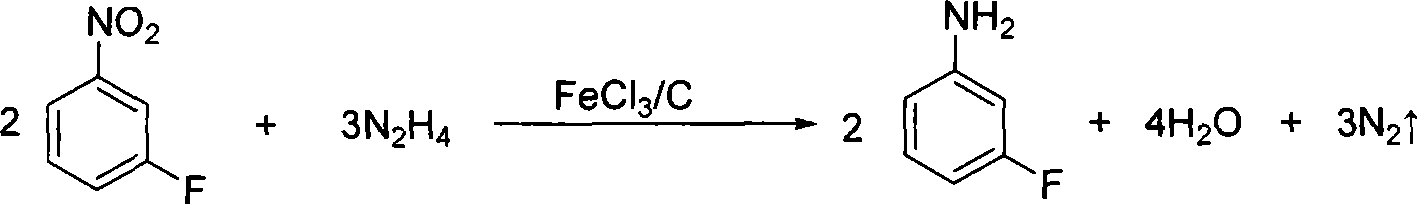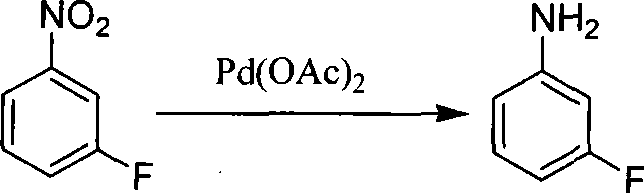Preparation method of m-fluoroaniline
A technology for fluoroaniline and m-aminoacetanilide hydrochloride, applied in the field of preparation of m-fluoroaniline, can solve the problems of expensive CsF, difficult to prepare, low reaction yield and the like, and achieves the effects of easy industrialization, easy acquisition and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of diazonium salt:
[0031] In a 500 mL four-necked bottle, add 60 mL of water and 54.7 g of m-aminoacetanilide hydrochloride. Stir, add ice-water bath to cool to -5~0°C, add 30mL of 25% NaOH aqueous solution, neutralize to pH 6, stir until all raw materials are dissolved. Add 125 grams of 40% fluoboric acid aqueous solution and keep it at -5-0°C. Then slowly add 18.7 g NaNO 2 Solution dissolved in 30mL of water, the temperature in the bottle is controlled at -5~0°C, the dropwise addition time is about 25 minutes, after the dropwise addition is completed, react at -5~0°C for another 3 hours, and the diazotization reaction is completed.
[0032] Lower the temperature in the four-necked bottle to -5°C, quickly suction filter, discard the filtrate, and suck and filter the filter cake as dry as possible. The filter cake was beaten and washed with 60 mL of cold ethanol and 60 mL of cold ether, respectively, sucked and filtered as dry as possible, and dried ...
Embodiment 2
[0038] Preparation of diazonium salt:
[0039] In a 500 mL four-necked bottle, add 87 mL of water and 54.7 g of m-aminoacetanilide hydrochloride. Stir, add ice-water bath to cool to -10~-5°C, add 32mL of 25% NaOH aqueous solution, neutralize to pH 7, stir until all raw materials are dissolved. Add 115 grams of 40% fluoboric acid aqueous solution and keep it at -10~-5°C. Then slowly add 25 grams of NaNO 2 Solution dissolved in 30mL of water, control the temperature in the bottle at -10~-5°C, add dropwise for about 25 minutes, after the dropwise addition is completed, react at -10~-5°C for another 5 hours, and the diazotization reaction is completed.
[0040] Then in the same manner as in Example 1, 58 grams of m-acetamidoaniline diazonium fluoroborate were obtained.
[0041] 2. Thermal decomposition and hydrolysis of diazonium salt
[0042] Put 20 grams of the above-mentioned diazonium salt into a 500mL four-necked bottle, heat and decompose carefully, raise the temperature...
Embodiment 3
[0046] Preparation of diazonium salt:
[0047] In a 500 mL four-necked bottle, add 70 mL of water and 54.7 g of m-aminoacetanilide hydrochloride. Stir, add an ice-water bath to cool to 0-5°C, add 31 mL of 25% NaOH aqueous solution, neutralize to pH 6.5, and stir until all raw materials are dissolved. Add 153 grams of 40% fluoboric acid aqueous solution and keep it at 0-5°C. Then slowly add 21 grams of NaNO 2 Solution dissolved in 30mL of water, the temperature in the bottle is controlled at 0-5°C, the dropwise addition time is about 25 minutes, after the dropwise addition is completed, react at 0-5°C for another 3 hours, and the diazotization reaction is completed.
[0048] Then in the same manner as in Example 1, 56.6 grams of m-acetamidoaniline diazonium fluoroborate were obtained.
[0049] 2. Thermal decomposition and hydrolysis of diazonium salt
[0050] Put 20 grams of the above-mentioned diazonium salt into a 500mL four-neck bottle, heat and decompose carefully, rais...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com