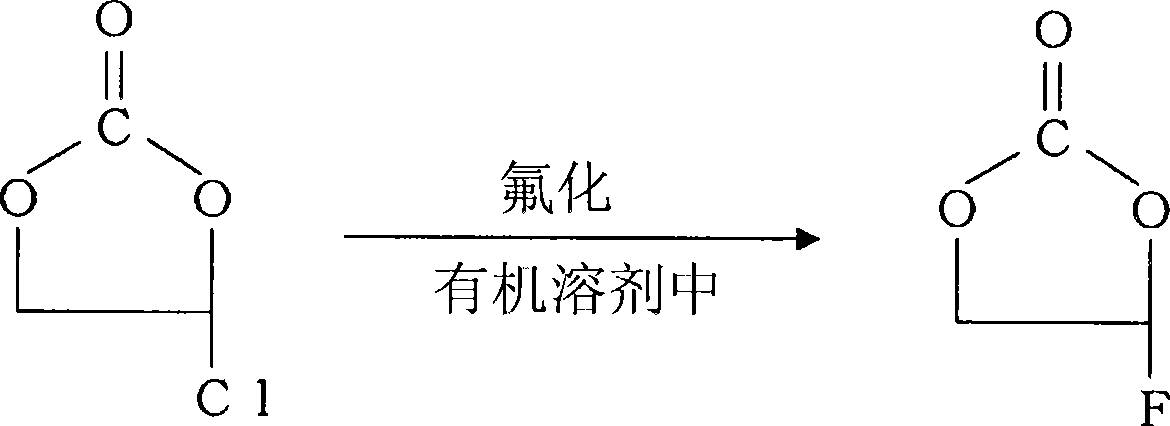
Preparation of 4-fluoro-1,3-dioxolane-2-one
A manufacturing method and technology of dioxolane, applied in the direction of organic chemistry, etc., can solve the problems of high dielectric constant, low cycle characteristics, chlorine radicals easily remaining in solvents or refined products, etc., and achieve high-purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
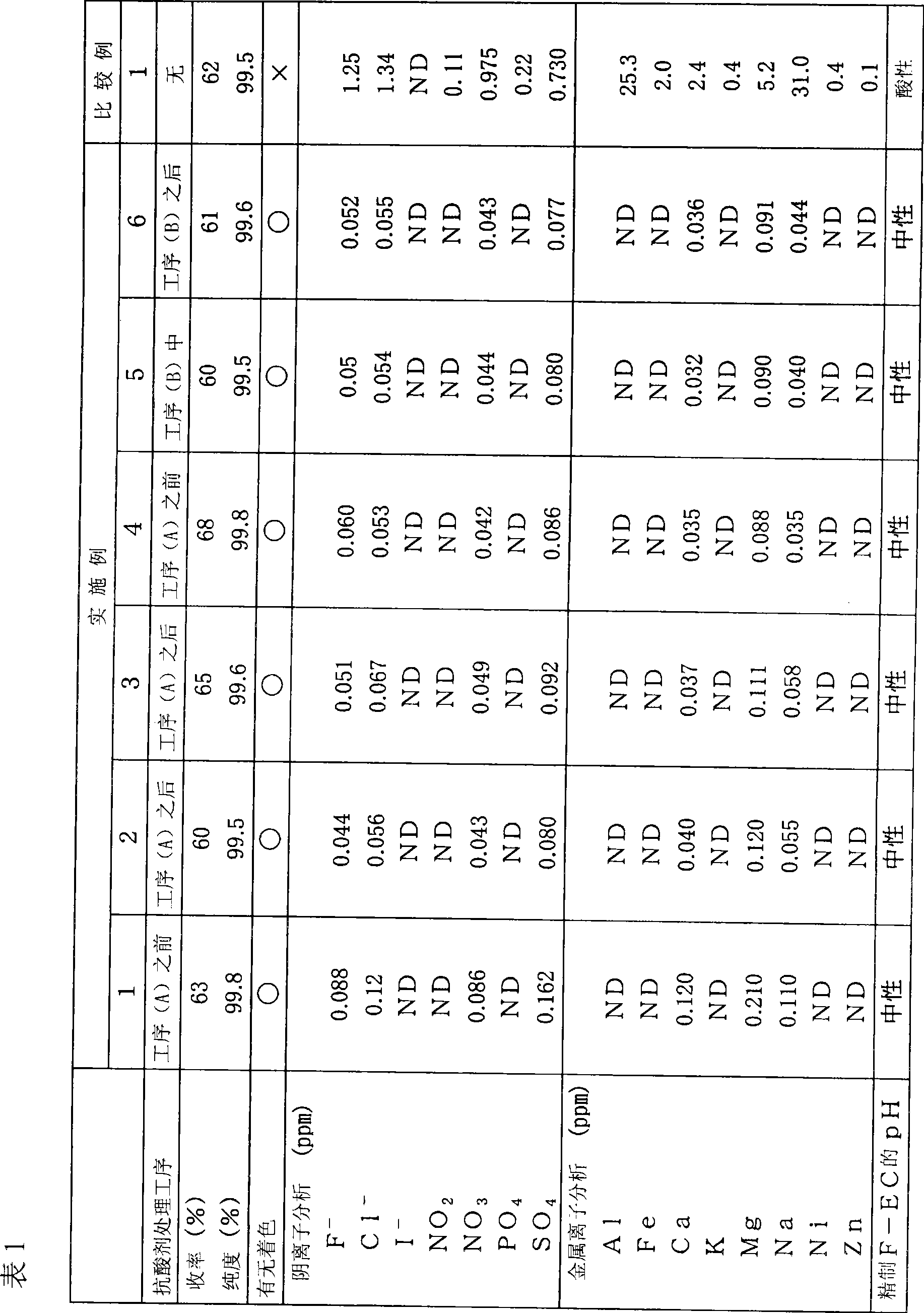
[0098] Example 1 (antacid treatment before step (A))
[0099] Antacid treatment process (C)
[0100] To 500g (4.08mol, pH1~2) Cl-EC (manufactured by Aldrich), add 100g of amorphous silica-alumina gel (secado KW produced by Shinagawa Chemical Co., Ltd., neutral silica gel, trade name), in Stir at room temperature for 2 hours.
[0101] Solid component removal process (E)
[0102] After the stirring was completed, the amorphous silica-alumina gel was removed by filtration to prepare antacid-treated Cl-EC (pH 6-7).
[0103] Fluorination process (A)
[0104] A reflux tube was attached to the upper part of a 3L glass three-necked flask equipped with a stirring device, 355 g (6.12 mol) of spray-dried potassium fluoride was added, and the water was removed by flame drying while stirring under vacuum. Then, 1.3 L of acetonitrile and 500 g (4.08 mol) of antacid-treated Cl-EC were added and stirred using a syringe. The reaction was carried out at a reaction temperature of 85°C, and ...
Embodiment 2
[0123] Example 2 (antacid treatment after step (A))
[0124] Fluorination process (A)
[0125] A reflux tube was attached to the upper part of a 3L glass three-necked flask equipped with a stirring device, 355 g (6.12 mol) of spray-dried potassium fluoride was added, and the water was removed by flame drying while stirring under vacuum. Then, 1.3 L of acetonitrile and 500 g (4.08 mol) of untreated Cl-EC were added and stirred using a syringe. The reaction was carried out at a reaction temperature of 85°C, and the progress was analyzed by gas chromatography (GC). After reacting for 6 hours, it was confirmed that the peak of the raw material disappeared, and the reaction ended. After the reaction, the salt in the reaction product was filtered to obtain a reaction product liquid (pH 1-2).
[0126] Antacid treatment process (C)
[0127] 100 g of silica gel (Silica gel 60 manufactured by Merck, trade name, particle size 0.063 to 0.200 mm) was added to the obtained filtrate, and...
Embodiment 3
[0135] Example 3 (antacid treatment after step (A))
[0136] In addition to using amorphous silica-alumina gel (secado KW manufactured by Shinagawa Kasei Co., Ltd., neutral silica gel, trade name) instead of the silica gel used in the antacid treatment step (C) of Example 2, In the same manner as in Example 2, colorless and transparent F-EC was obtained as a fraction at 74°C (1 mmHg), with a yield of 65% and a GC purity of 99.6%.
[0137] The purified F-EC was carried out in the same manner as in Example 1, and the presence or absence of coloration, anion analysis, metal ion analysis, and pH measurement were performed. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com