Preparation method of azoxystrobin intermediate
An intermediate, azoxystrobin technology, applied in the field of agricultural fungicides preparation, can solve problems such as long reaction time, low benzofuranone content, incomplete reaction, etc., achieves improved yield and purity, shortened reaction time, reduced The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
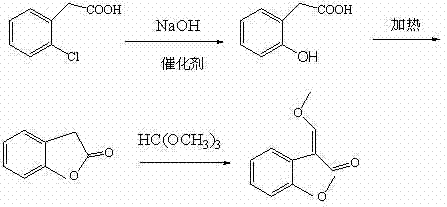
[0017] The preparation of the azoxystrobin intermediate according to the present embodiment comprises the following three steps:
[0018] (1) Preparation of o-hydroxyphenylacetic acid
[0019] 173g (99%, 1.00mol) of o-chlorophenylacetic acid, 800g (12.5%, 2.5mol) of sodium hydroxide aqueous solution, 6.6g (98%, 0.1mol) of copper powder were put into the autoclave, and the pressure was slowly increased to 7KPa ( Relative to atmospheric pressure), heat preservation reaction at 160°C for 3 hours, after the reaction is completed, cool down and filter, recover copper powder, add hydrochloric acid to the mother liquor to adjust pH, freeze and filter to obtain o-hydroxyphenylacetic acid, 140g after drying, content 97.5%.
[0020] (2) Preparation of benzofuranone
[0021] Put 140g (97.5%, 0.90mol) of o-hydroxyphenylacetic acid, 2g (98%, 0.02mol) of concentrated sulfuric acid into a 1L reaction flask equipped with a condenser tube and a receiving flask, turn on the vacuum, a...
Embodiment 2
[0025] The preparation of the azoxystrobin intermediate according to the present embodiment comprises the following three steps:
[0026] (1) Preparation of o-hydroxyphenylacetic acid
[0027] 173g (99%, 1.00mol) of o-chlorophenylacetic acid, 800g (12.5%, 2.5mol) of sodium hydroxide aqueous solution, 10.2g (98%, 0.1mol) of cuprous chloride were put into the autoclave, and slowly pressurized to 7KPa (relative atmospheric pressure), heat preservation reaction at 160°C for 3 hours, after the reaction is completed, cool down and filter to recover cuprous chloride, add hydrochloric acid to adjust the pH of the mother liquor, freeze and filter to obtain o-hydroxyphenylacetic acid, 141g after drying, content 97.3%.
[0028] (2) Preparation of benzofuranone
[0029] 141g (97.3%, 0.90mol) o-hydroxyphenylacetic acid, 2g (98%, 0.02mol) methanesulfonic acid, put into a 1L reaction flask equipped with a condenser tube and a receiving flask, turn on the vacuum, when the pressure ...
Embodiment 3
[0033] The preparation of the azoxystrobin intermediate according to the present embodiment comprises the following three steps:
[0034] (1) Preparation of o-hydroxyphenylacetic acid
[0035] 173g (99%, 1.00mol) of o-chlorophenylacetic acid, 800g (12.5%, 2.5mol) of sodium hydroxide aqueous solution, 12.7g (98%, 0.1mol) of copper carbonate were put into the autoclave, and slowly pressurized to 7KPa ( Relative atmospheric pressure), heat preservation reaction at 160°C for 3 hours, after the reaction is completed, cool down and filter to recover copper carbonate, add hydrochloric acid to adjust the pH of the mother liquor, freeze and filter to obtain o-hydroxyphenylacetic acid, 140g after drying, content 97.4%.
[0036] (2) Preparation of benzofuranone
[0037] Put 140g (97.4%, 0.90mol) o-hydroxyphenylacetic acid, 2g (98%, 0.02mol) concentrated sulfuric acid into a 1L reaction flask equipped with a condenser tube and a receiving flask, turn on the vacuum, and when the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com