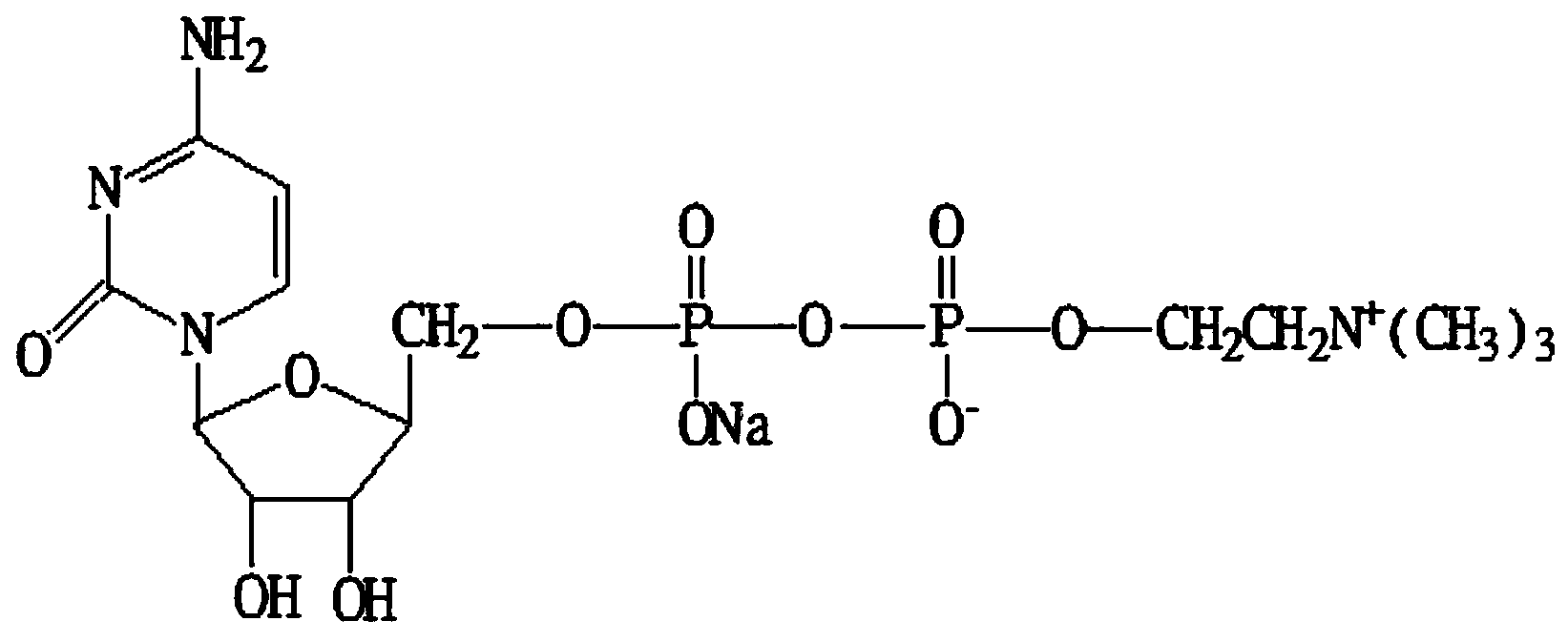
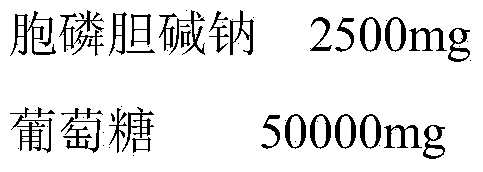Citicoline sodium glucose injection
A technology of glucose injection and citicoline sodium, applied in the field of citicoline sodium glucose injection, can solve the problems of poor thermal stability, high substance content, can not meet production and use, etc., achieve high stability, production The process is simple and the effect of reducing the production of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Citicoline Sodium Glucose Injection
[0021]
[0022] Use 0.1N sodium hydroxide to adjust the pH value to 5.5-5.6, and add water for injection to 1000ml.
[0023] Preparation Process:
[0024] 1. Dissolving glucose in water, adding activated carbon for adsorption and filtering, diluting the filtrate with water to an isotonic concentration, thereby obtaining an isotonic aqueous solution of glucose; preferably, the water is water for injection;
[0025] 2. Add citicoline sodium, glycine and citric acid to the isotonic aqueous solution obtained in step 1, stir to dissolve, then add activated carbon to absorb and filter, and use 0.1N sodium hydroxide to adjust the pH value to 5.5- 5.6, Microporous membrane filtration.
Embodiment 2
[0027] Citicoline Sodium 3000mg
[0028] Glucose 40000mg
[0029] Citric acid 1300mg
[0030] Use 0.1N sodium hydroxide to adjust the pH value to 5.5-5.6, and add water for injection to 1000ml.
[0031] Preparation process: with embodiment 1.
Embodiment 3
[0033] Citicoline Sodium 3000mg
[0034] Glucose 40000mg
[0035] Glycine 1800mg
[0036] Use 0.1N sodium hydroxide to adjust the pH value to 5.5-5.6, and add water for injection to 1000ml.
[0037] Preparation process: with embodiment 1.
[0038] The indicators of the Citicoline Sodium Glucose Injection prepared in the above examples meet the relevant provisions of the 2010 edition of the Chinese Pharmacopoeia Part II.
[0039] Properties: Colorless clear liquid.
[0040] PH value: 5.5-5.6
[0041] Visible foreign matter: qualified
[0042] Sterility test: qualified
[0043] Insoluble particles: qualified
[0044] Content: Qualified
[0045] Related substances: individual impurities do not exceed 0.3%, and other impurities do not exceed 0.5%.
[0046] Stability test of citicoline sodium glucose injection:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com