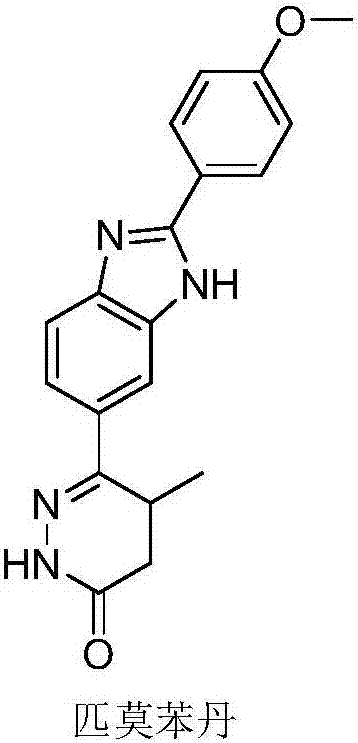
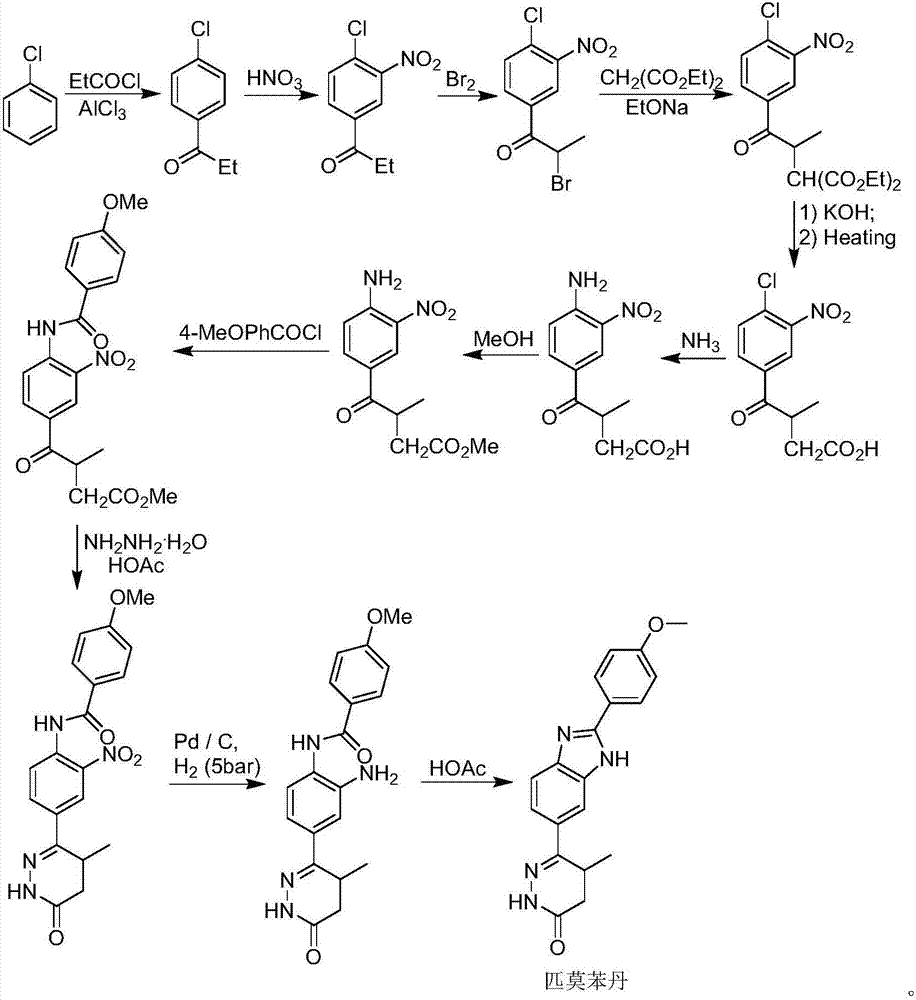
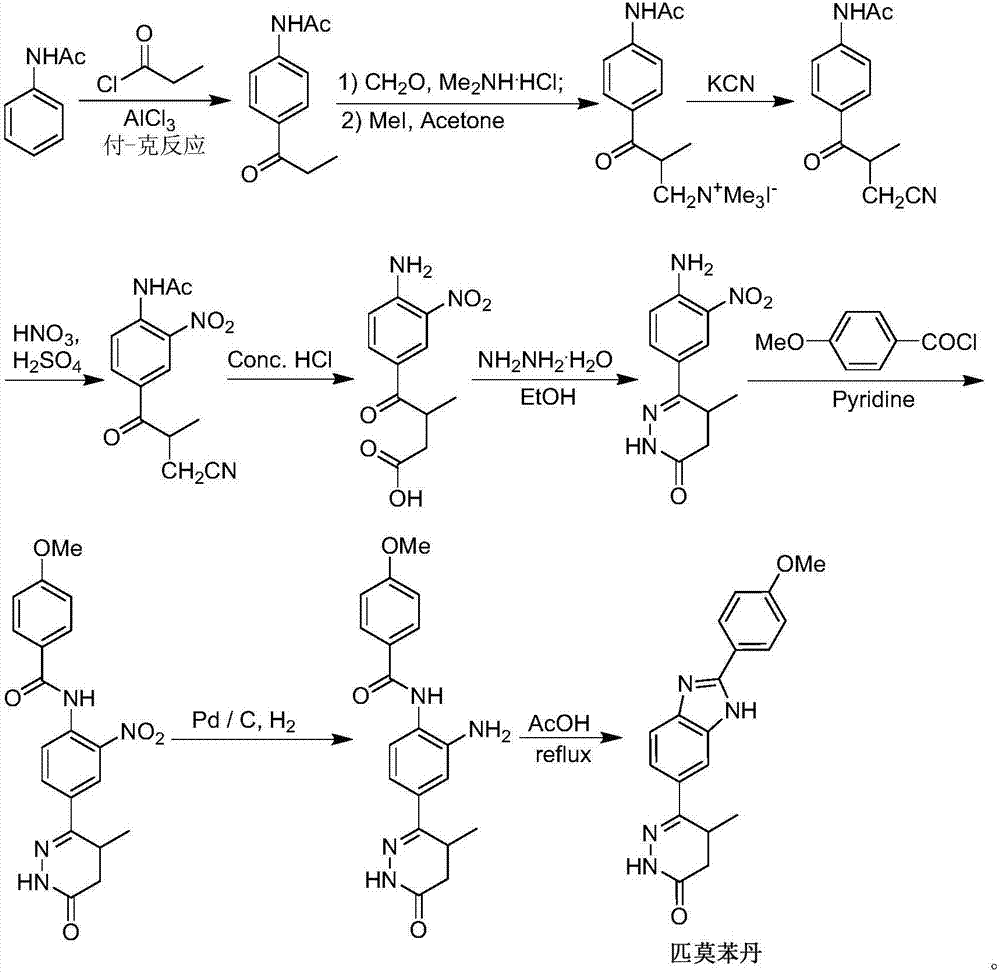
Preparation of pimobendan
A technology for the preparation of pimobendan, which is applied in the field of preparation of pimobendan, can solve the problems of being unsuitable for industrial scale-up production, and achieve the effects of short steps, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The formula III compound (R 1 =Me) The crude product (262g) was dissolved in anhydrous toluene (2000mL), the temperature of the system was cooled to 0-5°C in an ice-salt bath, and POCl was slowly added dropwise through the dropping funnel under nitrogen protection 3 (450mL), after the addition was complete, the system was slowly heated to reflux for 2 hours until the TLC spot plate tracked the disappearance of the compound of formula III. Slowly lower the temperature of the system to about 50°C, then carefully remove the solvent and unreacted POCl under reduced pressure 3 . CH was added to the residue 2 Cl 2 (1000mL) and H 2 O (500mL), after stirring for 10min, the system was carefully added saturated NaHCO 3 Adjust the pH of the system to about 6.5. Separate the organic phase and add CH to the aqueous phase 2 Cl 2 (2 x 500 mL) extraction. The organic phases were combined, washed twice with saturated brine (2×500 mL), dried over anhydrous sodium sulfate, filter...
Embodiment 5
[0052] Compound V (R1=Me) (95g, 231mmol) prepared in Example 5 was dissolved in EtOH (150mL), and then an aqueous solution of NaOH (37g NaOH dissolved in 520mL H2O, 4.0eq) was added to the system. After the addition was complete, the system was stirred overnight at room temperature. The ethanol solvent was carefully removed under reduced pressure in the system at 50°C, and the remaining aqueous solution was adjusted to pH 1.0-1.5 with 3M HCl. The system was extracted with EtOAc (3×500mL), the organic phase was washed twice with saturated brine (2×300mL), dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain compound VI (83.8g), without purification , used directly in the next step.
[0053] 8. Preparation of 4-(2-(4-methoxyphenyl)-1H-benzo[d]imidazol-6-yl)-3-methyl-4-oxobutanoic acid (formula VII)
Embodiment 7
[0054]Compound VI (83.8 g) prepared in Example 7 was dissolved in DMF (150 mL), and the system was slowly warmed up to 130-140° C., and kept for 3 hours for reaction. The system was cooled naturally, and then the organic solvent was removed under high vacuum with a rotary evaporator. The obtained residue was the compound of formula VII (72.1 g), which was directly used in the next reaction without purification.
[0055] 9. Preparation of Pimobendan
[0056] Ethanol EtOH (250 mL) and hydrazine hydrate (120 mL) were directly added to compound formula VII (72.1 g) prepared in Example 8, and the system was slowly heated to reflux for 12 hours after the addition was complete. The system was cooled naturally, and then the organic solvent and unreacted hydrazine hydrate were removed under high vacuum and reduced pressure. Add DMF (250mL) to the residue, slowly raise the temperature and stir until it dissolves, then slowly add H 2 O (250 mL), stirred for 1 hour after the dropwise a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com