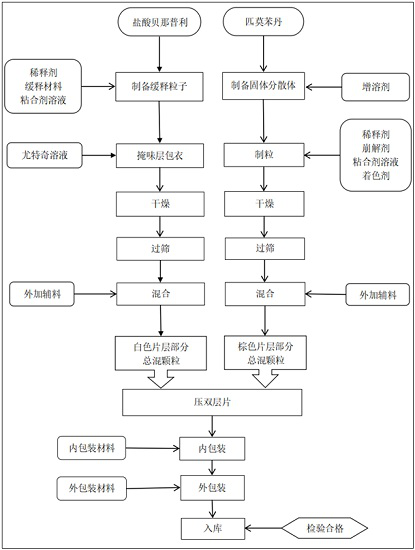
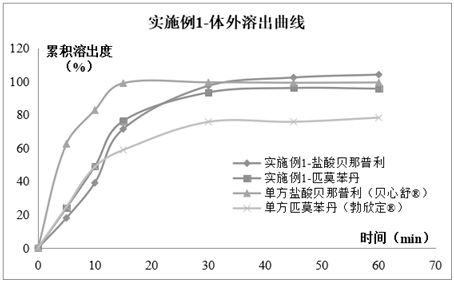
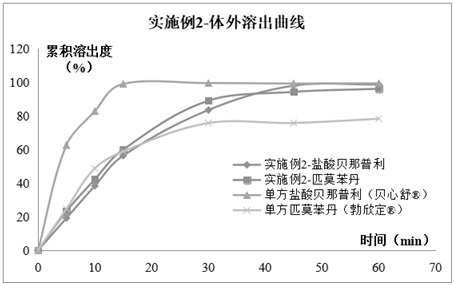
Compound pharmaceutical composition containing benazepril and pimobendan for pets and preparation method of compound pharmaceutical composition
A technology of pimobendan and benazepril hydrochloride, which can be used in drug combinations, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve problems such as exercise intolerance, fluid sensation, dyspnea, etc. , to achieve the effect of improving the survival rate, fast onset speed and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]A compound double-layer tablet containing benazepril and pimobendan for pets, comprising the following raw materials in weight percentage: benazepril hydrochloride 1.0%, pimobendan 0.5%, diluent 70.5%, sustained-release matrix 3 %, coating taste-masking material 10%, disintegrant 3%, binder 2%, solubilizer 5%, glidant 1%, lubricant 2%, flavoring agent 1% and pigment 1%.
[0054] In the present embodiment, the prescription adopted by the benazepril pimobendan hydrochloride tablet for pets is as follows:
[0055]
[0056] In this prescription, hypromellose is selected as the slow-release matrix.
[0057] The solubilizer is selected from succinic acid.
[0058] Eudragit E100 was selected as the taste-masking material for the coating.
[0059] The diluent is microcrystalline cellulose.
[0060] The disintegrating agent is selected crospovidone.
[0061] Adhesive is selected povidone for use.
[0062] The flavoring agent is beef flavor essence.
[0063] The glidant i...
Embodiment 2
[0086]
[0087] The preparation method of the pharmaceutical composition in embodiment 2 is the same as embodiment 1.
Embodiment 3
[0089]
[0090] The preparation method of the pharmaceutical composition in embodiment 3 is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com