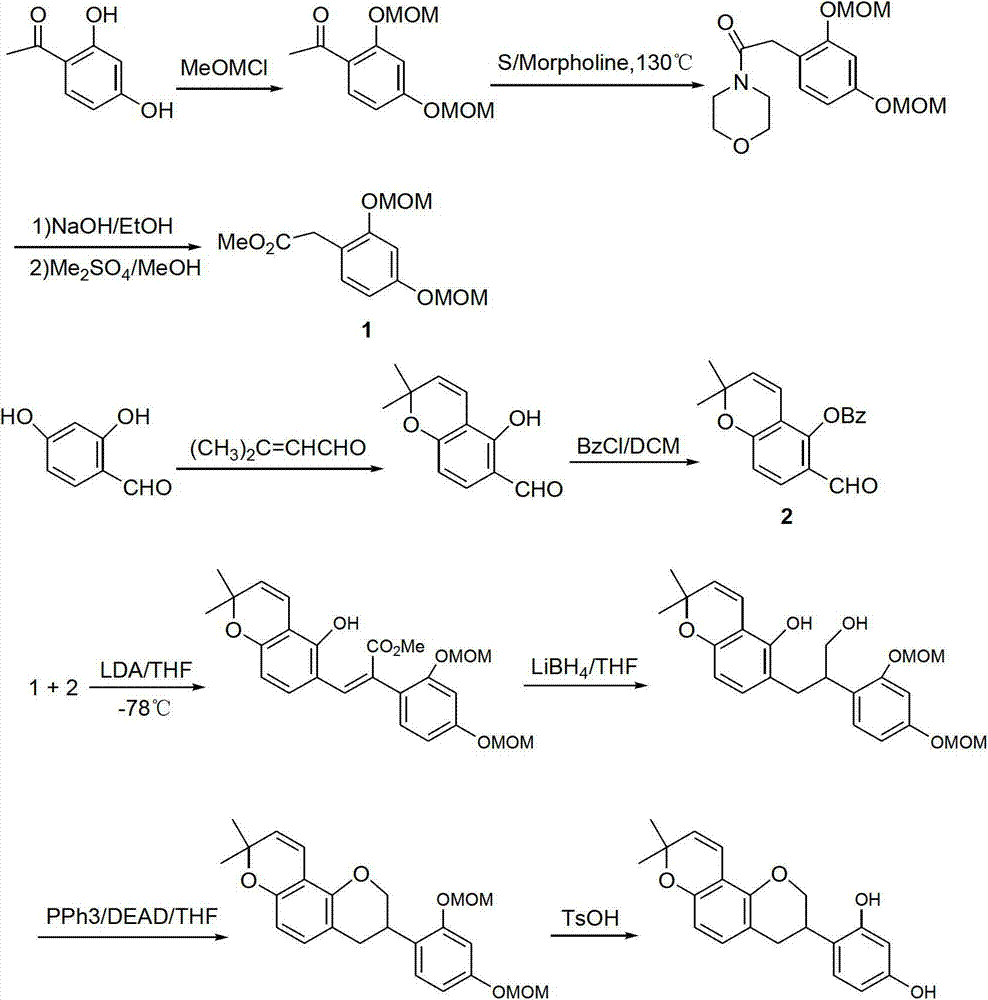
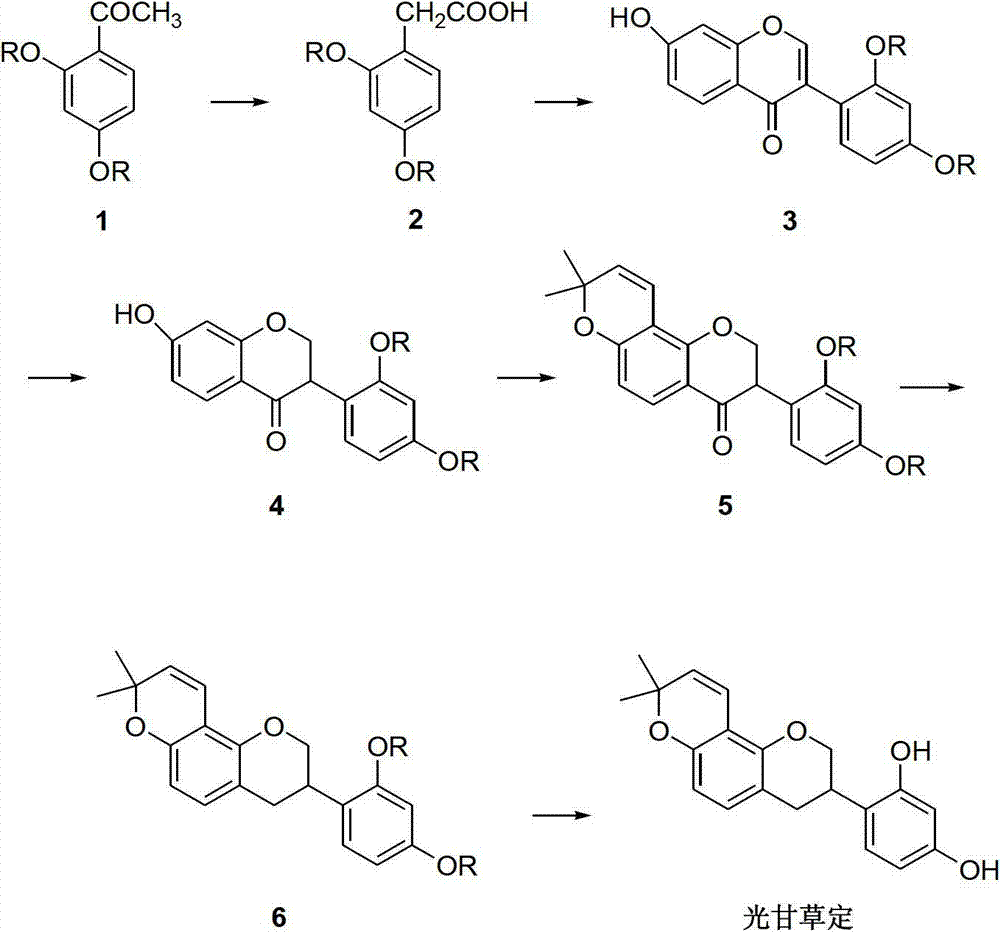
Method for synthesizing glabridin
A synthetic method, the technology of glabridin, is applied in the direction of organic chemistry, which can solve the problems that are not suitable for large-scale production, and achieve the effects of high yield, short route, and simple operation and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of 2,4-dimethoxyphenylacetic acid (compound 2)
[0034] Put the compound 2,4-dimethoxyacetophenone (0.1mol), sulfur powder (0.2mol), and morpholine (0.2mol) in a reaction flask, stir and heat to reflux for 7h, cool to room temperature, add 200mL dichloromethane Dissolve, wash with 6N hydrochloric acid (100mL×2), collect the organic phase and distill off the solvent under reduced pressure to obtain a brown oil. Add 20wt% sodium hydroxide solution, stir and reflux for 7 hours, cool to room temperature, adjust the pH to 1-2 with concentrated hydrochloric acid, collect the obtained solid by filtration, and obtain compound 2 with a yield of 85%.
[0035] (2) Synthesis of 7-hydroxy-2′,4′-dimethoxyisoflavone (compound 3)
[0036] Compound 2 (0.1mol) was mixed with resorcinol (0.1mol), and reacted at 85°C for 3h under the catalysis of fresh boron trifluoride diethyl ether (60mL) to generate a coupling product. After cooling to room temperature, add dry N,N-dimet...
Embodiment 2
[0046] The synthesis of compounds 2, 3, 4, 6 and glabridin is the same as that in Example 1, except that the preparation of compound 5 is different.
[0047] Preparation of Compound 5: Compound 4 (0.02mol) and 3-methyl-2-enbutanal (0.024mol) were mixed in anhydrous picoline (80mL), refluxed for 10h, cooled to room temperature, washed with 6N hydrochloric acid , extracted with ethyl acetate, dried over anhydrous sodium sulfate, and removed the solvent with steam to obtain a brown oil, which was dissolved in n-hexane and then frozen and crystallized for 12 hours to obtain a light yellow solid, namely compound 5, with a yield of 77%.
Embodiment 3
[0049] The synthesis of compounds 2, 3, 4, 6 and glabridin is the same as that in Example 1, except that the preparation of compound 5 is different.
[0050] Preparation of compound 5: under nitrogen protection, compound 4 (0.01mol), 3-methyl-2-enbutyraldehyde (0.012mol), phenylboronic acid (0.012mol), and 50mL of acetic acid were mixed in 250mL of toluene, and refluxed for 7h , The solvent was removed under reduced pressure, and the residue was subjected to silica gel column chromatography to obtain compound 5 with a yield of 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com