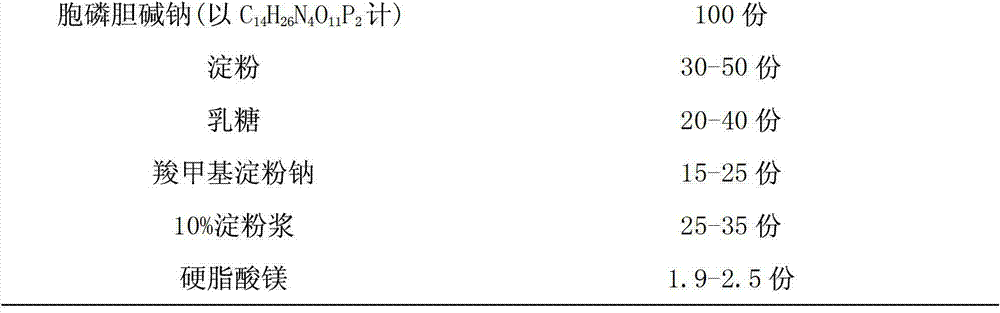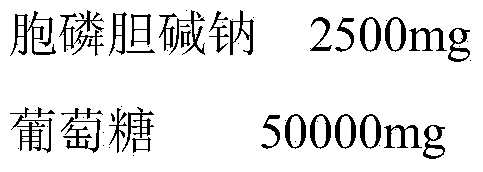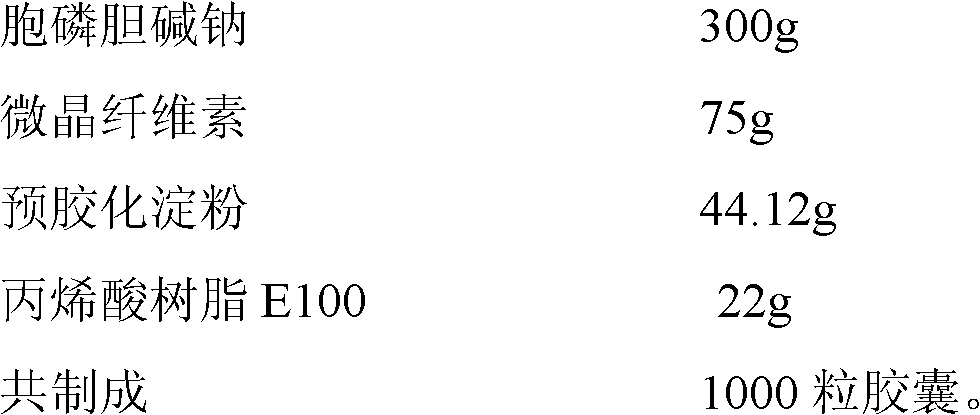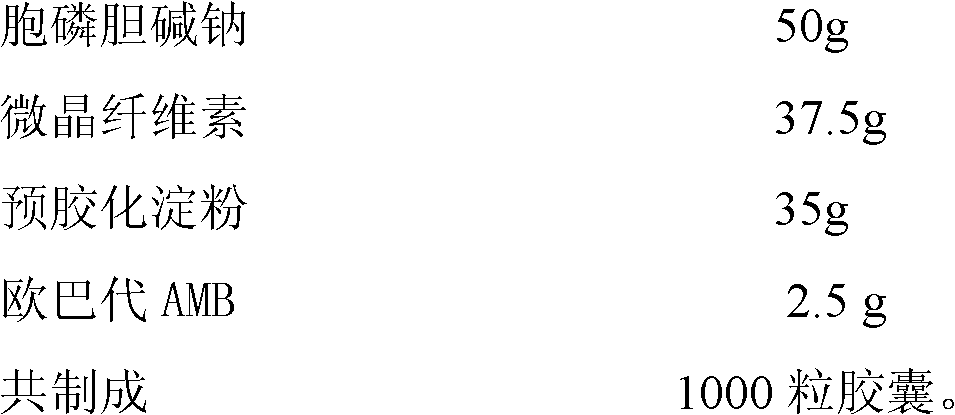Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
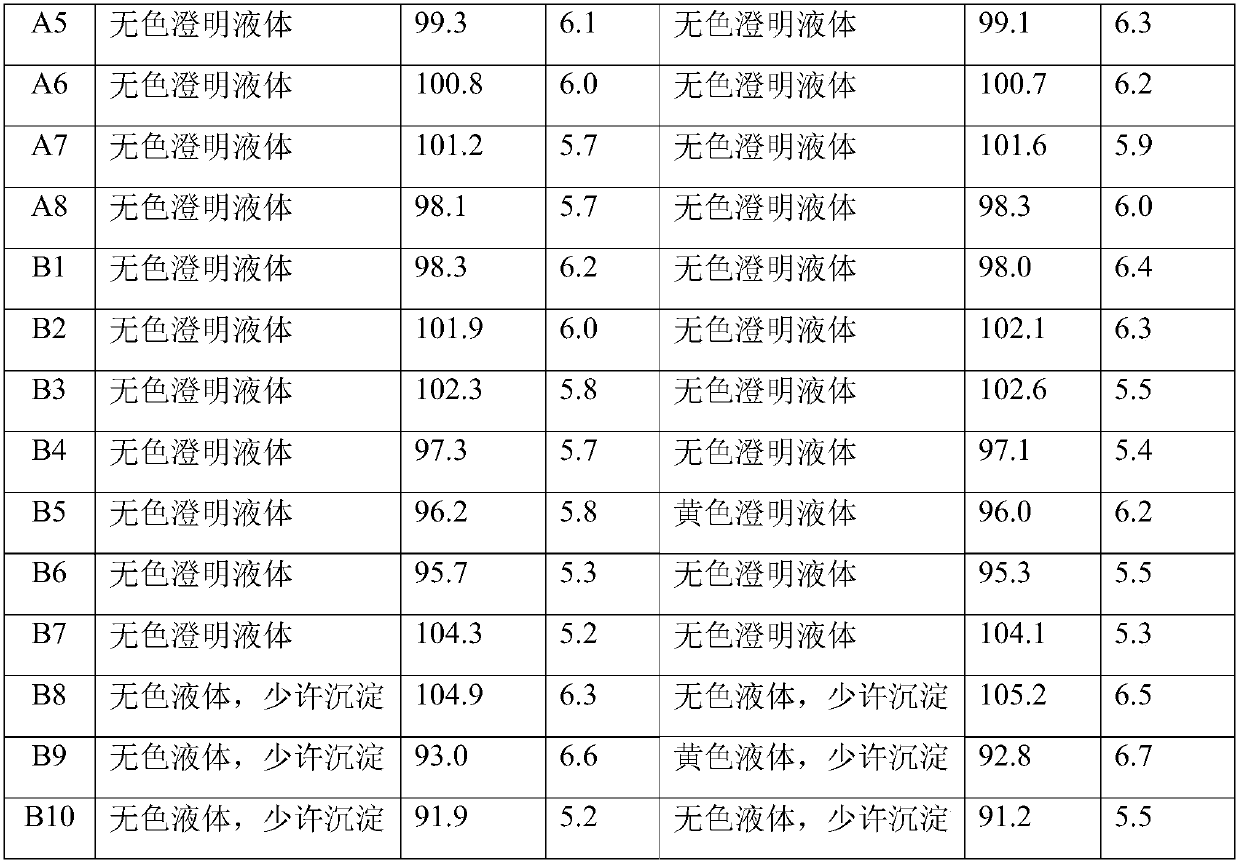
54 results about "Citicoline sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Citicoline sodium is a brain supplement that has received a lot of attention recently. More and more individuals are interested in using natural compounds to help improve their memory and cognitive power.
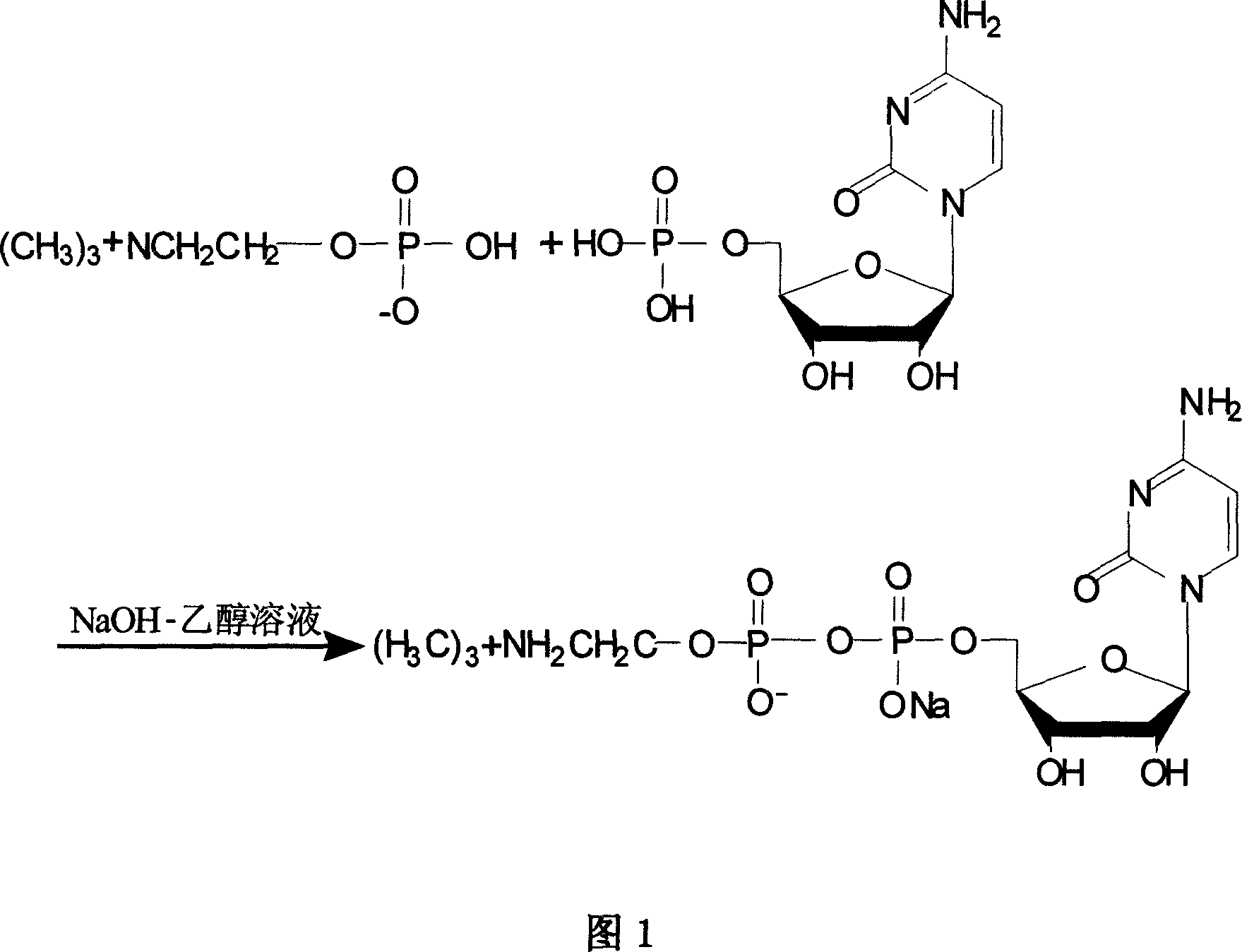
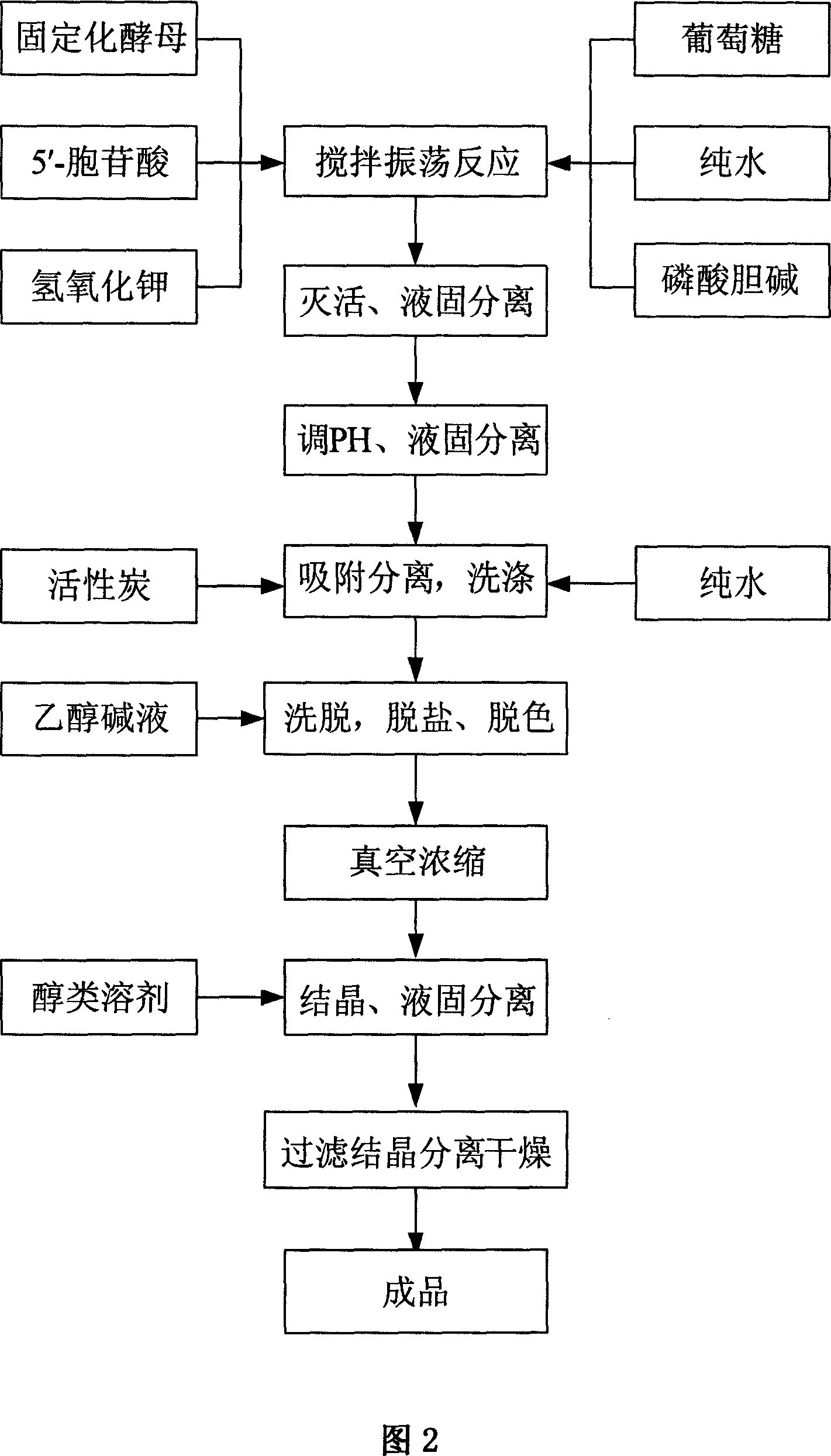
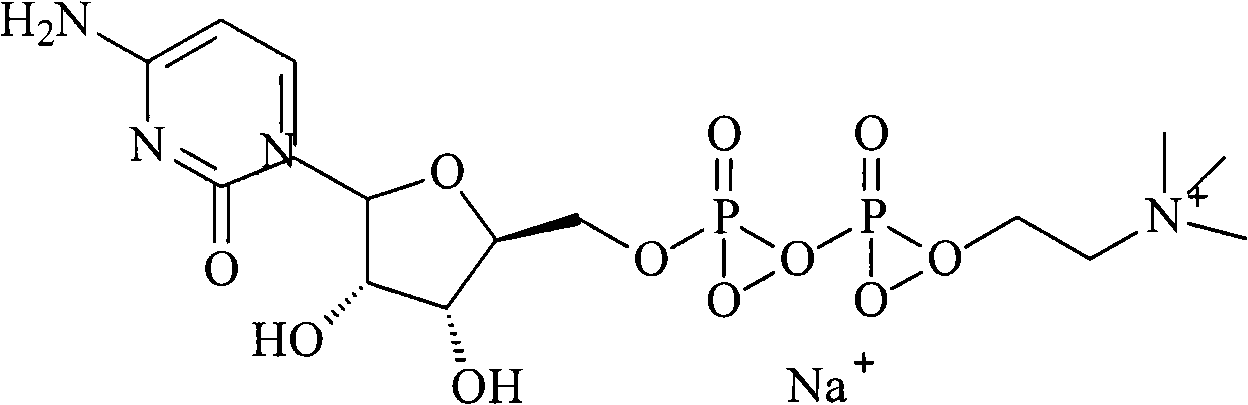
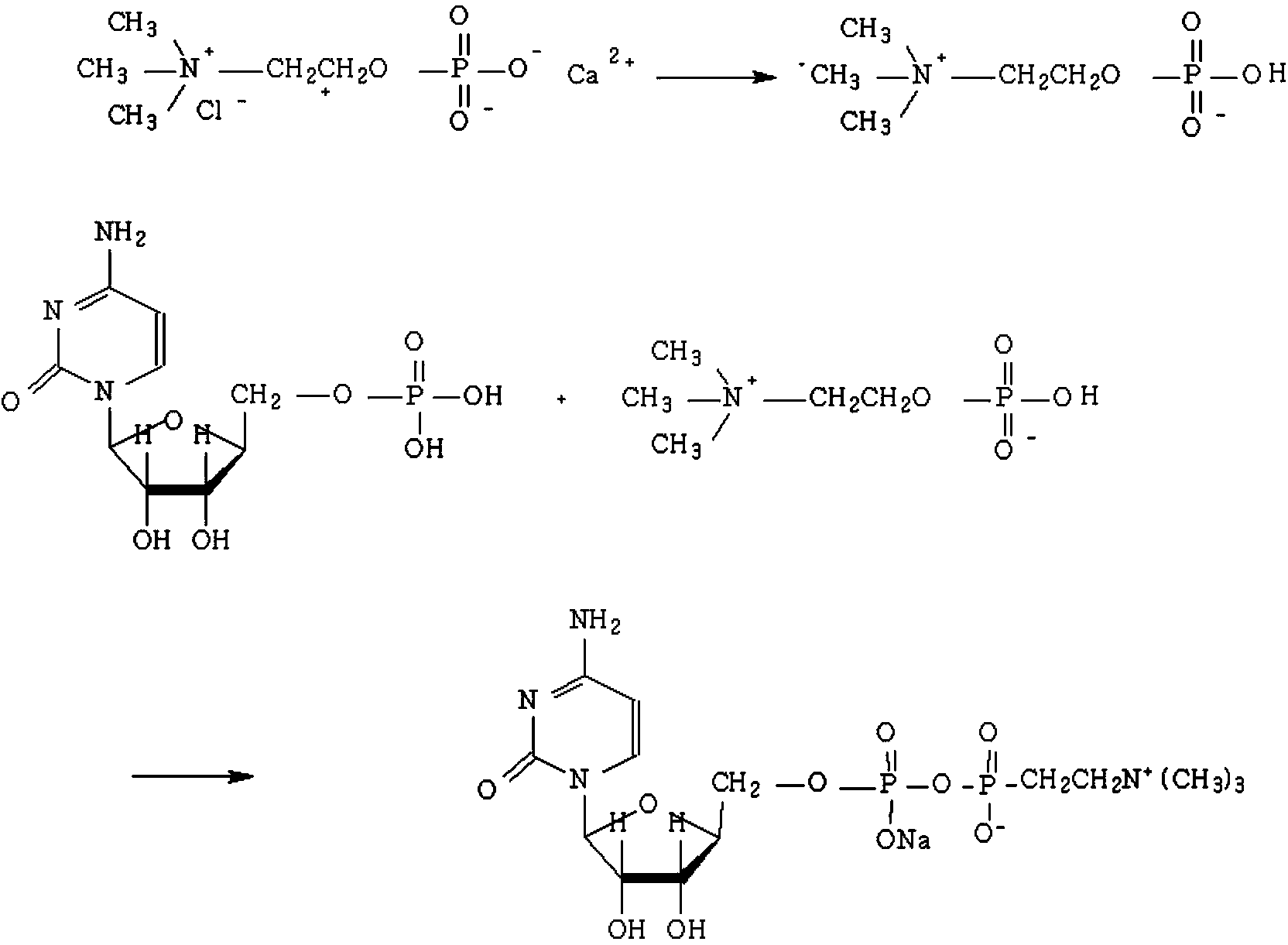
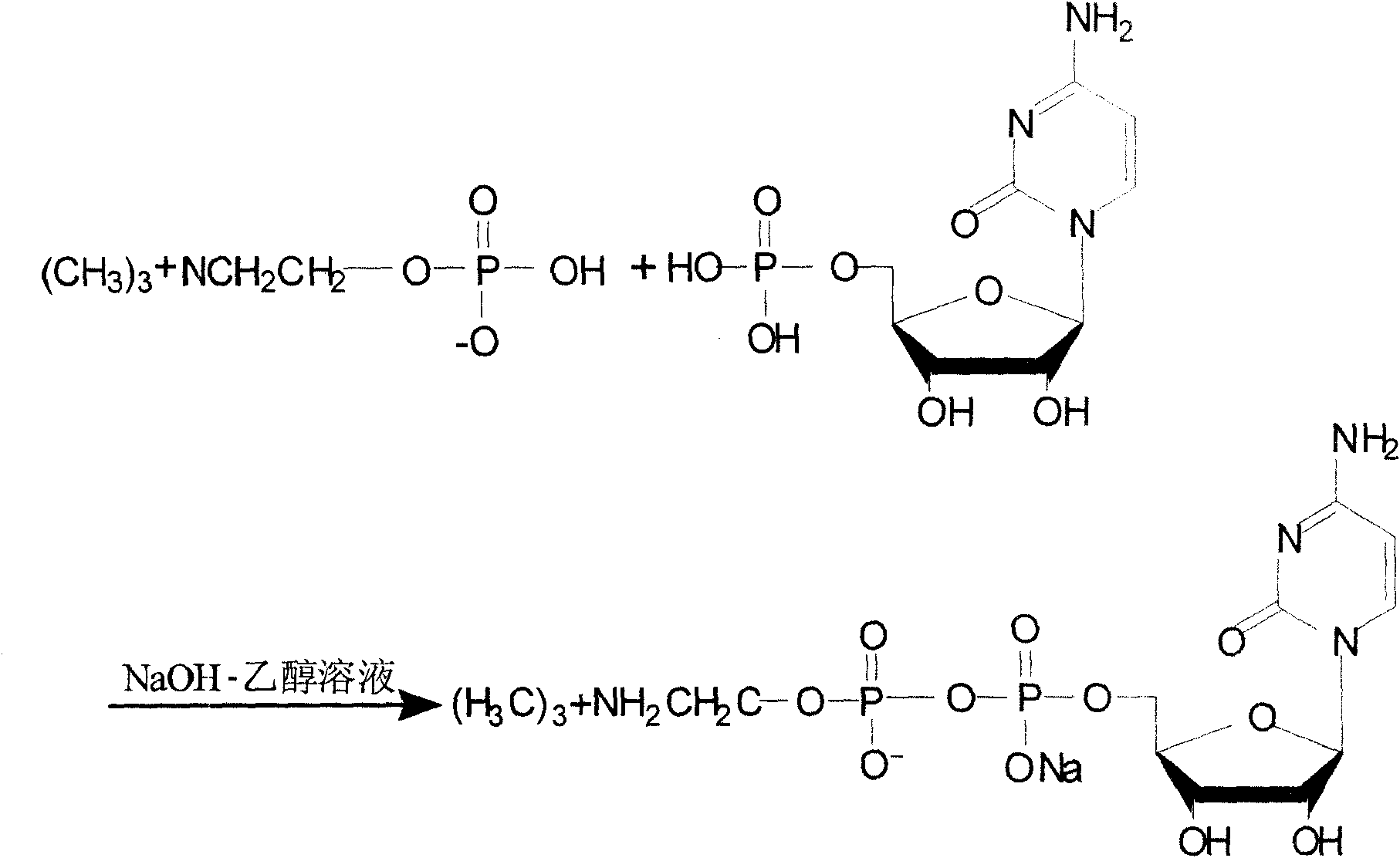
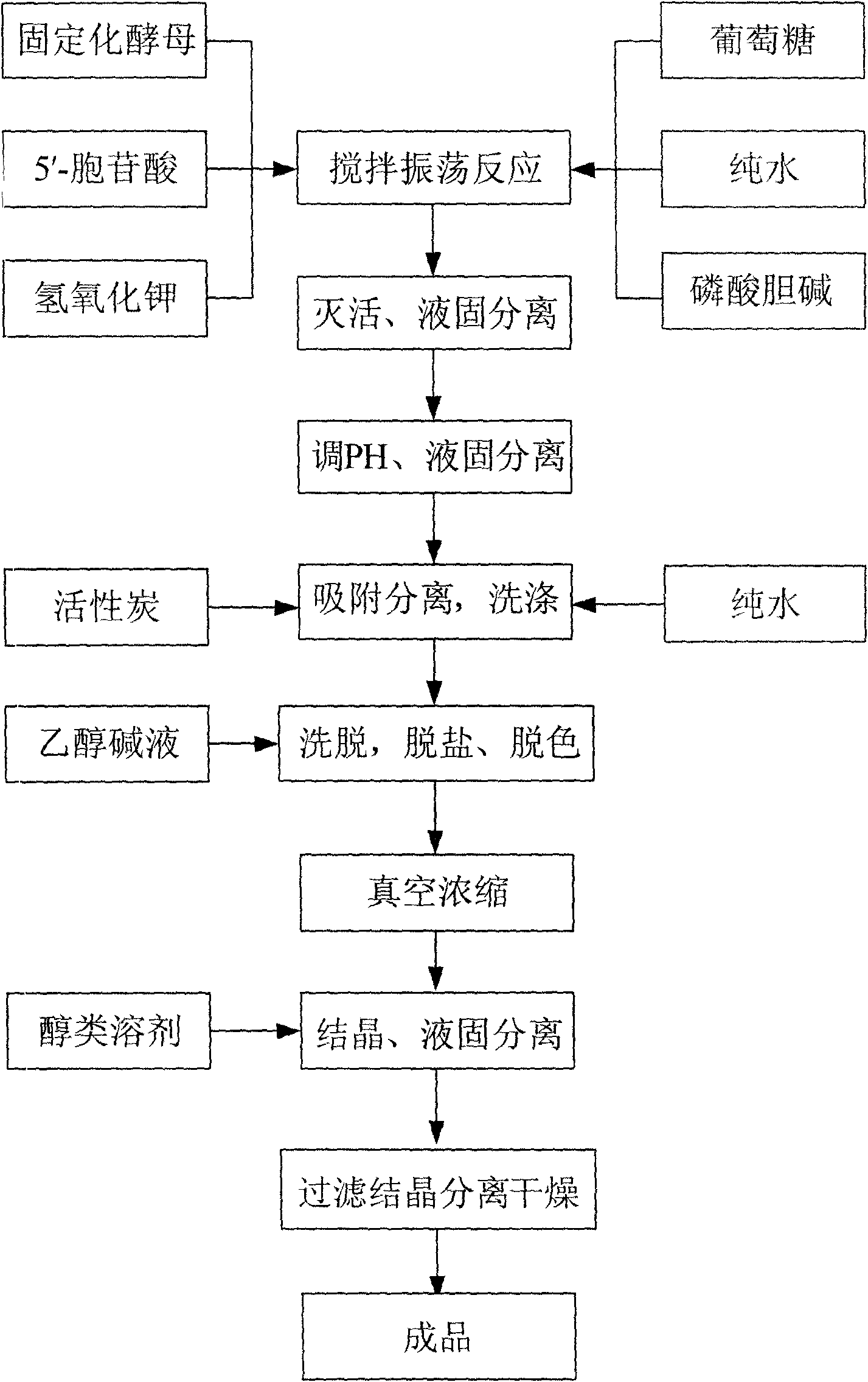
Process for preparing citicoline sodium
The present invention is process of preparing citicoline sodium. The preparation process includes the biotransformation of material including 5'-cytidylate, phosphorylcholine, potassium hydroxide and glucose with yeast as the biocatalyst; extraction and separation with active carbon as the adsorbing carrier, Cl- type ion exchange resin as the separating carrier and re-compounded water-alcohol mixture as the analyzing reagent; and product purification with alcohol solvent as the crystallizing solvent. Compared with available technology, the present invention has the advantages of high product yield and high product purity.
Owner:苏州正济药业有限公司
Salting-out and solvating-out crystallization method for citicoline
ActiveCN101538300AHigh yieldGood hygroscopicitySugar derivativesCardiovascular disorderFiltrationSalting out
The invention discloses a salting-out and solvating-out crystallization method for citicoline. The method comprises the following steps: adding inorganic sodium salt which accounts for 1 to 20 percent of citicoline and a solvating agent which is 1.0 to 4.0 times the volume of a staring citicoline aqueous solution to a citicoline aqueous solution with a pH value between 5.0 and 8.0 and a concentration between 10 and 400 g / L; stirring the materials at a rotating speed controlled between 20 and 230 rpm at a temperature of between 10 and 40 DEG C; performing pumping filtration after complete crystallization; washing the obtained product with ethanol; drying the obtained product in vacuum; and obtaining the citicoline sodium salt crystals with the purity higher than 99 percent. The method has the advantages that the method obviously improves the quality of final products, raises crystallization yield stability, can control the particle size of the crystals by changing crystallization temperature or the states of a flow field, and is simple to operate, good in repeatability and suitable for the industrial production of the citicoline.
Owner:NANJING HIGH TECH UNIV BIOLOGICAL TECH RES INST CO LTD
Citicoline sodium tablets and preparation method thereof
ActiveCN102028664AIncrease productionLow costOrganic active ingredientsNervous disorderNervous systemCiticoline sodium
The invention provides citicoline sodium tablets which are medicinal preparations for treating sequela of a nervous system caused by a craniocerebral injury or a cerebrovascular accident, and a preparation method thereof. Every 1,000 tablets comprise 100.0 to 300.0g of citicoline sodium, 20.0 to 70.0g of starch, 50.0 to 160.0g of microcrystalline cellulose, 0.6 to 3g of hydroxypropyl methyl cellulose, 1.7 to 5.5g of magnesium stearate, 0 to 10g of sodium starch glycolate and 0 to 6.0g of pregelatinized starch. The preparation method of the citicoline sodium tablets mainly comprises the steps of sieving, weighing, proportioning, premixing, preparing a soft material, preparing wet granules, drying, granulating, mixing, tabletting, performing aluminum-plastic-aluminum packaging and outer packaging and the like. The tablets prepared by the method have the advantages of high yield, low cost, accurate divided dose, relatively stable medicinal physicochemical property and longer storage period and are convenient to carry and use.
Owner:四川梓橦宫药业股份有限公司
Citicoline sodium glucose injecta and preparation process thereof
ActiveCN102144963AImprove quality stabilityImprove safety and effectivenessOrganic active ingredientsNervous disorderCiticoline sodiumBULK ACTIVE INGREDIENT
The invention discloses a citicoline sodium glucose injecta and a preparation process thereof. The citicoline sodium glucose injecta mainly contains the active ingredients: citicoline sodium, isotonic agent glucose, a stabilizer, a pH regulator and water for injection, wherein the stabilizer is selected from one or two of malic acid, sale compounds thereof and sulfite compounds; the pH regulator is one or more of sodium hydroxide, hydrochloric acid, malic acid and apple sodium; the weight ratio of the active ingredients of the Citicoline sodium to the isotonic agent glucose is 1:(20-100), theweight ratio of the active ingredients of the citicoline sodium to the stabilizer is 1:(0.02-2), and the stabilizer is added to solve the problem of unstable heating and excessive related substances of the injecta in the production and storage process and improve the quality stability and the safe validation of medicaments.
Owner:HUNAN KELUN PHARMA
Ubelin manufacturing technique
InactiveCN101130797ASolve pollutionHigh activityMicroorganism based processesFermentationUltrafiltrationCholine Phosphate
The present invention relates to a production process of citicoline. It is characterized by that said production process includes the following steps: firstly, adding water and glucose into a reaction tank, then adding yeast, making fermentation, after fermentation adding inorganic salt and choline phosphate, the adding cytidine monophosphate solution, an adding cane sugar, heating, then quickly-cooling by using water, pressing reaction liquid, washing by using water, removing salt from obtained clear liquor; then pressing said clear liquor and making said clear liquor be fed into a carbon column, rinsing said carbon column by using pure water, then using ethyl alcohol solution to make elution, collecting citicoline sodium; then vaporizing and concentrating eluent, diluting concentrate and making it be fed into a macroporous ion exchange resin column, then washing said column by using water and making elution, concentrating eluent, heating the collected citicoline sodium solution, removing impurity by utilizing ultrafiltration device, decolouring, adding ethyl alcohol and stirring them, storing the above-mentioned material in a refrigeration house and staying overnight; then making crystal solution undergo the processes of centrifugal separation, vacuum drying, bagging, weighing, sealing and dry-storage.
Owner:张剑
Citicoline sodium compound and new method thereof
InactiveCN102010454AHigh purityIncrease contentOrganic active ingredientsSenses disorderActivated carbonState of art
The present invention provides a citicoline sodium compound and new method thereof, wherein purification is performed via active carbon adsorption, acidification reaction and preparing chromatographic column, finally a citicoline sodium compound with high purity is obtained; the shortage of low raw material purity in the prior production is recovered, simultaneously the quality of preparation product is improved, and side effects are reduced. Compared with the prior art, the method has the advantages of simple and easy technology, low cost, high yield and high product purity; the method is suitable for industrial production; and the citicoline sodium prepared by the refining method of the present invention is suitable for being used in preparation of neural activation agent pharmaceuticals.
Owner:HAINAN MEIDA PHARMA
Citicoline sodium tablet and preparation method thereof
ActiveCN103191079AImprove securityEnsure safetyOrganic active ingredientsNervous disorderCiticoline sodiumSlurry
The invention provides a citicoline sodium tablet and a preparation method thereof. The citicoline sodium tablet provided by the invention contains citicoline sodium, starch, lactose, sodium carboxymethyl starch, 10% starch slurry and magnesium stearate. The prescription of a coating solution includes stomach-soluble coating powder and ethanol. The preparation method comprises the steps of: (1) preparation of an uncoated tablet: uniformly mixing main drugs with the lactose, grinding the mixture to pass through a 80-mesh sieve, respectively adding the starch and the sodium carboxymethyl starch and carrying out uniform mixing, passing through the 80-mesh sieve twice, adding the 10% starch slurry, adequately and uniformly mixing to prepare soft materials, carrying out pelletizing by utilizing a 20-mesh sieve, drying at 60 DEG C, adding the magnesium stearate, and carrying out uniform mixing, tabletting and testing; and (2) coating. The citicoline sodium tablet has the advantages of good stability, low cost, high active ingredient content and the like.
Owner:JINAN LIMIN PHARMA
Method for preparing citicoline through biological enzyme catalysis
The invention discloses a method for preparing citicoline through biological enzyme catalysis. The method is characterized by comprising the steps that a single artificially-transformed gene engineering strain is used as an enzyme source, an ammonium chloride catalyst, orotic acid, choline phosphate and other chemical substances are subjected to a reaction to generate citicoline, and then the citicoline is extracted from the reaction solution. Compared with the prior art, single strain is adopted for fermentation, the production technology is simple, the production period is short, the production cost is low, and the method for preparing citicoline through biological enzyme catalysis can be widely applied to industrial production of citicoline.
Owner:苏州正济药业有限公司
Moisture-proof coating citicoline sodium capsule and preparation method thereof
ActiveCN102525997AGood granularityGood disintegrationOrganic active ingredientsNervous disorderCiticoline sodiumMoisture absorption
The invention provides a moisture-proof coating citicoline sodium capsule and a preparation method thereof. The citicoline sodium capsule is composed of a capsule casing and quick-release moisture-proof micro pills or granules. All components in each capsule containing citicoline sodium quick-release moisture-proof micro pills or granules by percentage are 40% to 70% of citicoline sodium, 10% to 30% of microcrystalline cellulose, 10% to 30% of pregelatinized starch and 2% to 10% of moisture-proof coatings. The moisture-proof micro pills or granules are obtained by being extruded and rounded or pressed and granulated to be coated with moisture-proof coatings. The citicoline sodium capsule is good in moisture-proof function and resolves the problems that the capsule is prone to absorb waterdue to the fact that citicoline sodium is strong in moisture absorption, the capsule casing is prone to be fragile, and the like.
Owner:QILU PHARMA CO LTD
Method for preparing citicoline sodium
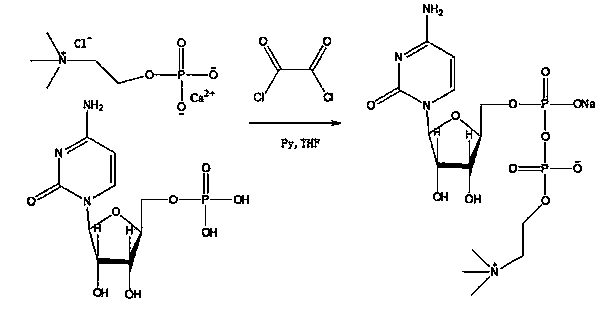
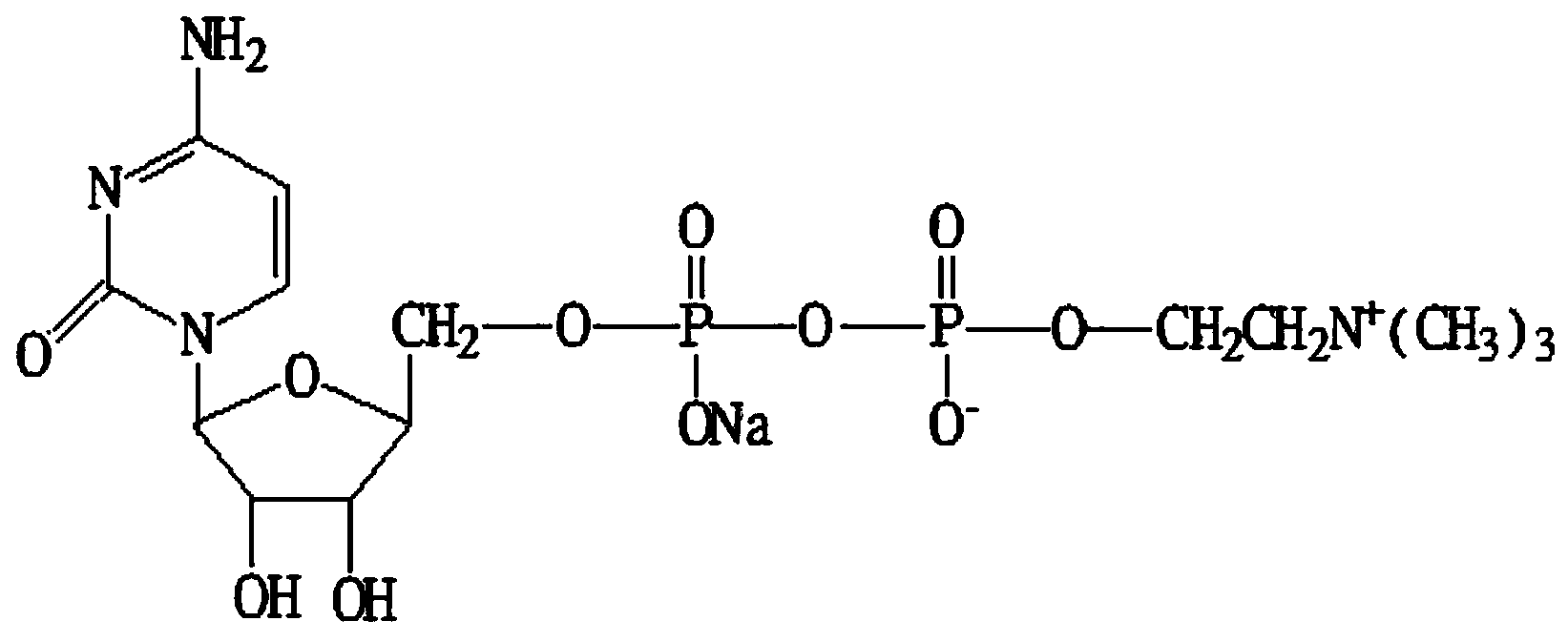
ActiveCN104031105ALow costAvoid swappingSugar derivativesSugar derivatives preparationCiticoline sodiumPhosgene
The invention discloses a method for preparing citicoline sodium. The method comprises the following steps: adding oxalic acid into a chlorination calcium water solution, removing the precipitate and water, reacting with cytidine monophosphate under the action of triphosgene, and subsequently purifying the citicoline sodium by using a recrystallization method.
Owner:安庆回音必制药股份有限公司
Method for preparing citicoline sodium by utilizing oxalyl chloride
ActiveCN104004040ALow costGood atom economySugar derivativesSugar derivatives preparationBenzeneCalcium biphosphate
The invention discloses a method for preparing citicoline sodium by utilizing oxalyl chloride. The method is characterized by comprising the following steps of: by taking choline chloride calcium phosphate (P-choline) as a raw material, dissolving in organic amine not containing reactive hydrogen, adding oxalyl chloride for reacting for 0.5-2 hours after adding benzene for removing water in an azeotropic manner, and then, adding cytidylic acid (5'-CMP) for reacting.
Owner:回音必集团抚州制药有限公司
Citicoline sodium glucose injection
ActiveCN104027304AReduce generationEasy to useOrganic active ingredientsNervous disorderGlycineCiticoline sodium
The invention relates to citicoline sodium glucose injection, belonging to the field of pharmaceutical preparations. The citicoline sodium glucose injection provided by the invention is composed of 3 g of citicoline sodium, 40 g of glucose, 1.8 g of glycine, and 1.3 g of citric acid; the pH value is adjusted to 5.5-5.6 by using sodium hydroxide; and water for injection is added till the citicoline sodium glucose injection is 1000 ml.
Owner:HUIYINBI GRP JIANGXI EAST ASIA PHARMA CO LTD
Method for catalytically producing citicoline sodium with immobilized enzyme
ActiveCN103849666AAvoid pollutionAvoid processing powerTransferasesMicroorganism based processesEscherichia coliPhosphorylcholine
The invention provides a method for catalytically producing citicoline sodium with an immobilized enzyme. The method comprises the following steps: by utilizing engineered escherichia coli of a molecularly cloned cytidine phosphotransferase gene for fermentation, preparing cytidine phosphotransferase, and preparing cytidine phosphotransferase liquid through purification; immobilizing cytidine phosphotransferase; by using phosphorylcholine and cytidine disodium triphosphate as raw materials, catalytically generating citicoline with immobilized cytidine phosphotransferase. The method is simple in production process, short in production cycle and low in production cost and can be widely applied to industrial production of citicoline.
Owner:KAIPING GENUINE BIOCHEM PHARMA
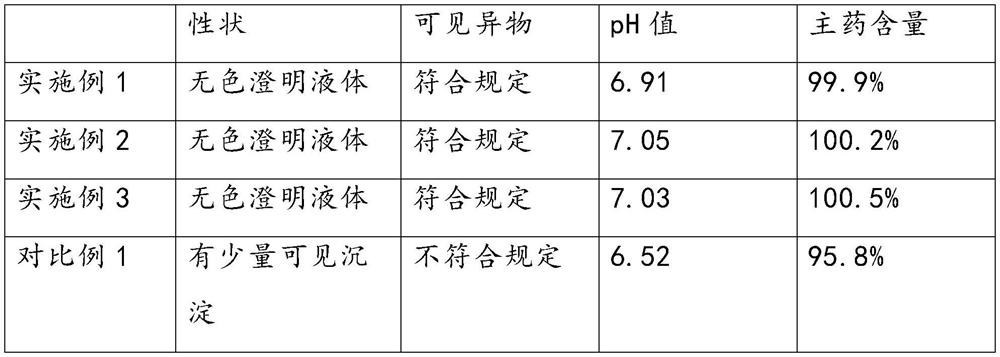
Citicoline Sodium injection pharmaceutical composition and preparation method thereof
The invention relates to a citicoline sodium injection pharmaceutical composition and a preparation method thereof. Particularly, the invention belongs to the technical field of medicines, relates to a pharmaceutical composition for treating consciousness disturbance caused by acute craniocerebral trauma and brain surgery and particularly relates to an injection pharmaceutical composition for treating consciousness disturbance caused by acute craniocerebral trauma and brain surgery. More particularly, the invention relates to the citicoline sodium injection pharmaceutical composition and a preparation method of citicoline sodium injection. The citicoline sodium injection comprises citicoline sodium and injection water, wherein the injection water serves as a solvent, and the concentration of citicoline sodium is 0.01g / ml-1g / ml. The citicoline sodium injection has excellent pharmaceutical properties explained in the specification.
Owner:CHENGDU TIANTAISHAN PHARMA
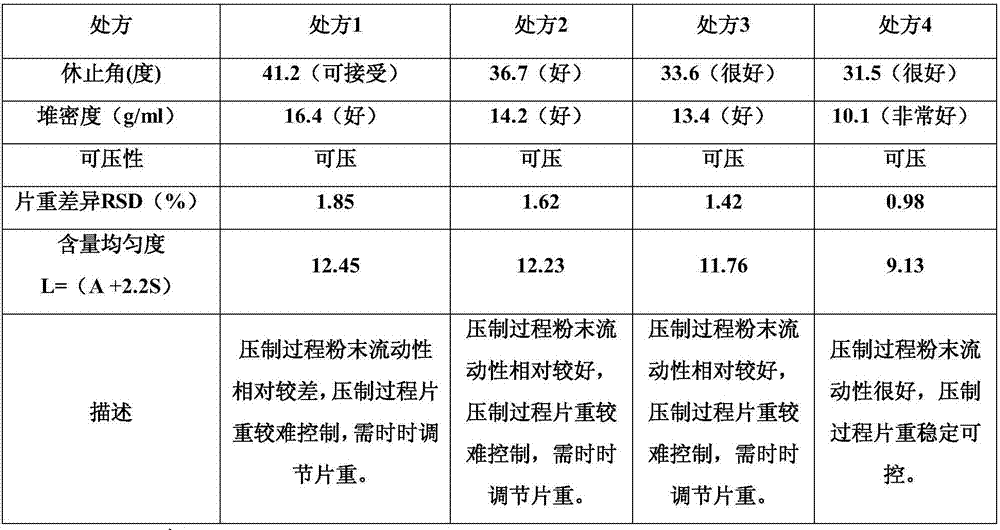
Citicoline sodium tablets and preparation method for directly compressing powder of citicoline sodium into tablets
ActiveCN107496369ASolve easy hydrolysisImprove securityOrganic active ingredientsNervous disorderCiticoline sodiumDissolution
The invention provides citicoline sodium tablets and a preparation method for directly compressing powder of citicoline sodium into tablets. The citicoline sodium tablets provided by the invention are prepared from citicoline sodium, cellactose 80, sodium carboxymethyl starch, magnesium stearate and micro-powder silica gel. The tablets provided by the invention are prepared by adopting a powder direct tablet compression method and the problem that citicoline sodium granulated by a traditional wet process is easy to hydrolyze is solved; meanwhile, the conditions that the tablet weight different is relatively great and the dissolution uniformly is relatively poor, which are easily caused by direct powder compression, are solved through screening prescriptions and setting technological parameters; the quality stability and the safety of products are greatly improved.
Owner:FUJIAN MINDONG REJUVENATION PHARMA
Citicoline sodium injection and preparation method thereof
ActiveCN102462659APollution controlControl Endotoxin LevelsOrganic active ingredientsSenses disorderCiticoline sodiumCiticoline
The invention discloses a citieoline sodium injection with good quality and low cost, and provides a method for preparing the injection simultaneously. The citieoline sodium injection provided by the invention is prepared from 1,000-5,000g of citieoline sodium, 5g of versene disodium and 20,000ml of water for injection, and is 2-5ml per tube. The preparation method comprises the following steps of: weighing each component; heating the water for injection to 28-30 DEG C, adding 0.02-0.05 percent of injection active carbon, adsorbing for 20-30 minutes, and filtering; and heating a feed liquid to 60-65 DEG C, preserving heat for 8-10 minutes, adding 0.02 percent of injection active carbon, stirring, dissolving, decarburizing, filtering, lowering temperature of the feed liquid to be below 40 DEG C, and performing split charging. Due to the adoption of the method, the pollution levels and endotoxin levels of relevant substances of a product and microorganisms are effectively controlled, and the stability and light inspection yield of the product are increased.
Owner:NORTH CHINA PHARMA COMPANY
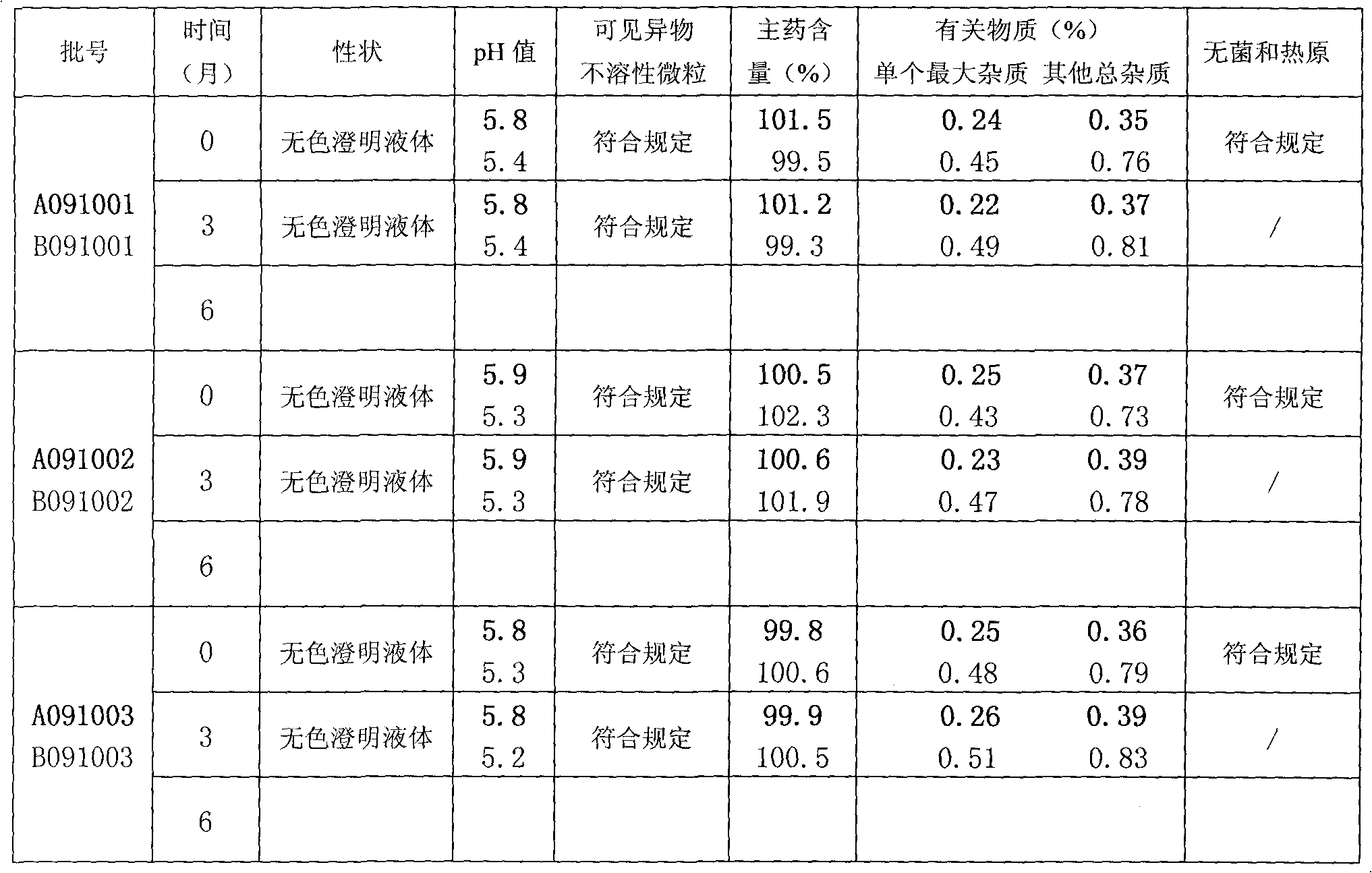
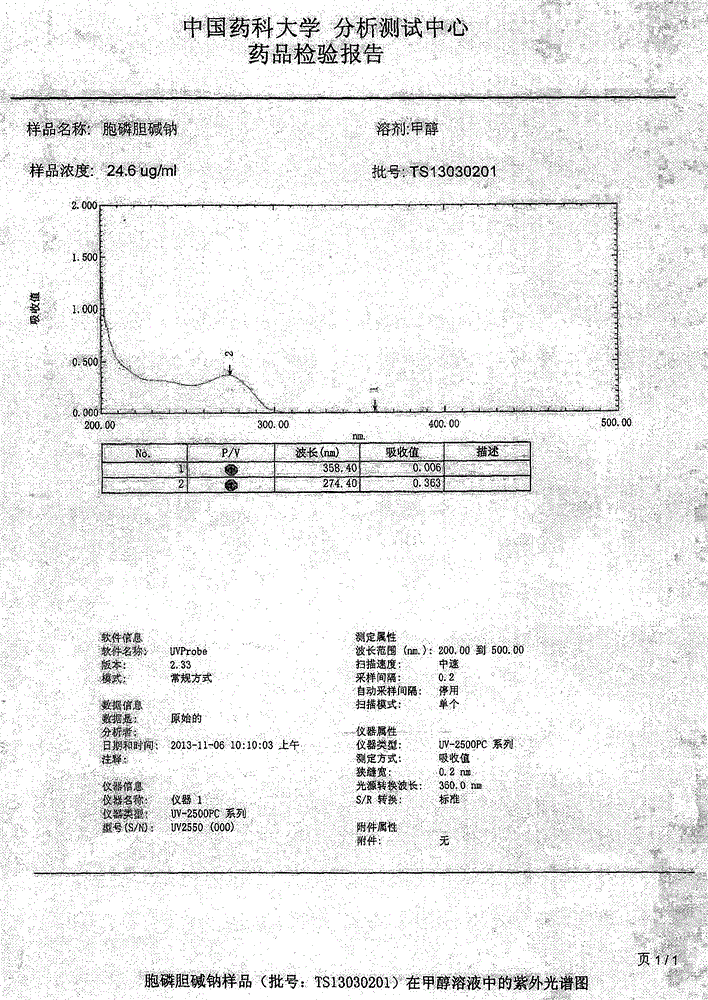
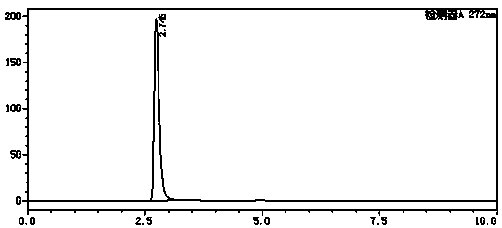
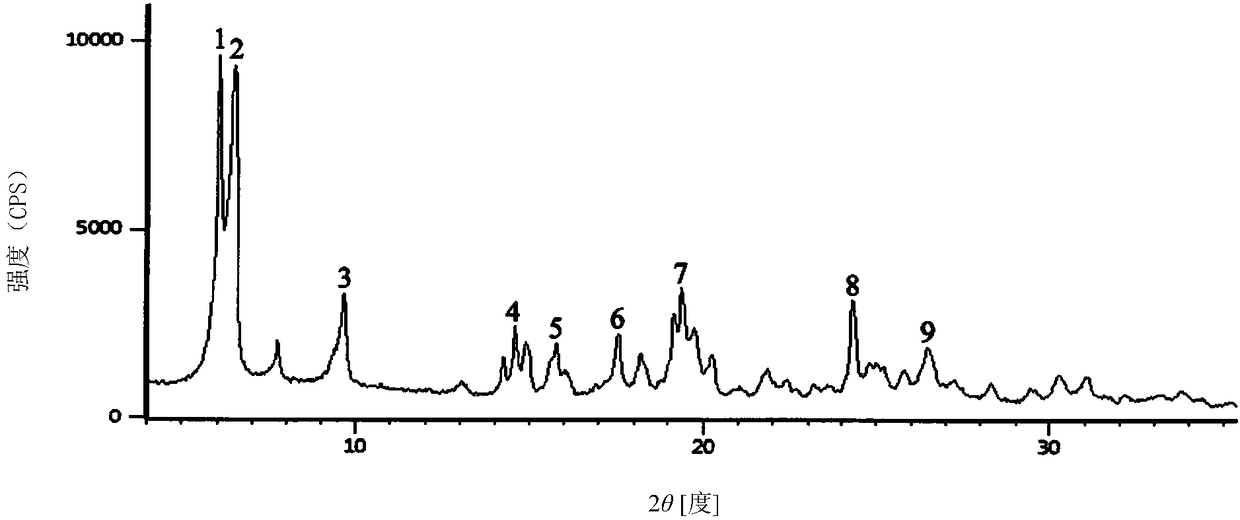
Method for detecting related substances in pharmaceutical preparation containing citicoline sodium
InactiveCN109580834AEfficient separationImprove applicabilityComponent separationTheoretical plateRetention time
The invention relates to a method for detecting related substances in a pharmaceutical preparation containing citicoline sodium. High performance liquid chromatography is adopted to analyze a system applicability solution, and the separation degree and theoretical plate number of citicoline sodium peak among the citicoline sodium peak and adjacent impurities and each known impurity in a chromatogram are recorded; the separation degree among citicoline sodium and adjacent impurities and each known impurity is greater than 1.5, when the theoretical plate number of the citicoline peak is greaterthan 2000, then a test solution and a contrast solution are analyzed and detected, the chromatogram is recorded for three times of the retention time of a main component peak, and the impurity number,type, relative percentage content, peak area and separation degree between the peaks are read and calculated from the recorded chromatogram; and the peak area in the chromatogram is calculated to obtain the content of each impurity. Compared with the prior art, the method for detecting the related substances in the pharmaceutical preparation containing citicoline sodium is simple and convenient,quick and good in durability, the impurities in the citicoline sodium can be effectively detected, and the method for detecting the related substances in the pharmaceutical preparation containing citicoline sodium is good in applicability, and high in accuracy and precision.
Owner:CISEN PHARMA
Method for separating and purifying citicoline sodium from microbial fermentation liquor
InactiveCN109836468AEfficient extractionImprove adsorption capacitySugar derivativesSugar derivatives preparationFiltrationUltrafiltration
The invention discloses a method for separating and purifying citicoline sodium from microbial fermentation liquor. The method comprises the following steps: (1) heating and flocculating citicoline sodium fermentation liquor, performing solid-liquid separation to obtain a clear liquid, and performing ultrafiltration and nanofiltration continuous pretreatment on the clear liquid to obtain a pretreating liquid; (2) pretreating the pretreating liquid on a strong-base anion exchange resin pre-column, adsorbing effluent through a strong-base anion exchange resin spin column, eluting with a sodium chloride solution, mixing high-purity eluting liquids, and performing vacuum concentration and alcohol-precipitation crystallization to obtain a citicoline sodium crude product; and (3) decolorizing the citicoline sodium crude product with activated carbon, performing filtration, concentration, alcohol-precipitation crystallization and vacuum drying to obtain a citicoline sodium finished product which has good quality, high yield, low cost and small pollution.
Owner:SUZHOU BIOSYNTHETICA CO LTD
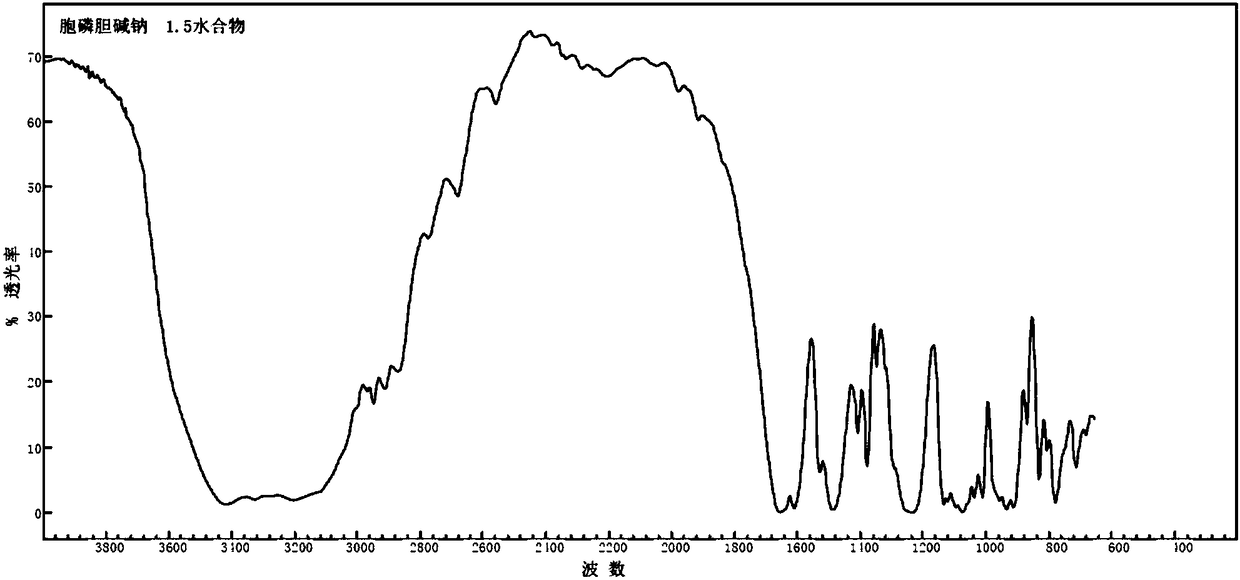
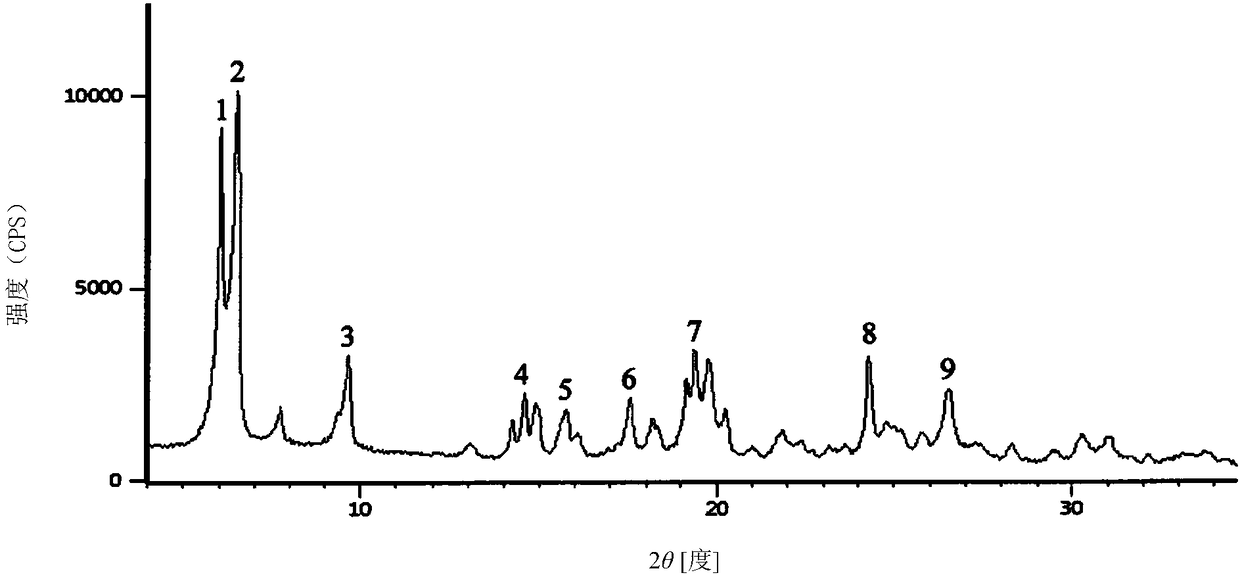
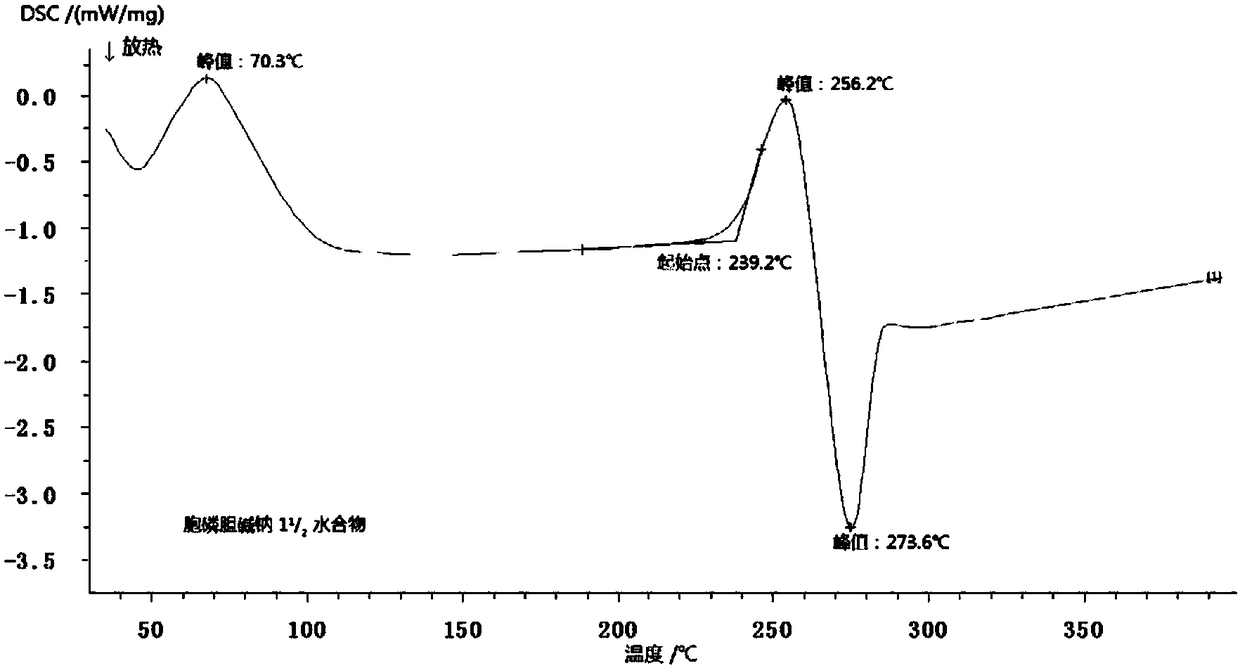
11/2 water citicoline sodium compound and pharmaceutical composition preparation
InactiveCN109160932AStable in natureNot easy to absorb moistureOrganic active ingredientsSenses disorderActivated carbonAlcohol
The invention discloses a 11 / 2 water citicoline sodium compound and a preparation method of the 11 / 2 water citicoline sodium compound, wherein each molar of citicoline sodium contains 11 / 2 molar of water. The citicoline sodium compound is prepared by dissolving a crude product of citicoline sodium into purified water, stirring, dissolving; adding an activated carbon, decarburizing; regulating thepH by using an acid, stirring evenly; slowly adding ethyl alcohol dropwise, adding a seed crystal after finishing adding dropwise, controlling the temperature, leaving to stand, crystalizing, filtering, washing a filtered material with ethyl alcohol, and drying in vacuum. The 11 / 2 water citicoline sodium compound is low in hygroscopicity and impurity content and good in stability, and has a broader application prospect. Meanwhile, the invention further discloses a pharmaceutical composition preparation of the 11 / 2 water citicoline sodium compound. The pharmaceutical composition preparation ofthe 11 / 2 water citicoline sodium compound is better in stability compared with the previous preparation.
Owner:刘兆娟
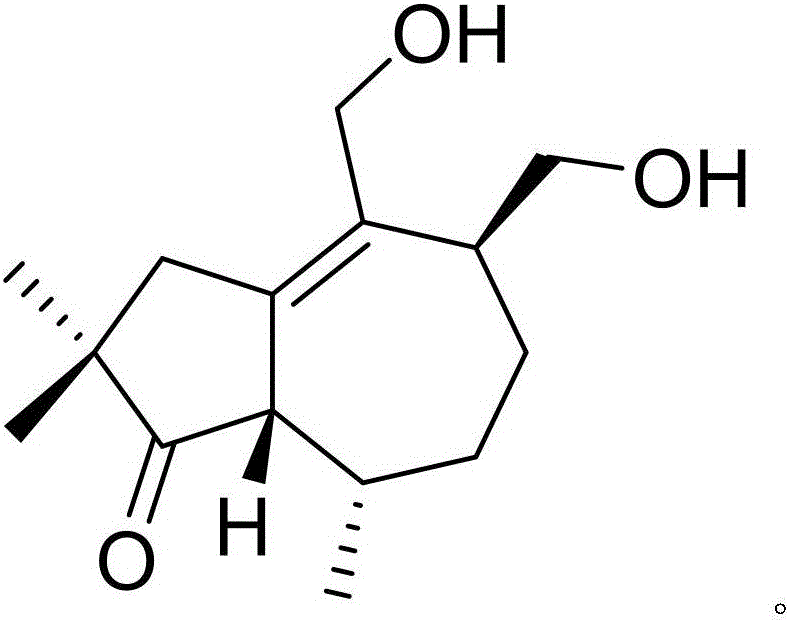
Drug composition of citicoline sodium and medical application of drug composition
InactiveCN105753681AHas anti-tumor effectImprove anti-tumor effectOrganic active ingredientsAntineoplastic agentsNatural productChemical compound
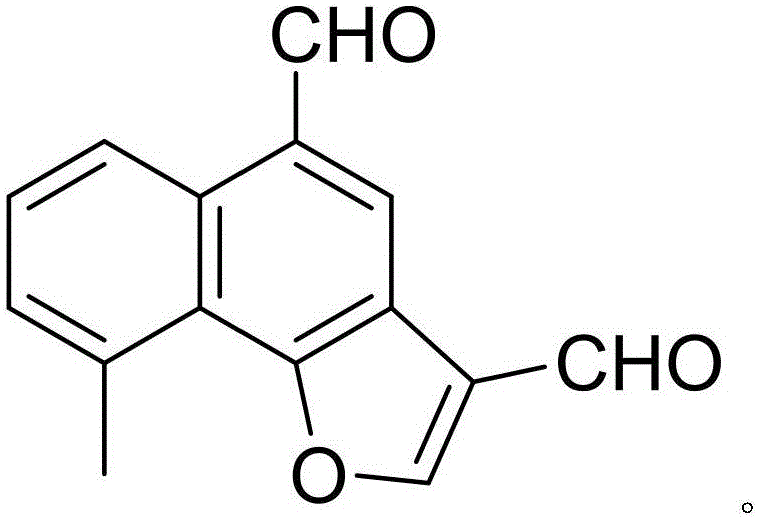
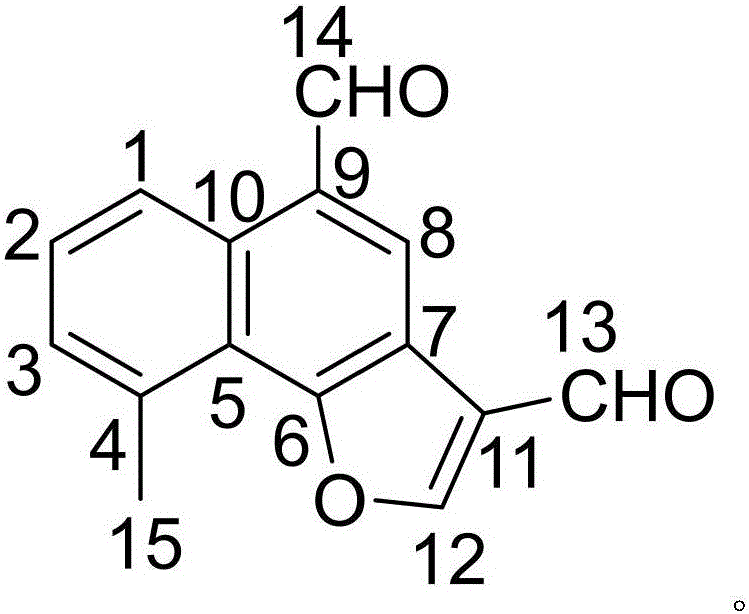
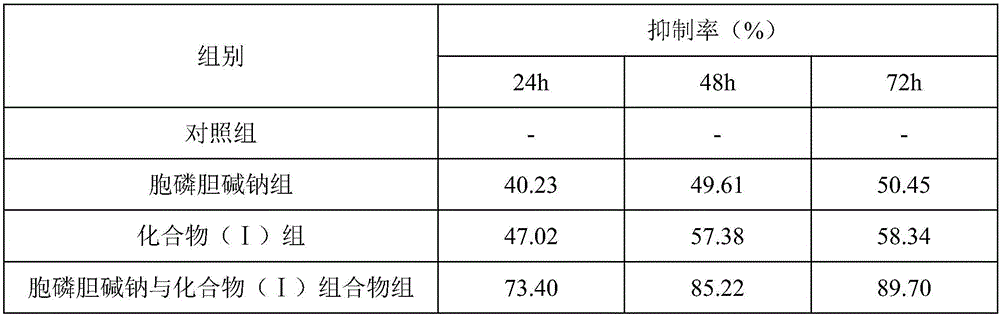
The invention discloses a drug composition of citicoline sodium and medical application of the drug composition.The drug composition of the citicoline sodium comprises the citicoline sodium and a natural product chemical compound (I) of a novel structure, and the citicoline sodium and the chemical compound (I) have an inhibiting effect on transplanted tumor when acting independently; the citicoline sodium and the chemical compound (I) have a more remarkable inhibiting effect on transplanted tumor when acting jointly, the safety is high, the toxicity is low, the drug for inhibiting tumor can be developed, and compared with the prior art, the drug composition has prominent substantive features and remarkable advancement.
Owner:吴国春
Synthetic method of citicoline sodium
InactiveCN111808899AAvoid disadvantages that need to be dealt withEnzyme source stabilityTransferasesFermentationCholine PhosphateCiticoline sodium
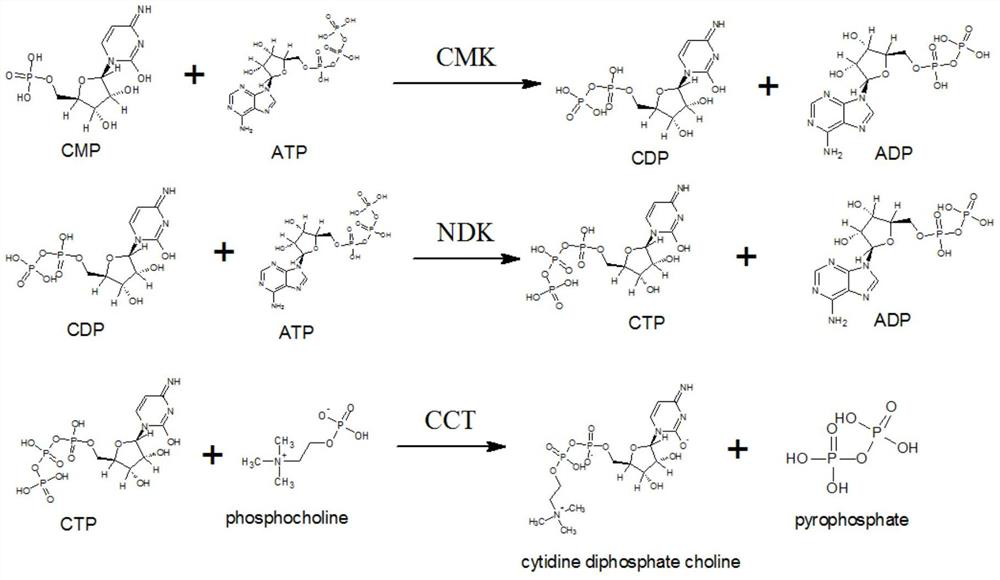
The invention discloses a synthetic method of citicoline sodium. The synthetic method comprises the following steps: adding 5'-cytidylic acid, calcium phosphorylcholine chloride, ATP, an aqueous magnesium chloride solution and a Tris-HCl buffer solution into a reaction vessel, and carrying out uniform mixing; then, adjusting a pH value to 6.0-8.0, adding CMP phosphokinase, nucleoside diphosphate kinase and choline phosphate-cytidyltransferase, and carrying out a stirring reaction for 4-10 hours at a temperature of 25-50 DEG C; and conducting high-speed centrifugation, and taking a supernatantto obtain citicoline sodium. Compared with the traditional citicoline sodium synthesis process, the synthetic method provided by the invention is environment-friendly in a synthesis route, stable in yield and is beneficial for reducing energy consumption and protecting the environment, so the synthetic method is suitable for large-scale industrial production.
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
Citicoline sodium medicine composition and pharmaceutical application of citicoline sodium medicine composition to preventing and treating ovarian cancer
InactiveCN105777682AImprove the effect of prevention and controlHighlight substantiveOrganic active ingredientsOrganic chemistryNatural productCiticoline sodium
The invention discloses a citicoline sodium medicine composition and pharmaceutical application of the citicoline sodium medicine composition to preventing and treating ovarian cancer. The citicoline sodium medicine composition contains citicoline sodium and a natural product compound (I) which is separated from dried roots of radix polygalae and is of a novel structure. Only common effects of preventing and treating the ovarian cancer can be realized when the citicoline sodium and the natural product compound (I) are individually used, but effects of preventing and treating the ovarian cancer can be obviously improved when the citicoline sodium and the natural product compound (I) are used with each other, and accordingly the citicoline sodium medicine composition can be developed to obtain medicines for preventing and treating the ovarian cancer. Compared with the prior art, the citicoline sodium medicine composition and the pharmaceutical application have the advantages of outstanding substantial characteristics and obvious advancement.
Owner:钱浩
Preparation method of citicoline sodium
InactiveCN106146590AEasy to removeEasy to operateSugar derivativesSugar derivatives preparationDistillationCiticoline sodium
The invention discloses a preparation method of citicoline sodium, and belongs to the technical field of biopharmacy. The preparation method comprises the steps that phosphorylcholine chloride calcium salt serving as a raw material and benzene are subjected to cbinary azeotrope to remove crystallization water in phosphorylcholine chloride calcium salt; phosphorylcholine chloride calcium salt reacts with sodium carbonate to generate a calcium carbonate precipitate, and filtering is conducted to obtain viscous materials; the viscous materials react with acetylchloride, a product reacts with cytidine monophosphate, and reduced pressure distillation is conducted to obtain residues; crystallization and recrystallizzation are conducted through sodium hydroxide and ethyl alcohol to obtain the product. According to the preparation method, operation is easy, acetylchloride is easy to remove by adding water, all the solvents can be recycled, better environmental friendliness and higher economic benefits are achieved, the purity of the product is high and reaches 98%, and the product is suitable for pharmaceutical purposes.
Owner:陈建峰
Citicoline sodium glucose injecta and preparation process thereof
ActiveCN102144963BImprove quality stabilityImprove safety and effectivenessOrganic active ingredientsNervous disorderCiticoline sodiumBULK ACTIVE INGREDIENT
The invention discloses a citicoline sodium glucose injecta and a preparation process thereof. The citicoline sodium glucose injecta mainly contains the active ingredients: citicoline sodium, isotonic agent glucose, a stabilizer, a pH regulator and water for injection, wherein the stabilizer is selected from one or two of malic acid, sale compounds thereof and sulfite compounds; the pH regulator is one or more of sodium hydroxide, hydrochloric acid, malic acid and apple sodium; the weight ratio of the active ingredients of the Citicoline sodium to the isotonic agent glucose is 1:(20-100), theweight ratio of the active ingredients of the citicoline sodium to the stabilizer is 1:(0.02-2), and the stabilizer is added to solve the problem of unstable heating and excessive related substances of the injecta in the production and storage process and improve the quality stability and the safe validation of medicaments.
Owner:HUNAN KELUN PHARMA
Citicoline sodium injection and preparation method thereof
ActiveCN109529016AImprove stabilityExtended storage timeOrganic active ingredientsNervous disorderCiticoline sodiumBULK ACTIVE INGREDIENT
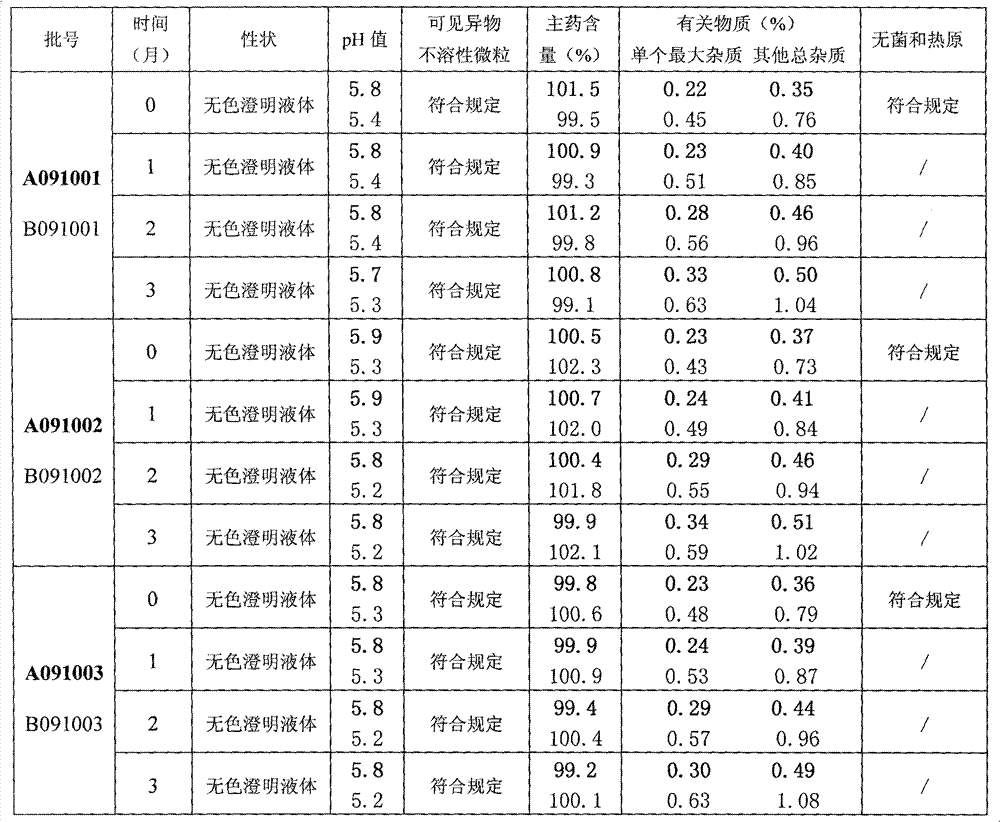
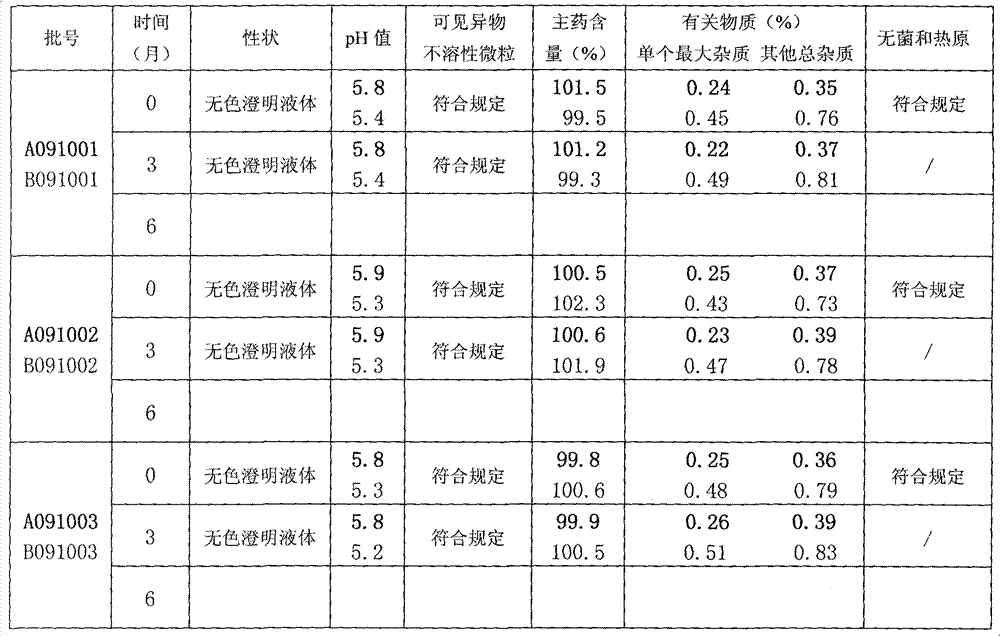
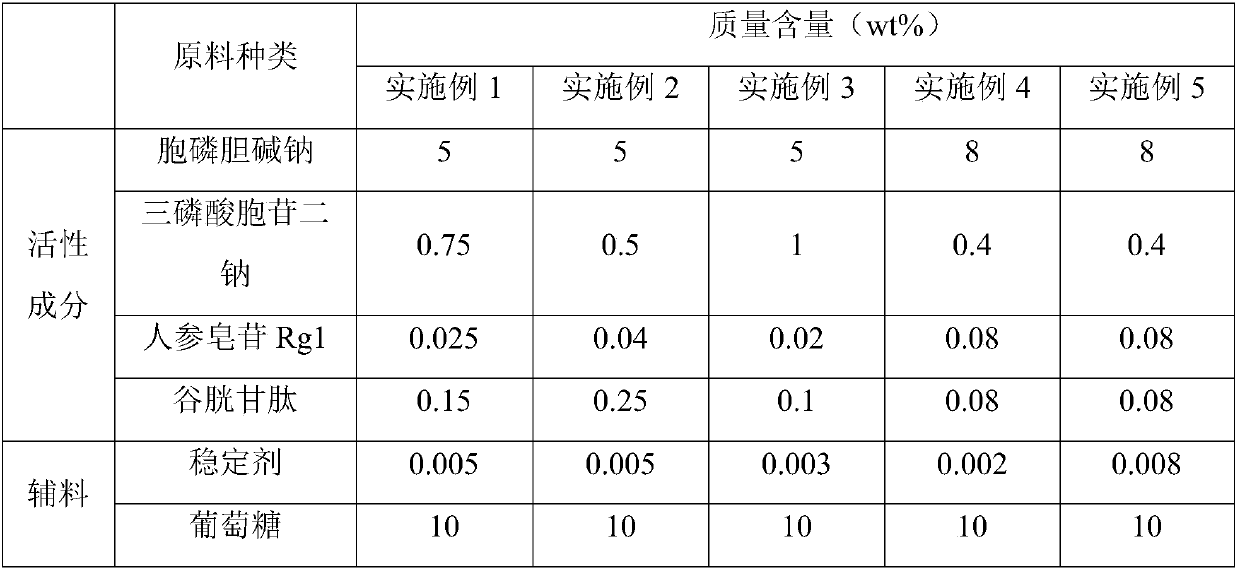
The present invention relates to the technical field of pharmaceutical preparation and particularly relates to a citicoline sodium injection and a preparation method thereof. The citicoline sodium injection comprises active ingredients, auxiliary materials and water for injection, the active ingredients comprise citicoline sodium, cytidine disdoium triphosphate, ginsenoside Rg1 and glutathione, and the auxiliary materials comprise a stabilizer, an isotonic agent and a pH adjusting agent. By adding the active ingredients of the cytidine disdoium triphosphate, ginsenoside Rg1 and glutathione tothe citicoline sodium injection, stability of the citicoline sodium injection can be improved and storage time is prolonged.
Owner:江西润泽药业有限公司
Moisture-proof coating citicoline sodium capsule and preparation method thereof
ActiveCN102525997BGood granularityGood disintegrationOrganic active ingredientsNervous disorderCiticoline sodiumMoisture absorption
The invention provides a moisture-proof coating citicoline sodium capsule and a preparation method thereof. The citicoline sodium capsule is composed of a capsule casing and quick-release moisture-proof micro pills or granules. All components in each capsule containing citicoline sodium quick-release moisture-proof micro pills or granules by percentage are 40% to 70% of citicoline sodium, 10% to 30% of microcrystalline cellulose, 10% to 30% of pregelatinized starch and 2% to 10% of moisture-proof coatings. The moisture-proof micro pills or granules are obtained by being extruded and rounded or pressed and granulated to be coated with moisture-proof coatings. The citicoline sodium capsule is good in moisture-proof function and resolves the problems that the capsule is prone to absorb waterdue to the fact that citicoline sodium is strong in moisture absorption, the capsule casing is prone to be fragile, and the like.
Owner:QILU PHARMA CO LTD
UPLC analysis method for simultaneously measuring citicoline sodium and nine related substances
ActiveCN110174482AShow detection advantageAccurate quality controlComponent separationOrganic solventPhosphate
The invention relates to the chemical field of medicine analysis, in particular to a UPLC analysis method for simultaneously measuring citicoline sodium and nine related substances. The method comprises the steps of performing chromatographic analysis by a reverse-phase chromatographic column, wherein the reverse-phase chromatographic column takes hydrophilic octadecyl silane bonded silica gel asa filling agent, phosphate buffer liquid as a moving phase A and an organic solvent as a moving phase B; performing gradient elution on a sample solution of the citicoline sodium; and measuring purityof the citicoline sodium and content of nine types of impurities according to an area normalization method. With the method provided by the technical scheme of the invention, the citicoline sodium impurity can be effectively separated, so that the quality of the citicoline sodium can be accurately controlled; and the detection advantage of a plurality of impurities of the citicoline sodium is displayed, and an accurate and high-efficiency detection method can be provided for measurement of the purity of the citicoline sodium and the impurity content.
Owner:苏州正济药业有限公司
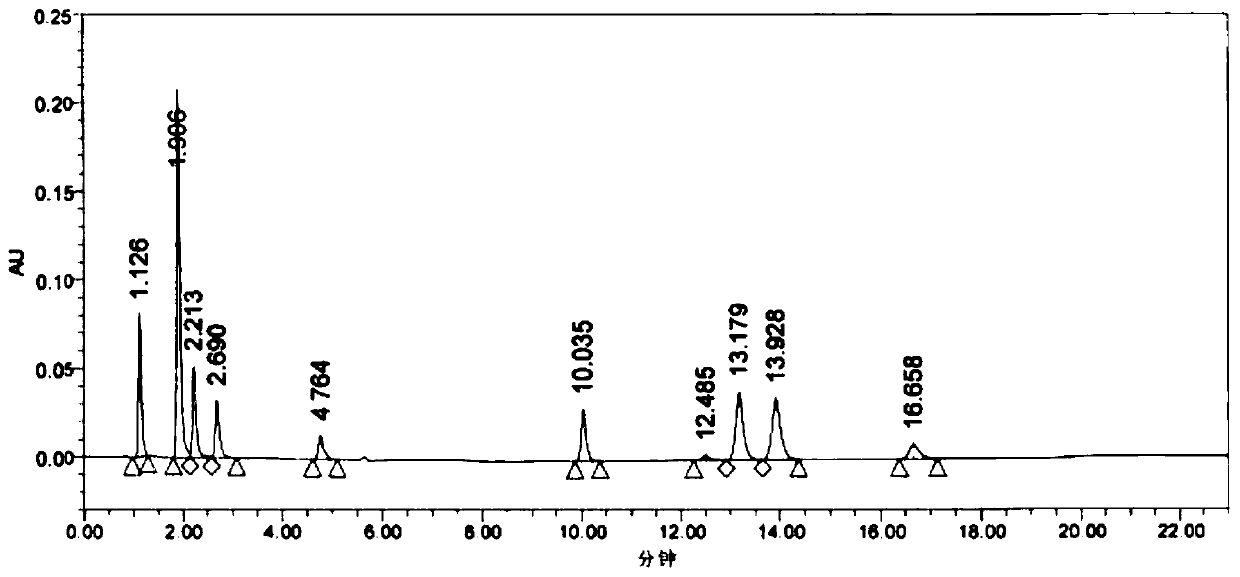
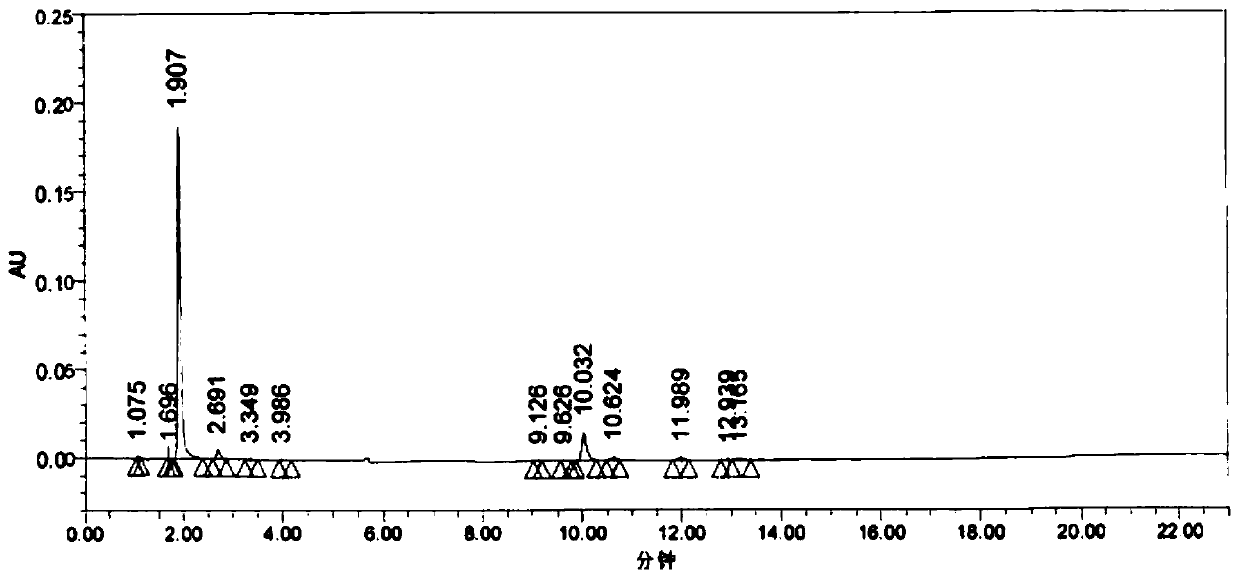
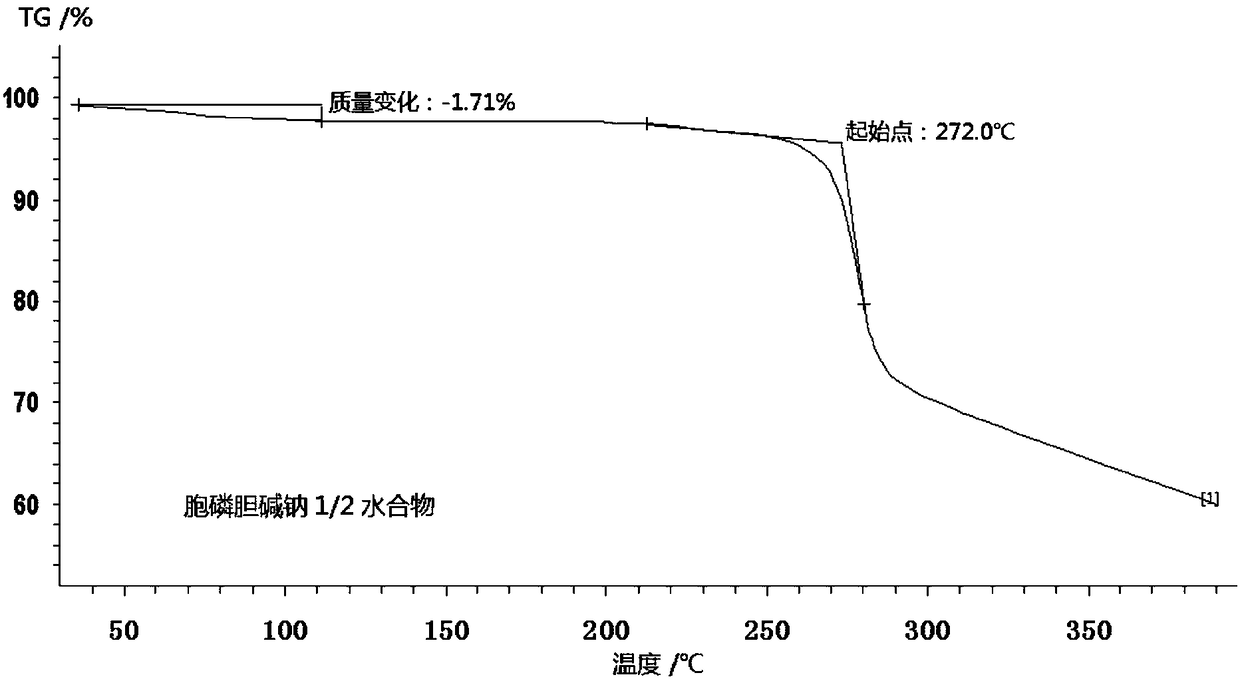
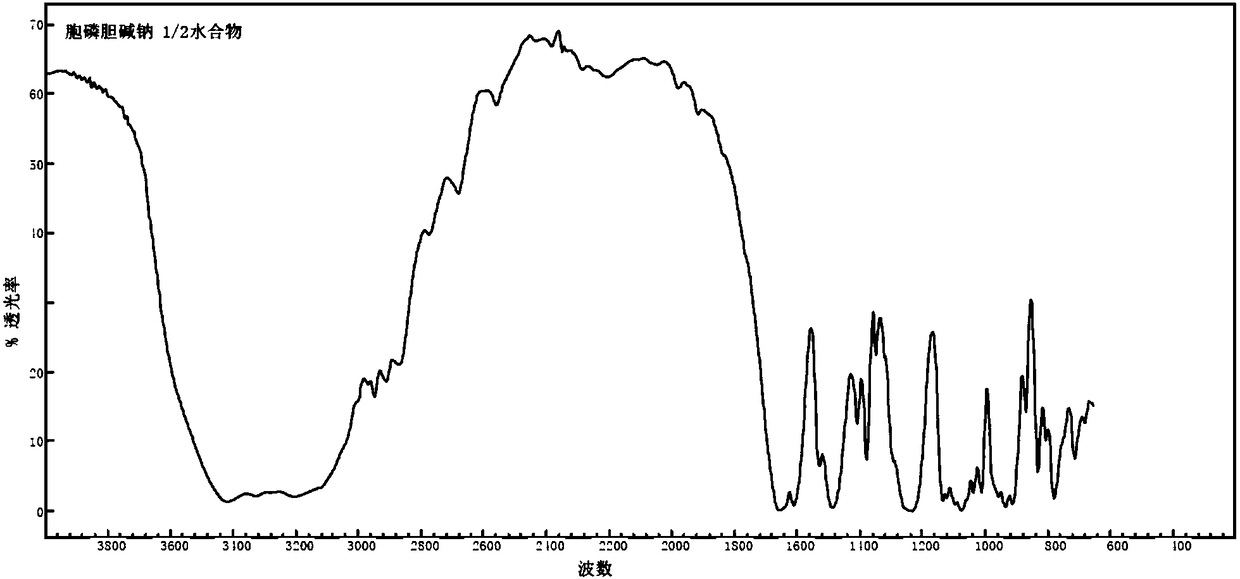
1/2 water citicoline sodium compound
The invention relates to a 1 / 2 water citicoline sodium compound and a preparation method of the 1 / 2 water citicoline sodium compound, wherein each molar of citicoline sodium contains 1 / 2 molar of water. Specifically, the preparation method is as follows: adding a crude product of citicoline sodium, sodium calcium edetate and sodium sulfite into a mixed solution of purified water and acetone, stirring and dissolving; adding activated carbon, stirring, adsorbing, filtering; using an acid to regulate the pH of a filtrate, stirring evenly; slowing adding isopropanol dropwise into the above solution, standing, crystalizing, filtering, washing a filtered material, and drying to obtain the 1 / 2 water citicoline sodium compound. The 1 / 2 water citicoline sodium compound is good in flowability, low in impurity content and hygroscopicity, fast in dissolution and good in stability, and has a broader application prospect.
Owner:王秀香
Citicoline sodium injection and preparation process thereof
PendingCN114224831ALow impurity contentReduce storage deteriorationOrganic active ingredientsNervous disorderDisodium EdetatePhysical chemistry
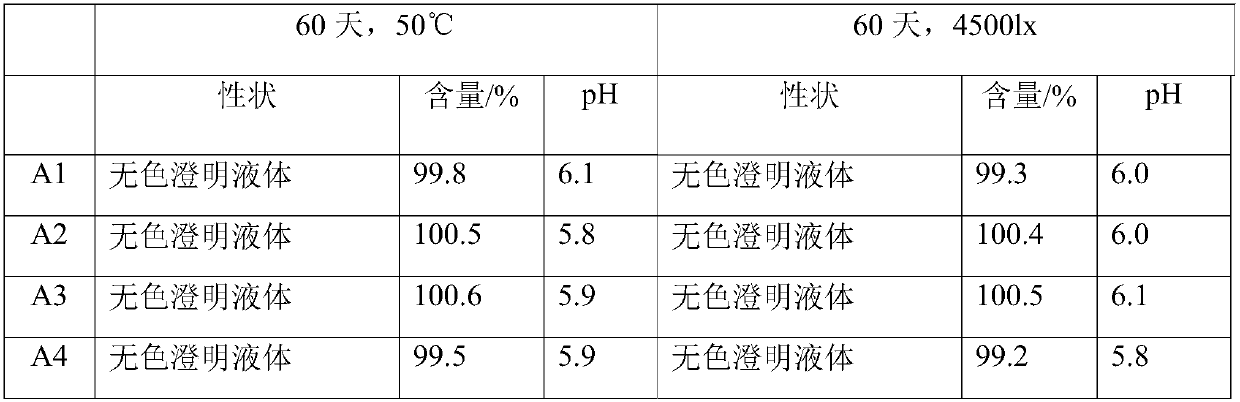
The invention relates to a citicoline sodium injection and a preparation process thereof, and belongs to the technical field of injection preparation. The injection comprises the following raw materials: citicoline sodium, sodium chloride, edetate disodium and water for injection. The main working procedures are as follows: proportioning, concentrated preparation and rough filtration, diluted preparation and fine filtration, detection and post-treatment. According to the preparation method, liquid is prepared through a two-step method of concentrated preparation rough filtration and diluted preparation fine filtration, reasonable technological parameter setting is matched, the impurity content in the prepared injection is low, storage deterioration of the injection is relieved, and it is tested that the prepared injection shows excellent stability in a 4000 lx hard light acceleration simulation experiment at the temperature of 40 DEG C + / -2 DEG C, and the injection has the good application prospect. The preparation process provided by the invention is suitable for mass production of citicoline sodium injection, and is suitable for stable production of 980000 ml injection.
Owner:ANHUI LIANYI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com