Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Benazepril Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
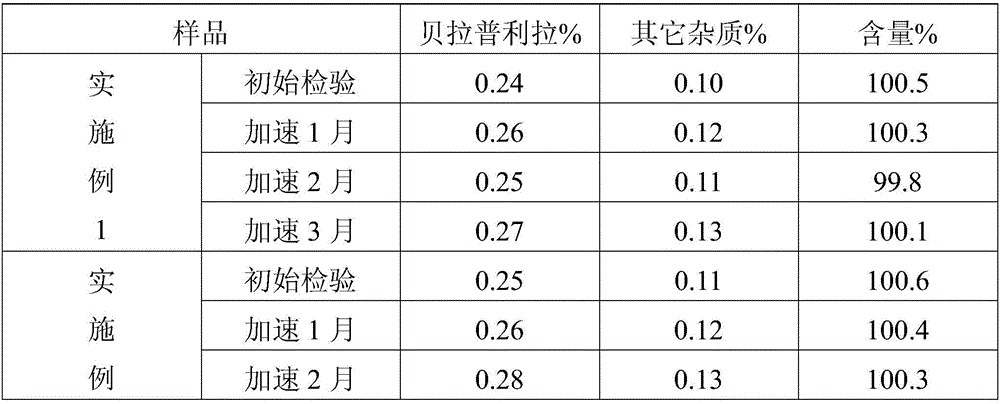
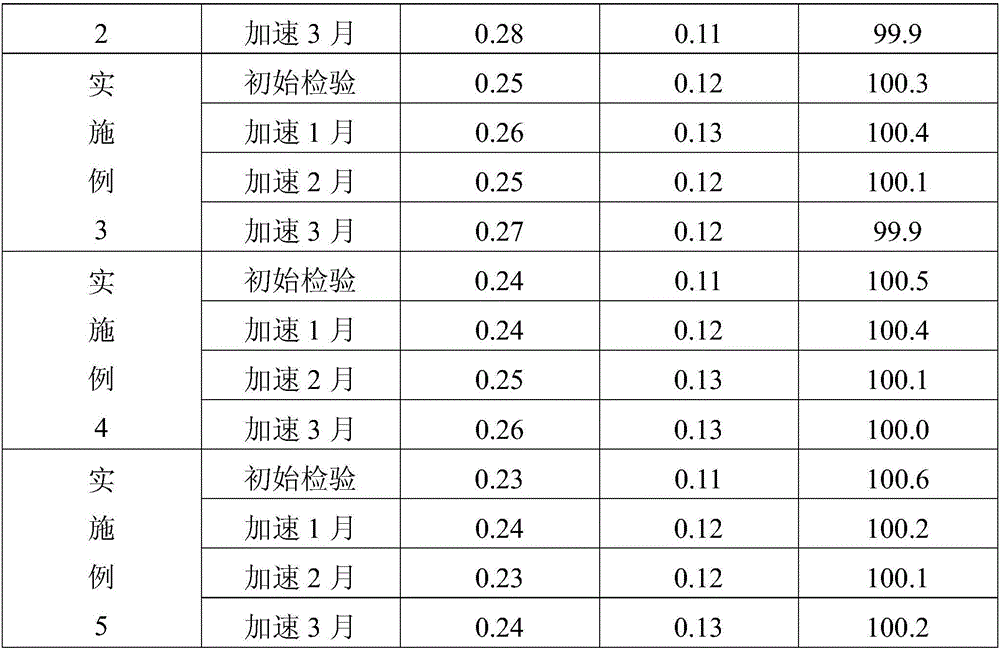
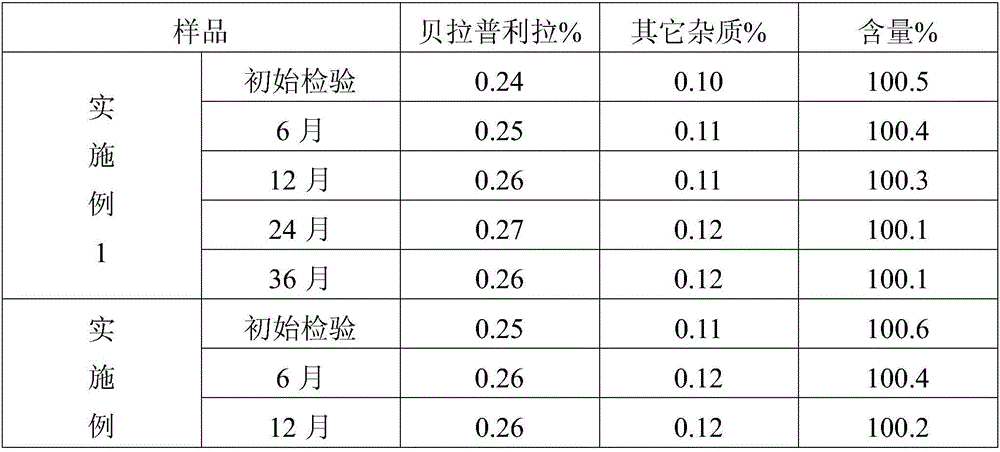
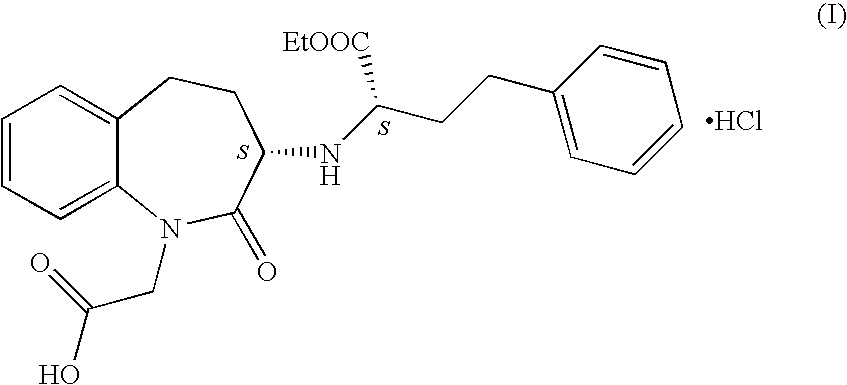
The hydrochloride salt of benazepril, a carboxyl-containing angiotensin-converting enzyme (ACE) inhibitor with antihypertensive activity. As a prodrug, benazepril is metabolized to its active form benazeprilat. Benazeprilat competitively binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This prevents the potent vasoconstrictive actions of angiotensin II, resulting in vasodilation. Benazeprilat also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow.
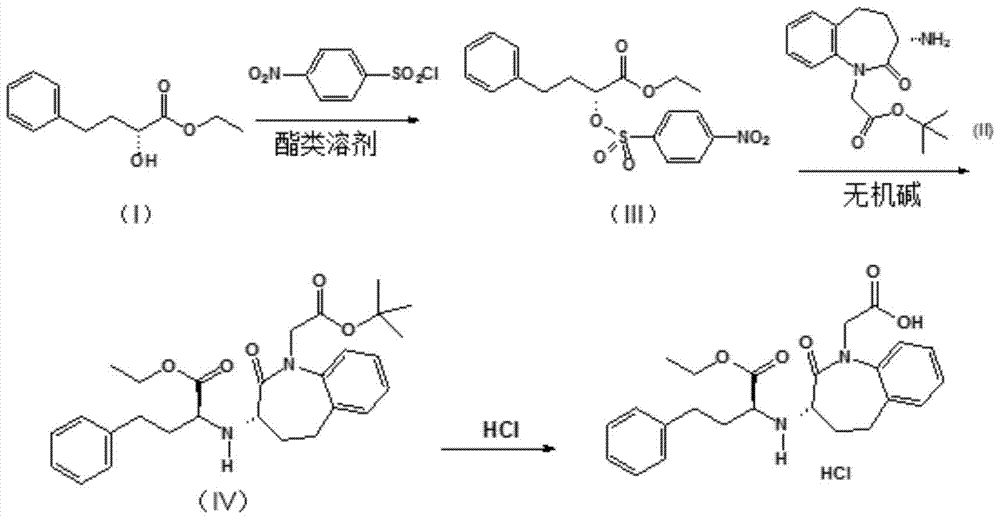
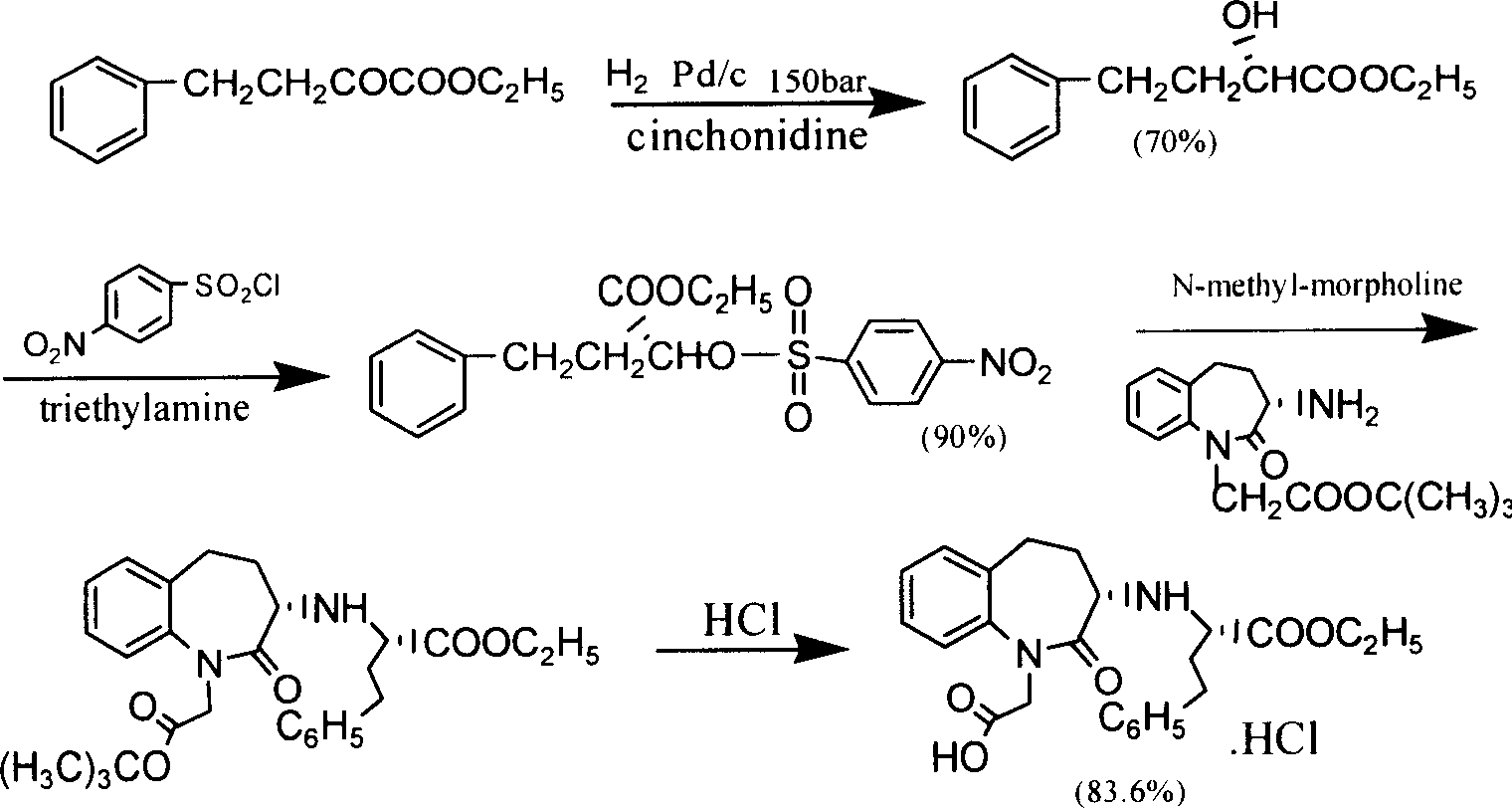
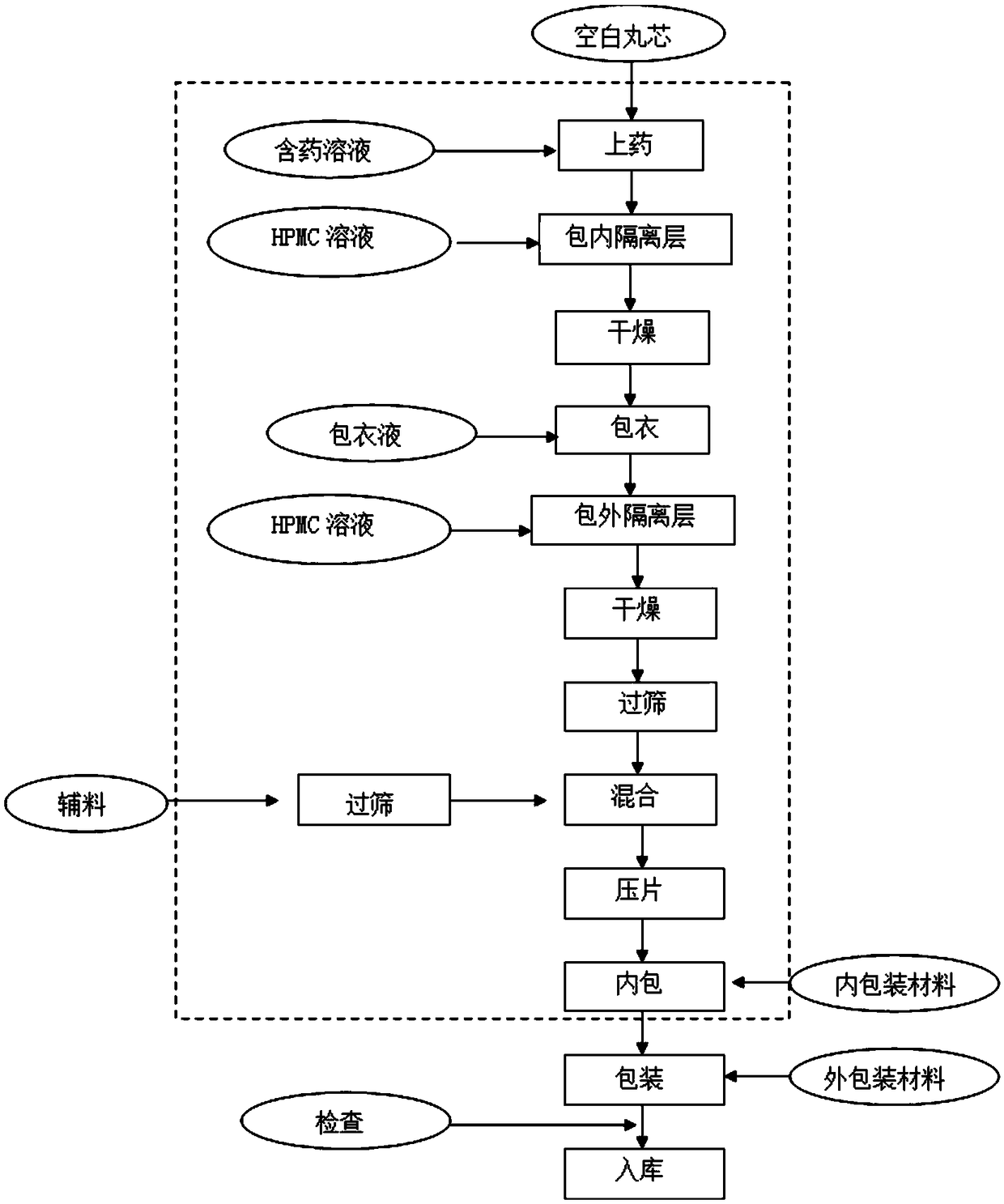
Process for preparing Benazepril hydrochloride materials
InactiveCN1844102AMild reaction conditionsThree wastes are easy to solveOrganic chemistryHeterocyclic compound active ingredientsAcetic acidBromine
The invention relates to a new method for preparation of benazepril hydrochloride, using bromobenzoaminocaprolactam as initial point, comprising synthesizing intermediates 4,5-dihydro-3-phthalimide-1H-1-benzazepin-2- (3H)-ketone, 2,3,4,5-tetrahydro-2-oxo-3-phthalimide-1H-1- benzazepin-1-acetic acid tertbutyl ester, 3- amidol-2, 3,4,5-tetrahydro-2-oxo-1H-1-benzazepin-1-acetic acid tertbutyl ester, (3S)-3-amidol-2, 3,4,5-tetrahydro-2-oxo-1H-1-benzazepin-1-acetic acid tertbutyl ester, (3S)-3-amidol-2, 3,4,5-tetrahydro-2-oxo-1H-1-benzazepin-1-acetate and finally getting benazepril hydrochloride, this route don't need special reactant, the reaction condition is temperate, the needed raw materials are all sold in domination, three wastes are easy to be resolved, the getted product is in conformity with the requirements by construction clear proof and is suit to industrial production.
Owner:大道隆达(北京)医药科技发展有限公司
Pharmaceutical composition using hydrochlorothiazide and benazepril hydrochloride as active ingredients, its preparation method and use
This invention is about a medicine composition taking hydrochlorothiazide and hydrochloric acid benapuli as active component and its preparing method and usages. It takes hydrochlorothiazide and hydrochloric acid benapuli as medicine active component, and is formed by mixing acceptable finding. It can be used to cure miscellaneous tapping hypertensions. It uses hydrochlorothiazide and hydrochloric acid benapuli as raw materials and adds some findings those have specified kinds and ratios. Developing troche, capsule, dispersing tablets, soft capsule, chewing tablets, buccal tablets, droplet, and other oral preparations according to the method in this invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Benazepril hydrochloride tablets for cats and dogs and preparation method for benazepril hydrochloride tablets
InactiveCN102525973AEasy to acceptImprove conveniencePill deliveryPharmaceutical non-active ingredientsMagnesium stearateLactose
The invention discloses benazepril hydrochloride tablets for cats and dogs and a preparation method for the benazepril hydrochloride tablets. The benazepril hydrochloride tablets consist of the following components in percentage by mass: 0.1 to 2 percent of benazepril, 30 to 70 percent of lactose, 10 to 50 percent of microcrystalline cellulose, 0.5 to 10 percent of hydroxypropyl cellulose, 0.5 to 9 percent of cross-linked polyvinylpyrrolidone, 1 to 10 percent of polyvinylpyrrolidone K30, 0.1 to 1 percent of aspartame and 0.2 to 1.5 percent of magnesium stearate. After being taken, the tablets can be quickly disintegrated, and active ingredients can be quickly dissolved; and the tablets have high palatability, and small irritation on intestines and stomach.
Owner:NANJING SBEED BIOTECH
Preparation method of benazepril hydrochloride tablets
ActiveCN102697749AGuaranteed liquidityGuaranteed anti-stickPill deliveryCardiovascular disorderMagnesium stearateStearic acid
The invention provides a preparation method of benazepril hydrochloride tablets. Benazepril hydrochloride which serves as an active component of the medicament is combined with auxiliaries filler, adhesive, disintegrating agent, lubricant magnesium stearate and a coating material to prepare particles, and the particles are made into tablets. The method is characterized in that the lubricant magnesium stearate is added after other components are granulated and coated, and the components are uniformly mixed and tableted. The preparation method can physically isolate benazepril hydrochloride from the magnesium stearate effectively, so that the problem that the variety over rises in the storage process, and the medicament quality is effectively controlled. The preparation method has high use value.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Benazepril hydrochloride soft chewable tablet and preparation method thereof
ActiveCN105963270AImprove palatabilitySolve the problem of difficult dissolution and disintegrationPill deliveryGranular deliveryPreservativeDissolution
A benazepril hydrochloride soft chewable tablet is prepared from, by weight, 1-5% of benazepril hydrochloride, 2-15% of hydrophobic excipient, 20-60% of a filling agent, 2-10% of a disintegrant, 10-40% of a corrigent, 0.2-1% of a preservative, 2-10% of a wetting agent and 0.01-0.3% of a coating material. Benazepril hydrochloride is used as an active ingredient of the chewable tablet, coated and then mixed with the hydrophobic excipient, the filling agent, the disintegrant and the corrigent, the preservative and the wetting agent are added, mixing is performed well, and a mixture is extruded. The benazepril hydrochloride soft chewable tablet is high in palatability and little in irritation to stomach and intestines when being taken, can quickly disintegrate and is quick in dissolution of the active ingredient and stable in quality.
Owner:QILU ANIMAL HEALTH PROD
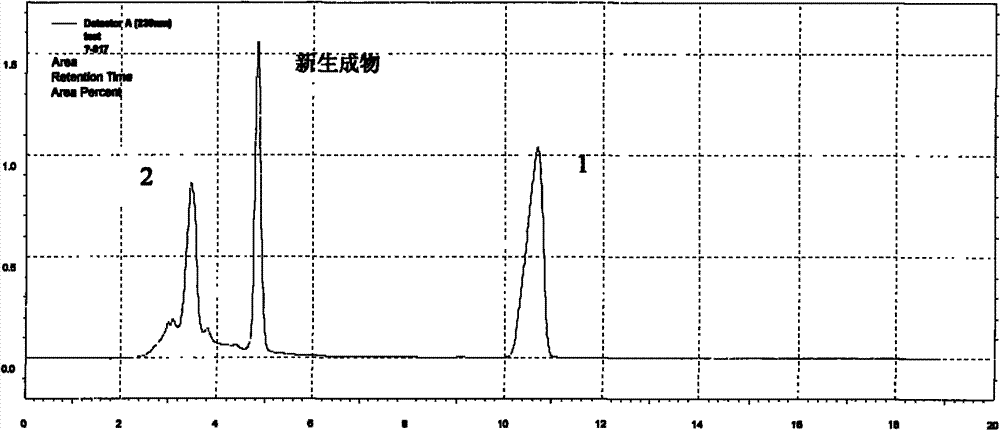
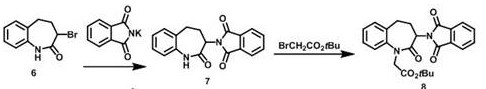
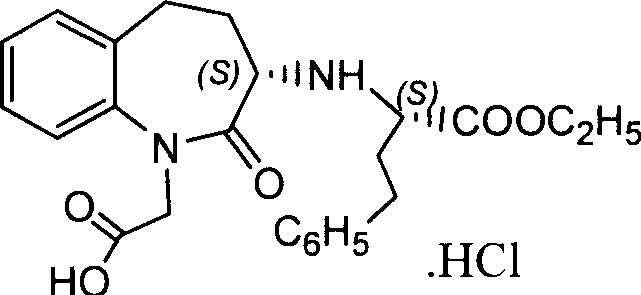
Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine
The invention relates to a preparation method of a key intermediate of a medicine benazepril hydrochloride, that is, 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine. 3-bromo benzo caprolactam and (S)-1-phenylethylamine are used as raw materials; an object product is obtained by amination and a solvent-free configuration transformation reaction; the preparation method has low production cost and simple operations, and has industrial production value.
Owner:INSIGHT HIGH TECH JIANGSU CO LTD
Benazepril hydrochloride chewable tablet for pet and preparation method of benazepril hydrochloride chewable tablet
InactiveCN108969496AImprove complianceEasy maintenanceInorganic non-active ingredientsUrinary disorderAdhesiveDissolution
The invention relates to a benazepril hydrochloride chewable tablet for a pet. The benazepril hydrochloride chewable tablet for the pet is prepared from the following raw materials in percentage by weight: 2.0-6.0 percent of benazepril hydrochloride, 2-5 percent of an adhesive, 30-60 percent of a diluent, 1.5-2.5 percent of a disintegrant, 2.5-6.5 percent of a glidant, 9-16 percent of a corrigentand 20-47 percent of coating smell masking material. The chewable tablet provided by the invention can mask smell without influencing dissolution. A benazepril hydrochloride drug composition preparedwith the method keeps all effective components of the raw materials, greatly improves the compliance after being orally taken by adding the diluent and an embedding skeleton and reaches better maintenance effect without generating larger stimulation to gastrointestinal tract.
Owner:河北远征药业有限公司
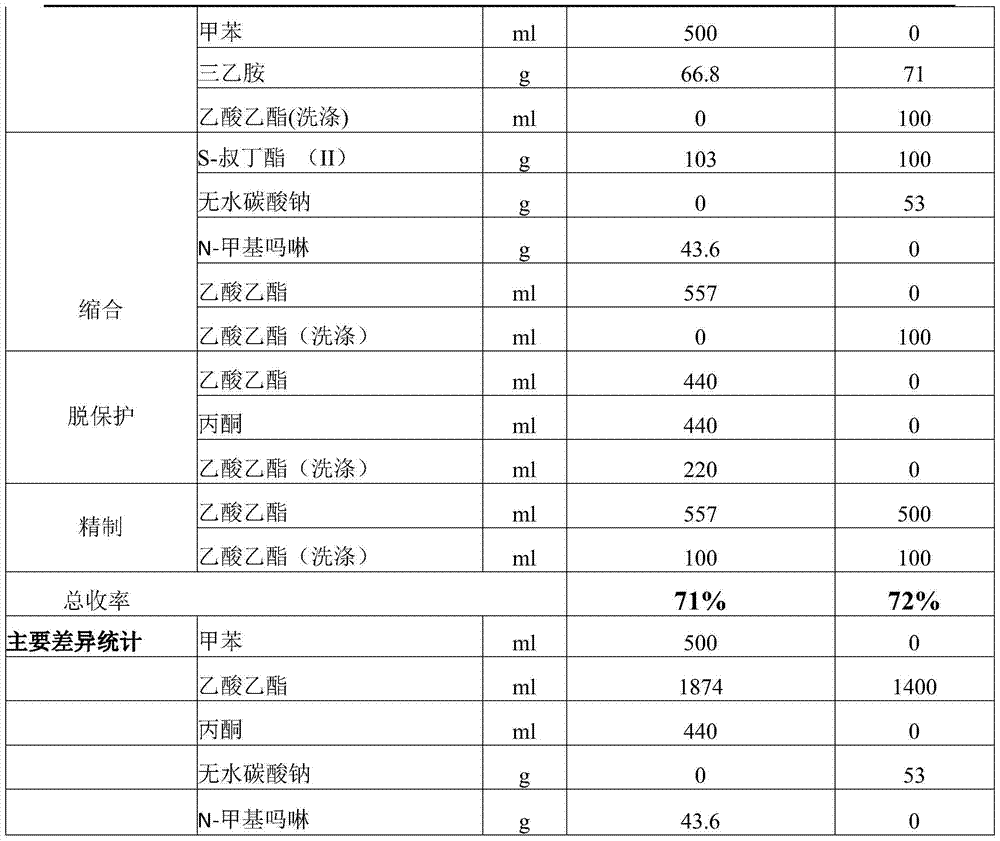
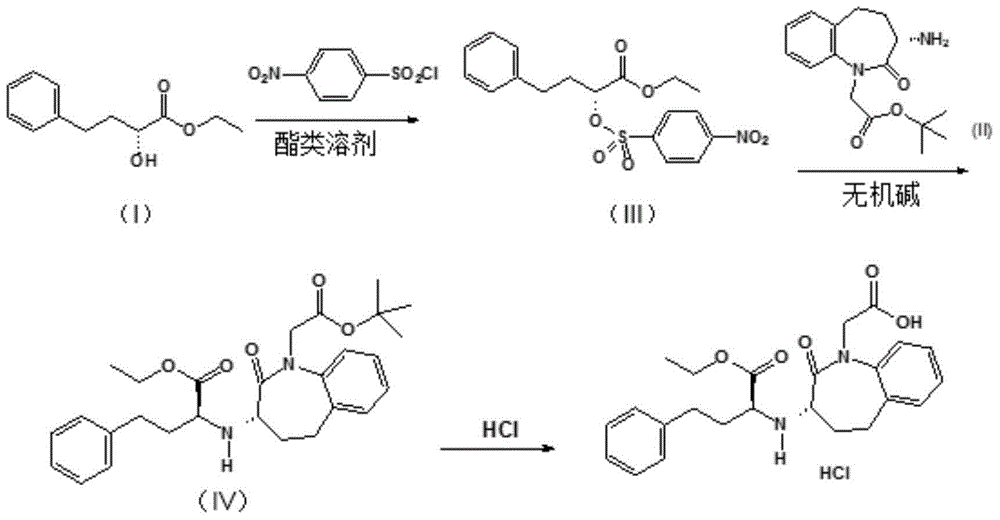
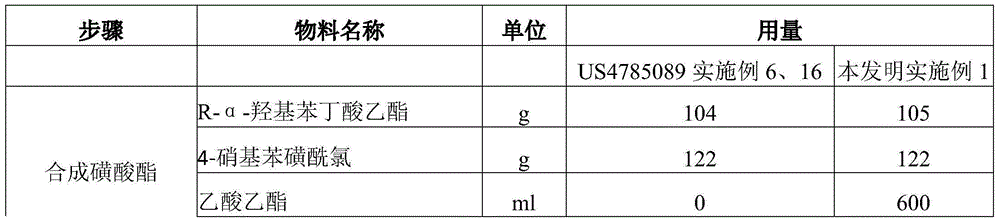
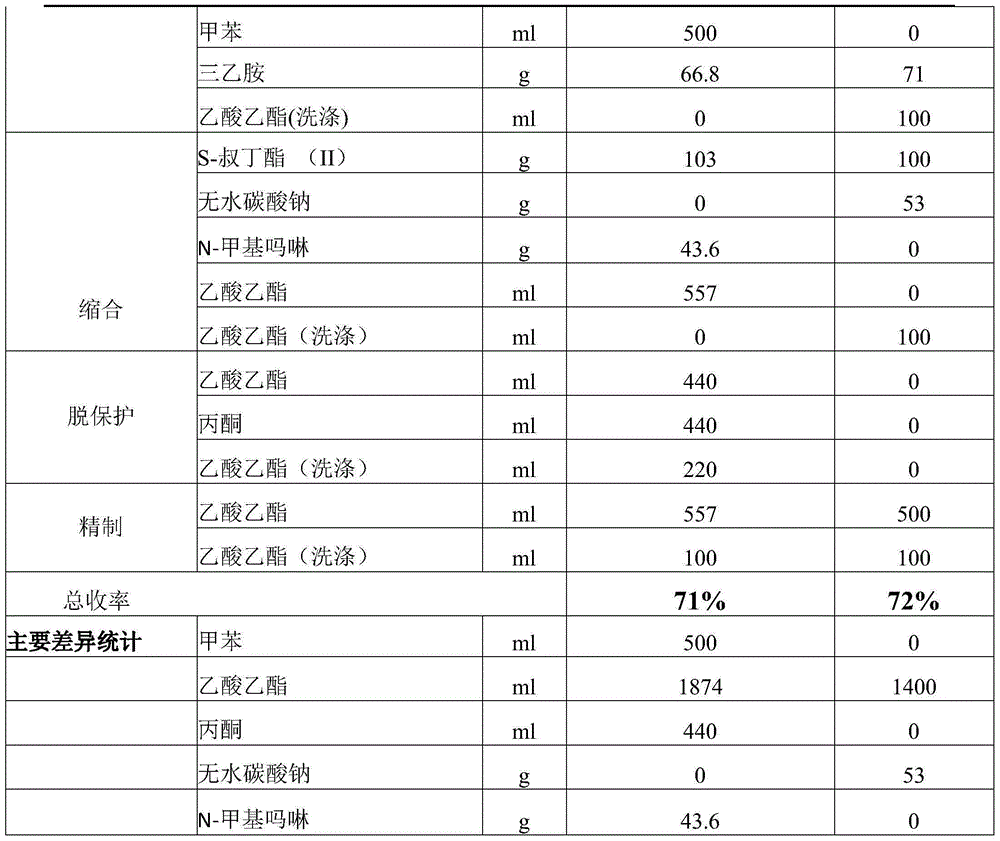
Improved preparation method of benazepril hydrochloride and pharmaceutical composition containing benazepril hydrochloride
ActiveCN105061312AReduce dosageLow costOrganic chemistryHeterocyclic compound active ingredientsMedicinal chemistryBenazepril Hydrochloride
The invention discloses an improved preparation method of benazepril hydrochloride and pharmaceutical composition containing the benazepril hydrochloride. With the adoption of the preparation method, the safety is high, the cost is low, the clean production value is high, industrial production is easy to realize, and meanwhile, the pharmaceutical composition is easy to prepare and use.
Owner:HUIZHOU XINLITAI PHARMA +2
Benazepril hydrochloride-hyacinth oil nanoemulsion antihypertensive drug
InactiveCN102727721AGood blood pressure effectStable buckPowder deliverySolution deliveryAdditive ingredientHalf-life
The invention discloses a benazepril hydrochloride-hyacinth oil nanoemulsion antihypertensive drug. Raw materials for the drug comprise, by mass percentage, 1% to 18% of benazepril hydrochloride, 15% to 40% of surfactant, 0 to 20% of cosurfactant and 1% to 20% hyacinth oil, with the balance being distilled water, wherein the sum of the mass percentage of the raw materials is 100%. Nanoemulsion provided in the invention has small and uniformly-distributed emulsion droplet particles, low viscosity and good fluidity. In the dosage form of nanoemulsion, the water-soluble drug benazepril hydrochloride and fat-soluble hyacinth oil are organically combined together, which improves the solubility and permeability of hyacinth oil and the stability and efficacy of benazepril hydrochloride. When the dosage form of nanoemulsion is prepared from benazepril hydrochloride and hyacinth oil, the advantages of both benazepril hydrochloride and hyacinth oil are combined; therefore, an antihypertensive effect of the drug provided in the invention is obviously improved, the half-life of the drug is prolonged, and administration frequency is reduced.
Owner:NORTHWEST A & F UNIV
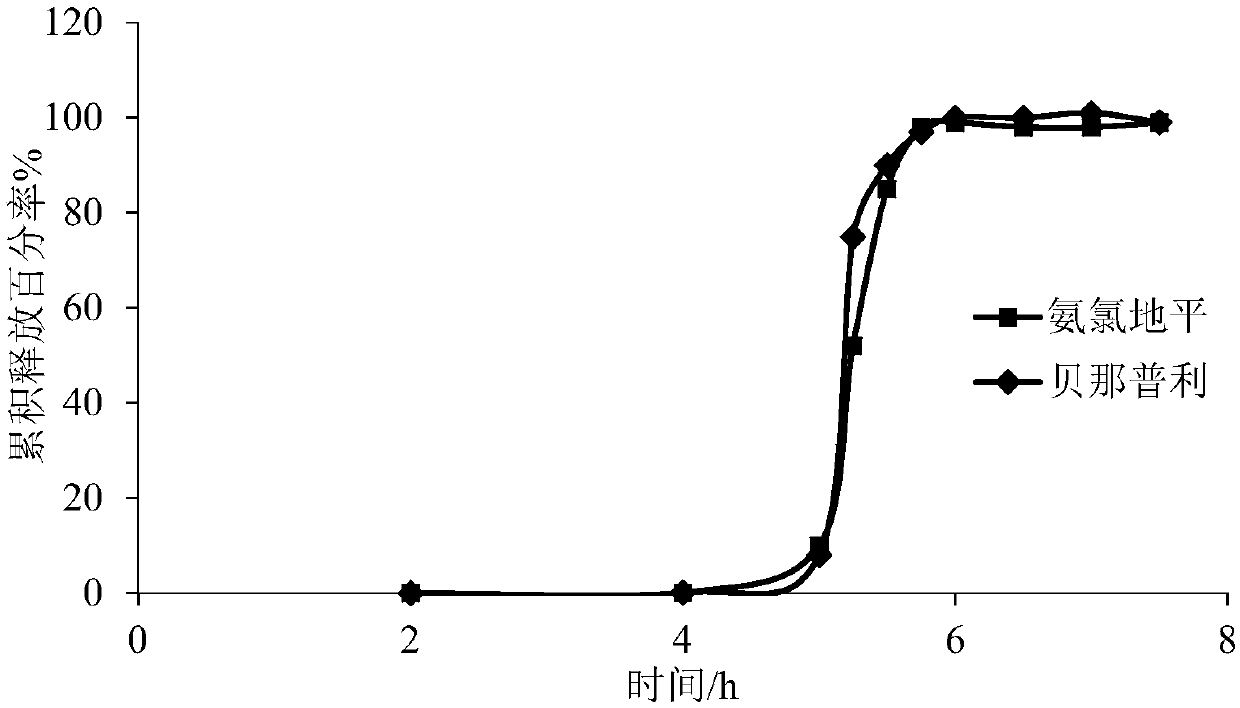
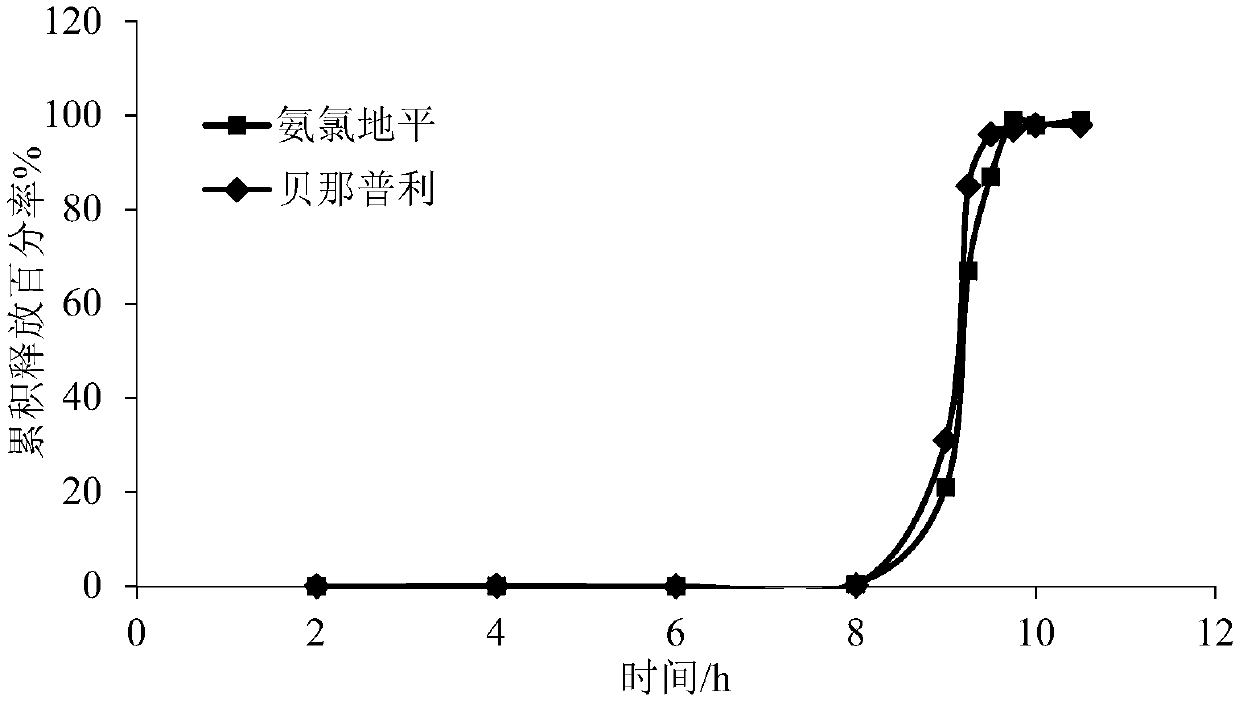
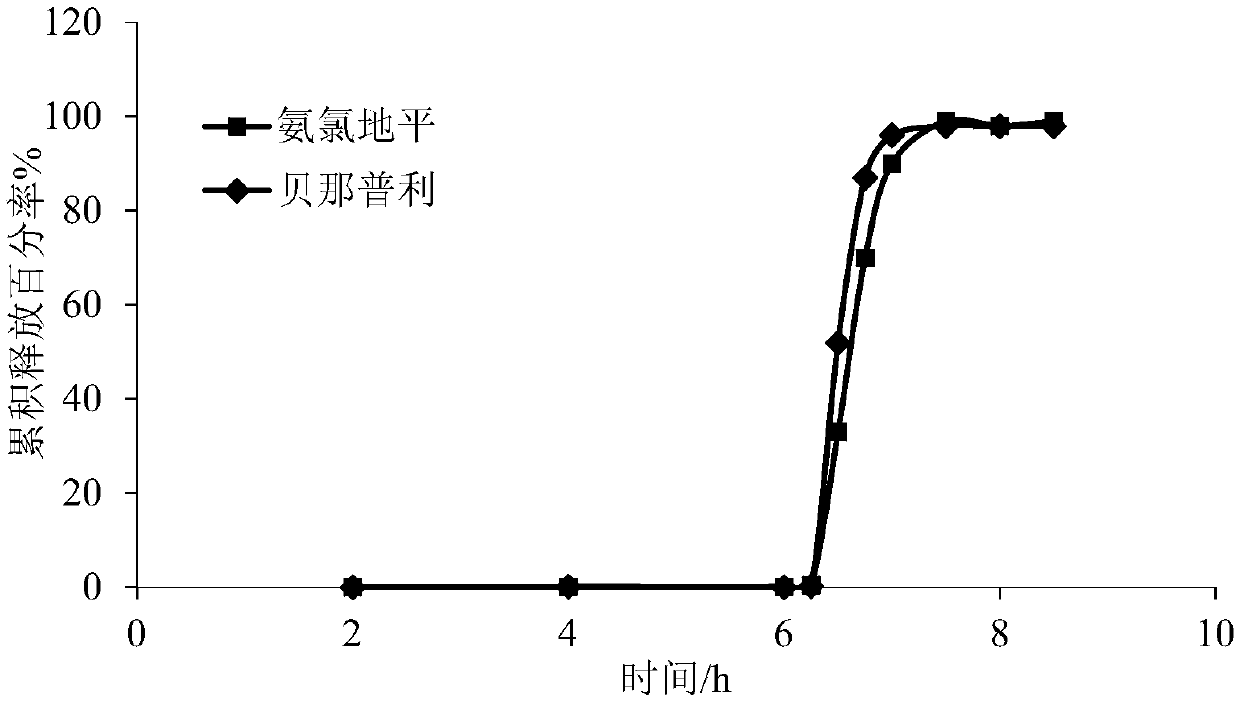
Amlodipine benazepril impulse tablet and preparation method thereof
InactiveCN109674794AGood treatment effectImprove medication compliancePharmaceutical non-active ingredientsCoatingsControl releaseTime lag
The invention discloses an amlodipine benazepril impulse tablet and a preparation method thereof. The amlodipine benazepril impulse tablet comprises a rapid-release tablet core and a controlled-release coating layer, wherein the rapid-release tablet core is prepared from a main drug and a tablet core auxiliary material, and the main drug is prepared from amlodipine besylate and benazepril hydrochloride. The preparation method comprises the following steps that (1) after the main drug and the tablet core auxiliary material are uniformly mixed, the rapid-release tablet core is prepared by adopting a direct powder compression method; (2) optionally, a swelling coating layer is formed on the rapid-release tablet core prepared in the step (1); and (3), the controlled-release coating layer is formed on the rapid-release tablet core prepared in the step (1) or the swelling coating layer prepared in the step (2) so as to obtain the amlodipine benazepril impulse tablet. According to the amlodipine benazepril impulse tablet and the preparation method thereof, the drug release time lag of the amlodipine benazepril impulse tablet can reach 1-10 h, the drug is completely and rapidly released, the drug release time lag accords with the pathogenetic time rule of hypertension and adverse reaction thereof, and the compliance of a patient is better; and in addition, a preparation process is simple, and relatively good industrial application values are achieved.
Owner:SHANGHAI SUNTECH PHARMA
Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine
The invention relates to a preparation method of a key intermediate of a medicine benazepril hydrochloride, that is, 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine. 3-bromo benzo caprolactam and (S)-1-phenylethylamine are used as raw materials; an object product is obtained by amination and a solvent-free configuration transformation reaction; the preparation method has low production cost and simple operations, and has industrial production value.
Owner:INSIGHT HIGH TECH JIANGSU CO LTD
Amlodipine benazepril pharmaceutical composition
ActiveCN102440993BImprove dispersion uniformityHelp releasePill deliveryPharmaceutical non-active ingredientsAmlodipine besilateSilica gel
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Benazepril hydrochloride tablets and preparation method thereof
InactiveCN106822012AAccelerate the degradation rateImprove stabilityPharmaceutical non-active ingredientsPill deliveryChemical reactionMagnesium stearate
The invention discloses benazepril hydrochloride tablets and a preparation method thereof and belongs to the technical field of pharmaceutical preparations. The benazepril hydrochloride tablets are prepared from the following components in parts by weight: 1.02 parts of benazepril hydrochloride, 3 to 5 parts of beta-cyclodextrin, 10 to 15 parts of microcrystalline cellulose, 0.6 to 1.8 parts of corn starch and 0.1 to 0.3 part of magnesium stearate. According to the benazepril hydrochloride tablets, the benazepril hydrochloride is subjected to inclusion protection by adopting a cyclodextrin inclusion technology, so that a condition that the acidic benazepril hydrochloride is in direct contact with the alkaline magnesium stearate is avoided; the problem that the content of benazeprilat and other impurities is improved, caused by the fact that the benazepril hydrochloride and the magnesium stearate have slow chemical reaction, is effectively avoided.
Owner:HUAYI PHARMA ANHUI CO LTD
Amlodipine benazepril tablet and preparation method thereof
ActiveCN102440993AImprove dispersion uniformityHelp releasePill deliveryPharmaceutical non-active ingredientsActive componentAmlodipine besilate
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Benazepril hydrochloride composition with effect of reducing blood pressure and preparation method thereof
The invention discloses a benazepril hydrochloride composition with effect of reducing blood pressure and a preparation method thereof. The preparation process comprises a solid fermentation method and a liquid fermentation method. Aspergillus CICC 2436 produces enzyme to hydrolyze benazepril hydrochloride, wherein the structure of part of benazepril hydrochloride is changed, namely an analog of benazepril hydrochloride. The original benazepril hydrochloride forms a composition containing the benazepril hydrochloride and the analog of the benazepril hydrochloride under the action of the enzyme, and the level of reducing the blood pressure by using the composition is superior to that of the benazepril hydrochloride with the same concentration.
Owner:BEIJING WBL PEKING UNIV BIOTECH
A kind of improved preparation method of benazepril hydrochloride and pharmaceutical composition containing the benazepril hydrochloride
ActiveCN105061312BReduce dosageLow costOrganic chemistryCardiovascular disorderImproved methodCleaner production
The invention discloses an improved preparation method of benazepril hydrochloride and pharmaceutical composition containing the benazepril hydrochloride. With the adoption of the preparation method, the safety is high, the cost is low, the clean production value is high, industrial production is easy to realize, and meanwhile, the pharmaceutical composition is easy to prepare and use.
Owner:HUIZHOU XINLITAI PHARMA +2
Compound preparation containing benidipine hydrochloride and benazepril hydrochloride, as well as application thereof
InactiveCN102389431AImprove antihypertensive effectLower doseHeterocyclic compound active ingredientsCardiovascular disorderRenal HypertensionsSide effect
The invention discloses a compound preparation containing benidipine hydrochloride and benazepril hydrochloride, as well as application thereof, wherein preparation containing benidipine hydrochloride and benazepril hydrochloride serve as effective components of the medicament. According to the compound preparation, the defects of large dose and high side effect of single drug are overcome, and the compound preparation disclosed by the invention is obtained by compounding the benidipine hydrochloride and benazepril hydrochloride. Oral preparations with different forms can be prepared by the preparation, due to synergic and complementary effects of medicaments, the compound preparation has reinforced treatment effect, the medicament dose is reduced in clinical application, adverse reaction of a patient is lightened, and the compound preparation can be accepted easily. The compound preparation has remarkable treatment effect in mild and severe hypertension, and is suitable for hypertension patient with cardiovascular remodeling, and patients with renal hypertension, hypertension with renal damage or with diabetic renal damage. Furthermore, the compound preparation has low price, simple preparation and easy popularization and application.
Owner:济南龙华医药技术有限公司
Medicine composition for treating congestive heart-failure and tablet and preparation method thereof
InactiveCN107970255AEffective treatmentGood curative effectPill deliveryCardiovascular disorderAngiotensin-converting enzymeCurative effect
The invention discloses a medicine composition for treating congestive heart-failure and a tablet and a preparation method thereof. The medicine composition is prepared from the following two components of benazepril hydrochloride and hyaluronic acid; the tablet is prepared from the medicine composition and auxiliary materials by a powder tabletting technology. The medicine composition has the advantages that the benazepril hydrochloride and the hyaluronic acid are jointly used, so that the effect of treating the congestive heart-failure of pet is better, the synergistic function is realized,and the angiotensin converting enzyme can be quickly and well inhibited; the concentration of angiotensin converting in blood plasma is quickly decreased, and the function of treating the heart failure, hypertension and the like is realized; the clinical effect is obvious, the adverse reaction is little, and the effect of treating the heart disease of the pet is effective.
Owner:QINGDAO AGRI UNIV
The preparation method of benazepril hydrochloride tablet
ActiveCN102697749BGuaranteed liquidityControl growth ratePill deliveryCardiovascular disorderCaptoprilBULK ACTIVE INGREDIENT
The present invention provides a kind of preparation method of benazepril hydrochloride tablet, by benazepril hydrochloride as the active ingredient of medicine, and pharmaceutical adjuvant filler, binding agent, disintegrant, lubricant magnesium stearate, The coating materials are combined into granules and made into tablets, which are characterized in that the lubricant magnesium stearate is added after the other components are granulated and coated, mixed and pressed into tablets. The invention can effectively physically separate the benazepril hydrochloride from the magnesium stearate, solves the problem that the related impurities of the variety rise too fast during storage, effectively controls the quality of the medicine, and has high use value.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Benazepril hydrochloride dropping balls and their production
InactiveCN1981767AIncrease the way of administrationImprove stabilityPill deliveryHeterocyclic compound active ingredientsMedicinePharmacology
A dripping pill of benazepril hydrochloride for treating hypertension and heart failure is prepared from benazepril hydrochloride and matrix. Its preparing process is also disclosed.
Owner:陈茜
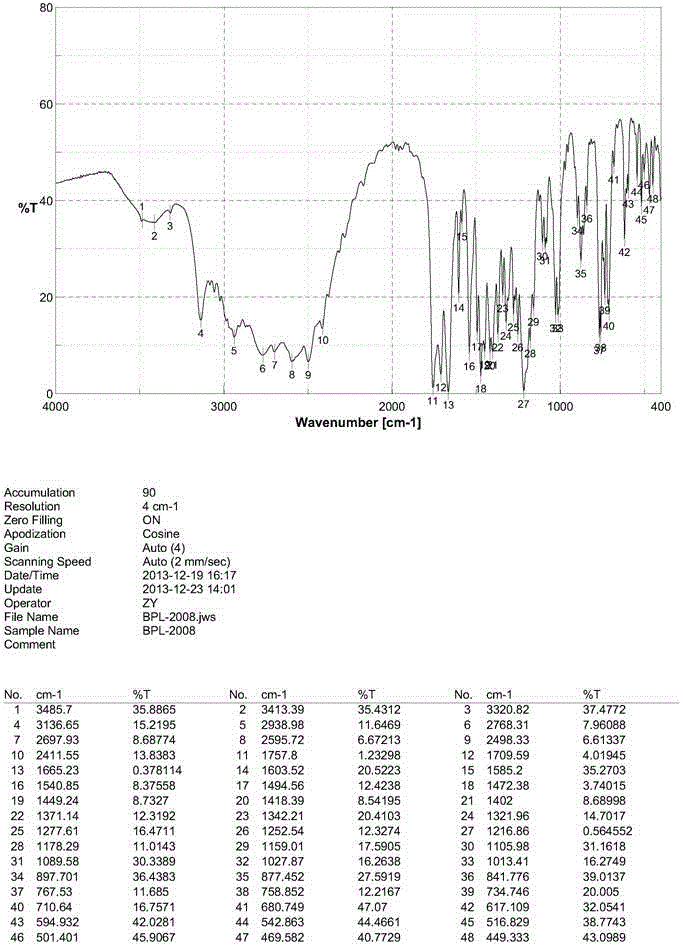
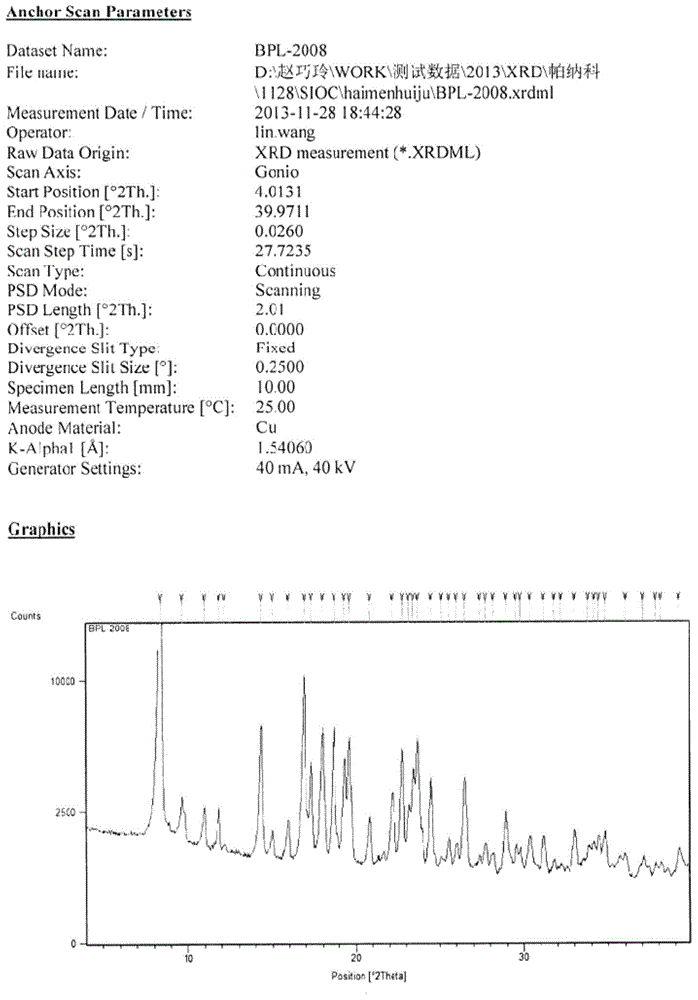
New crystal form of benazepril hydrochloride and preparation method thereof
InactiveCN104788376AOrganic chemistry methodsBulk chemical productionPharmaceutical drugBioavailability
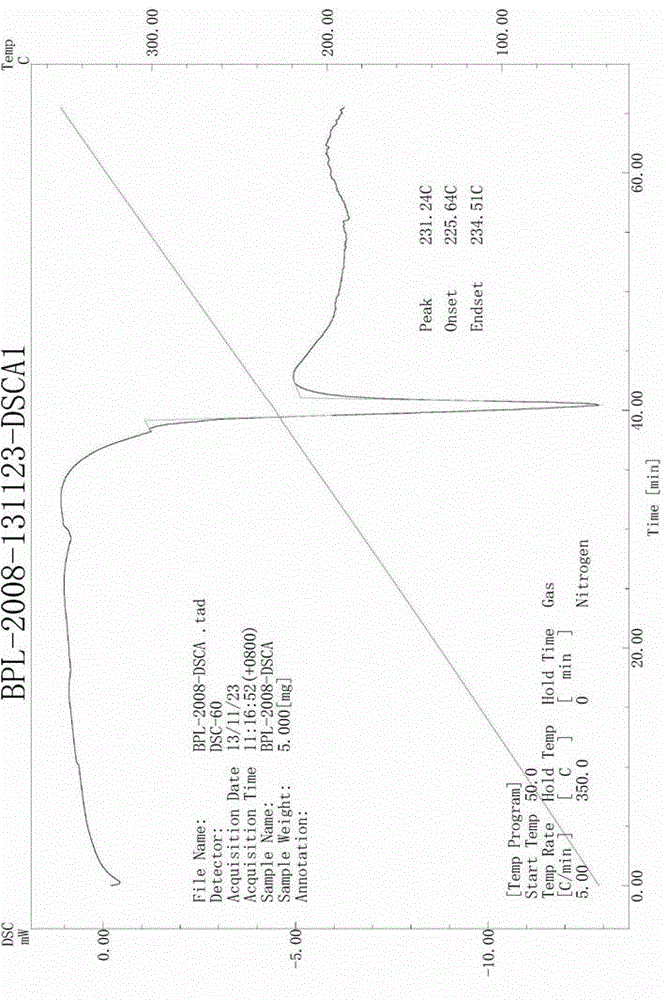
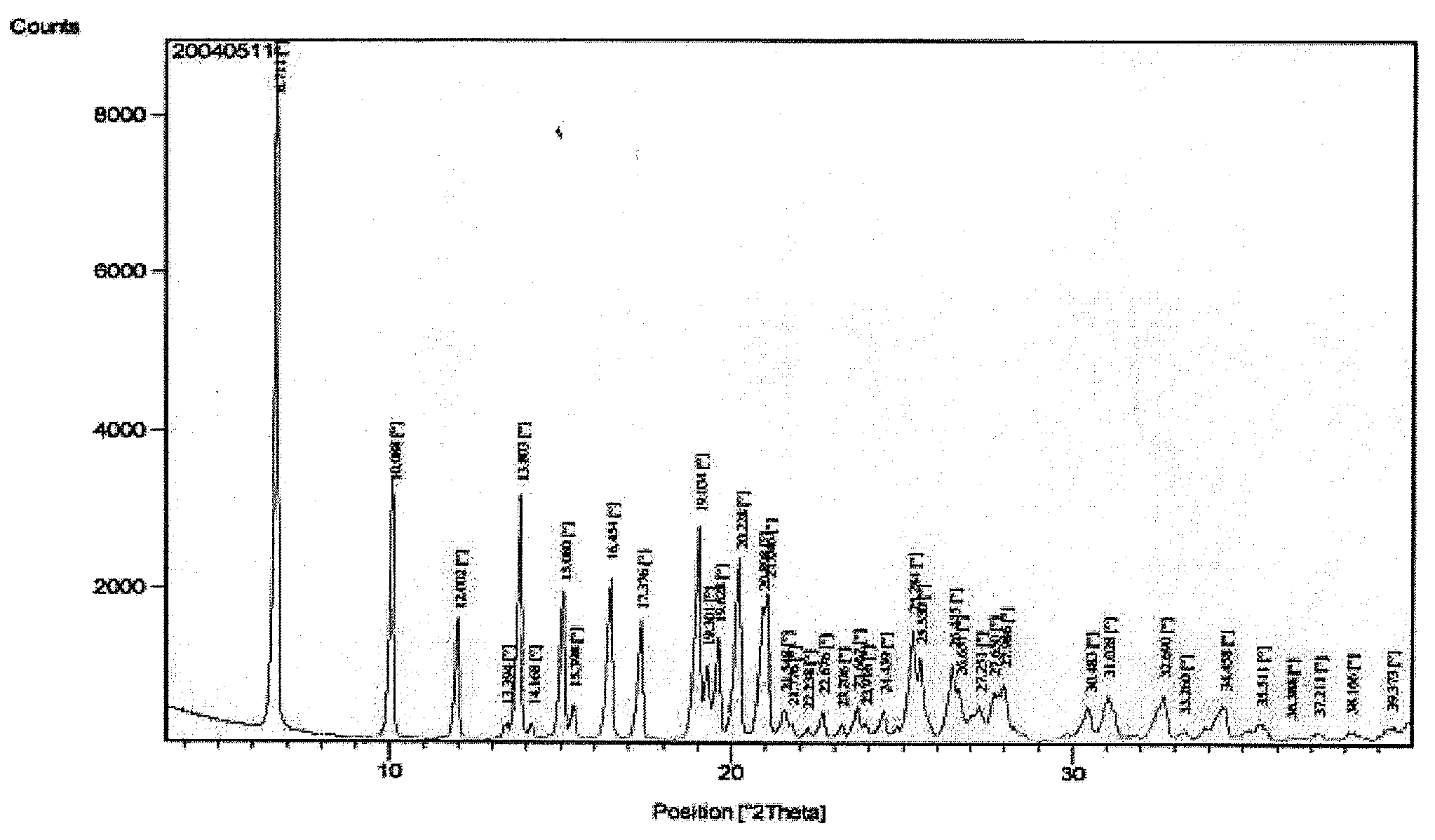
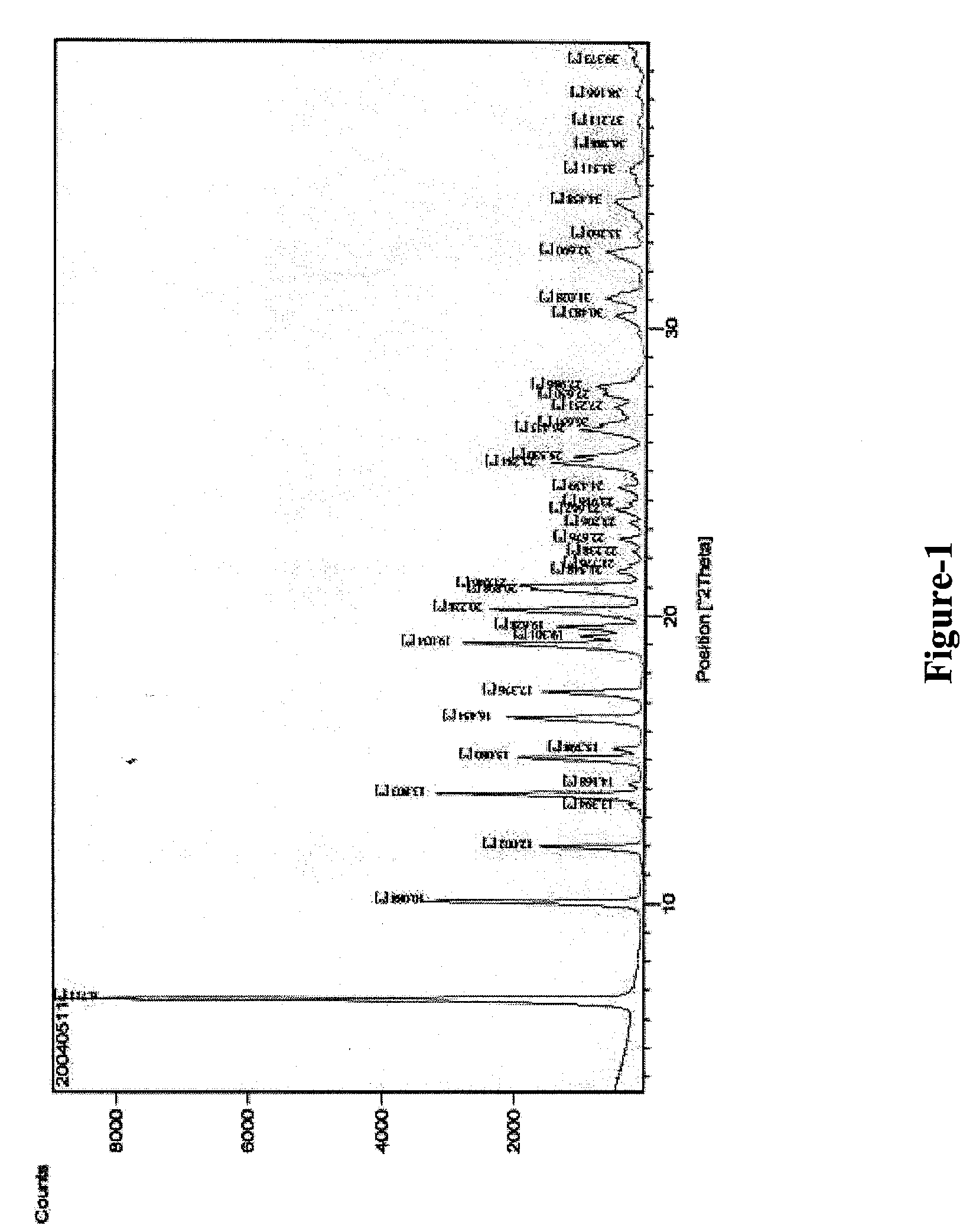
The invention relates to a new crystal form of benazepril hydrochloride and a preparation method and a drug application thereof. According to the crystal form, a sharp heat melting peak begins to appear at 225.6 DEG C. The invention is beneficial to researches on drug bioactivity and bioavailability, etc.
Owner:WISDOM PHARM CO LTD
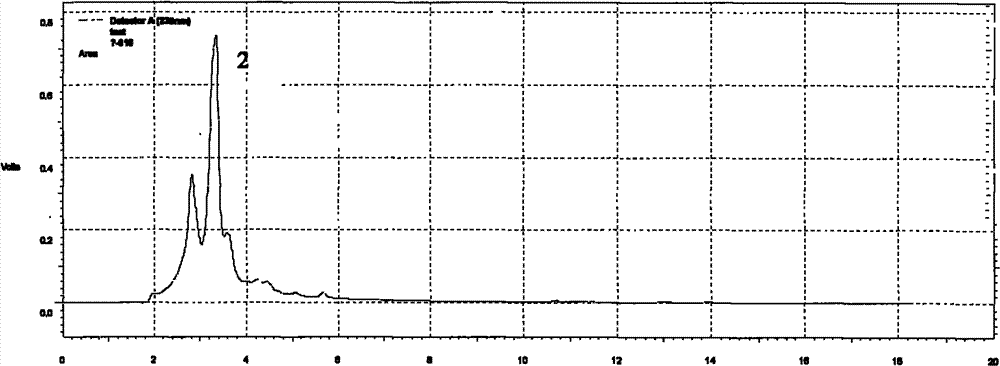
Ion chromatography-ultraviolet detection method for determining benazepril content in drug
InactiveCN102539561AAccurate determination of contentEasy to separateComponent separationIon chromatographyUltraviolet detectors
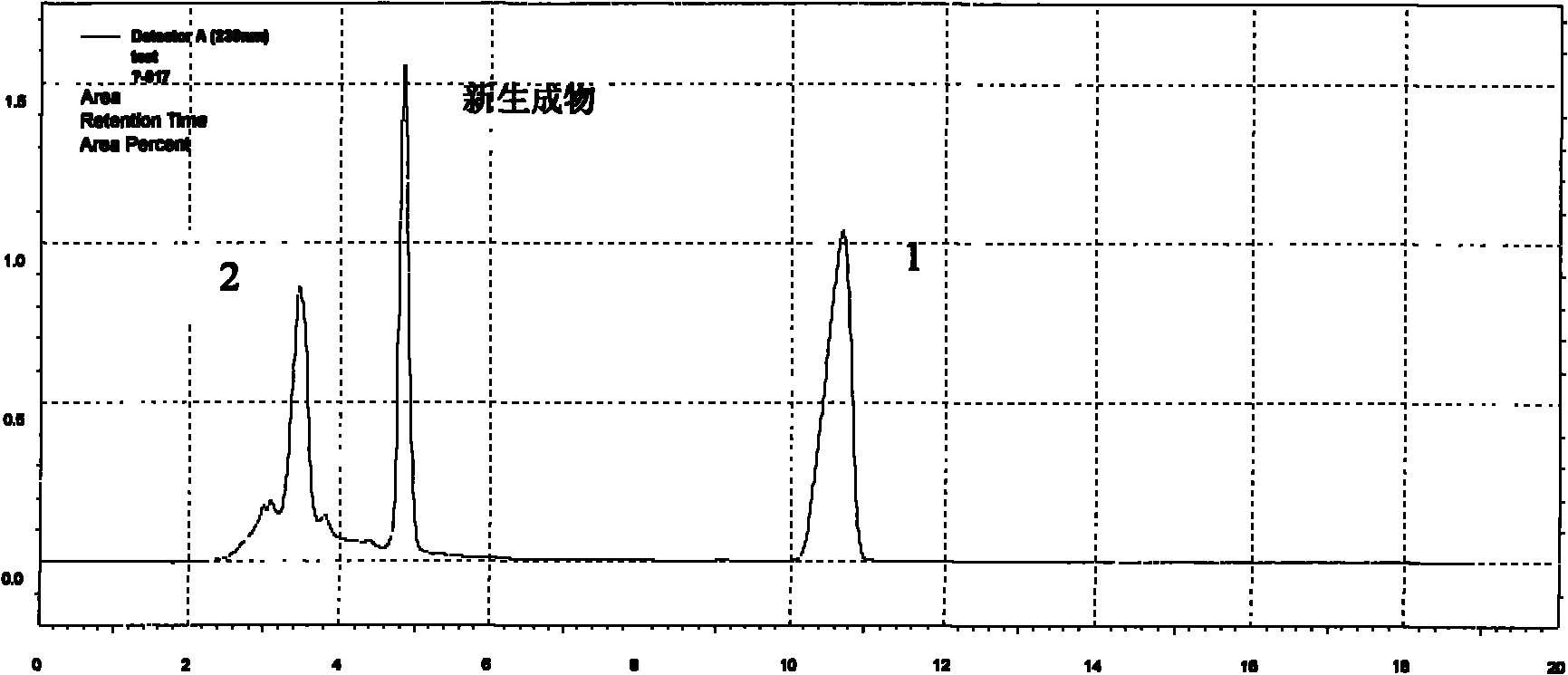
The present invention provides a new detection method for benazepril. According to the method, the combination of an ion chromatography cation exchange system and an ultraviolet detector is adopted, suitable separation conditions are selected according to the difference of retention behaviors of the materials on the chromatographic column, the optimal ultraviolet detection wavelength is selected, and the benazepril can be separated and detected at a high sensitivity. The method of the present invention can be used for detection of the benazepril content in the benazepril hydrochloride drug, human body fluid pharmacokinetic research, and the like.
Owner:ZHEJIANG UNIV
Synthesis method of (S)-amino compound
ActiveCN113292495AHigh yieldEmission reductionOrganic chemistry methodsPtru catalystChloroacetic acid
The invention relates to a synthesis method of a benazepril hydrochloride intermediate (S)-amino compound. According to the technical scheme, the preparation method of the (S)-amino compound comprises the following steps of by taking 2-aminobenzaldehyde 15 as an initial raw material, carrying out Aldol condensation under the action of alkali to prepare a compound 16, carrying out Pd / C catalytic hydrogenation on the compound 16 to prepare a compound 17, and carrying out DCC condensation on the compound 17 to generate a main structure compound 18 of the benazepril intermediate (S)-amino compound, carrying out substitution reaction on the compound 18 and tert-butyl chloroacetate to prepare a compound 19, and carrying out transamination reaction on the compound 19 under the catalytic action of a quinine-derived catalyst to generate the benazepril intermediate (S)-amino compound 1. The benazepril intermediate (S)-amino compound is synthesized by a method for directly constructing a chiral center through asymmetric catalysis, so that the reaction yield is increased.
Owner:迪嘉药业集团股份有限公司
Amlodipine/benazepril medicament composition liposome solid preparation
The invention discloses an amlodipine / benazepril medicament composition liposome solid preparation and a preparation method thereof. The liposome is prepared by combining active components of benzenesulfonic acid amlodipine, benzepril hydrochloride and specific combination of phosphatidyl ethanolamine, dioleoyl phosphatidylglycerol, cholesterol and deoxysodium cholate, so that the stability, dissolution and bioavailability of the medicament are greatly improved, and the effect is stable and durable, and the curative effect is obvious. By utilizing the amlodipine / benazepril medicament composition liposome solid preparation, the quality of the product is improved, and the toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Amlodipine/benazepril medicament composition liposome solid preparation
InactiveCN102600173APill deliveryPharmaceutical non-active ingredientsCholesterolAmlodipine besilate
The invention discloses an amlodipine / benazepril medicament composition liposome solid preparation and a preparation method thereof. The liposome is prepared by combining active components of benzenesulfonic acid amlodipine, benzepril hydrochloride and specific combination of phosphatidyl ethanolamine, dioleoyl phosphatidylglycerol, cholesterol and deoxysodium cholate, so that the stability, dissolution and bioavailability of the medicament are greatly improved, and the effect is stable and durable, and the curative effect is obvious. By utilizing the amlodipine / benazepril medicament composition liposome solid preparation, the quality of the product is improved, and the toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
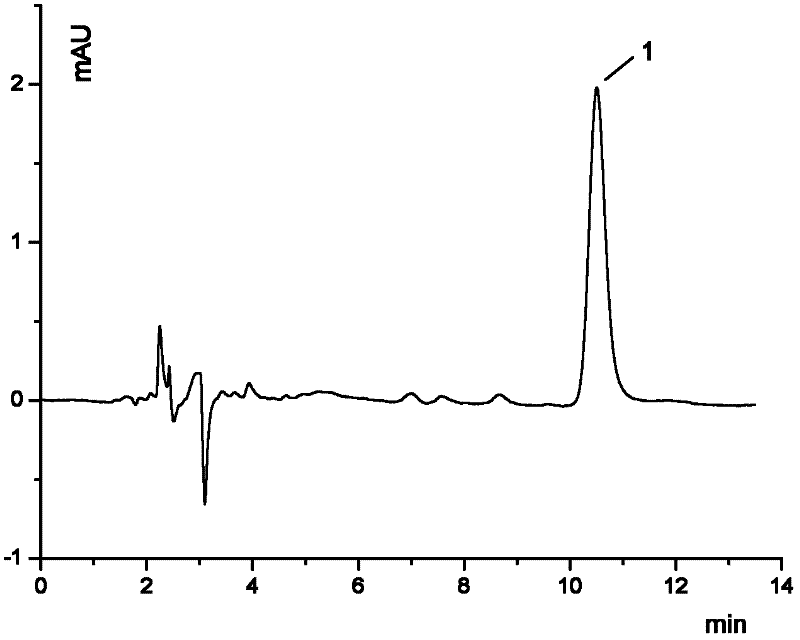
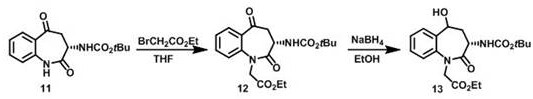
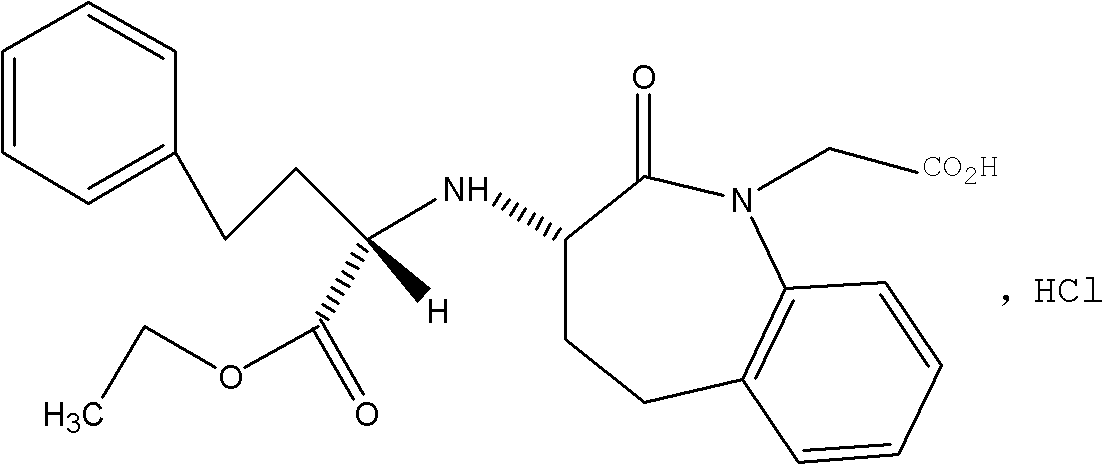
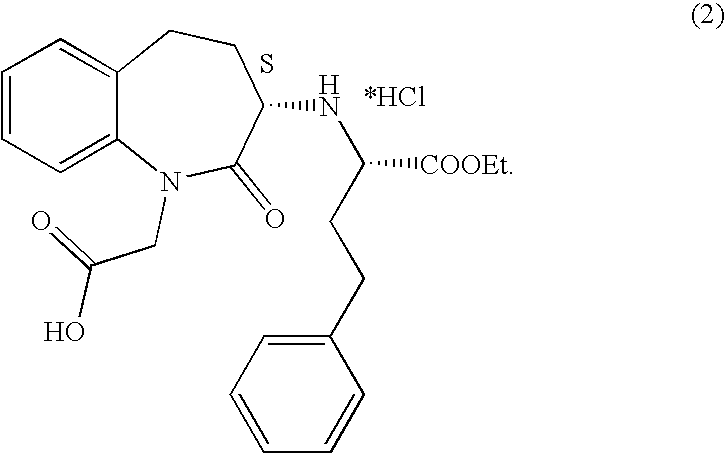
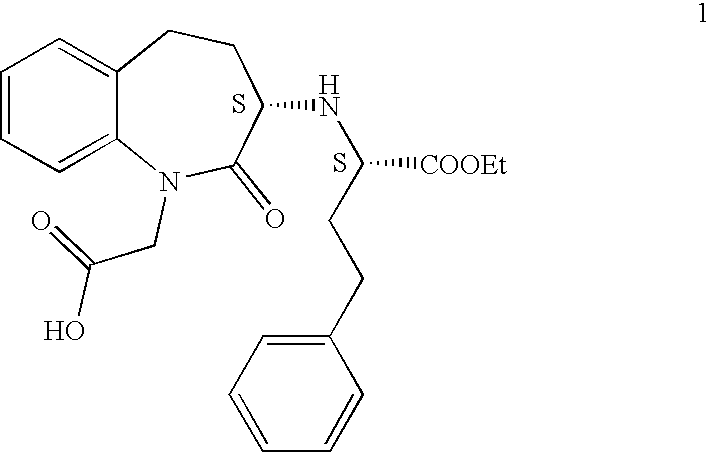
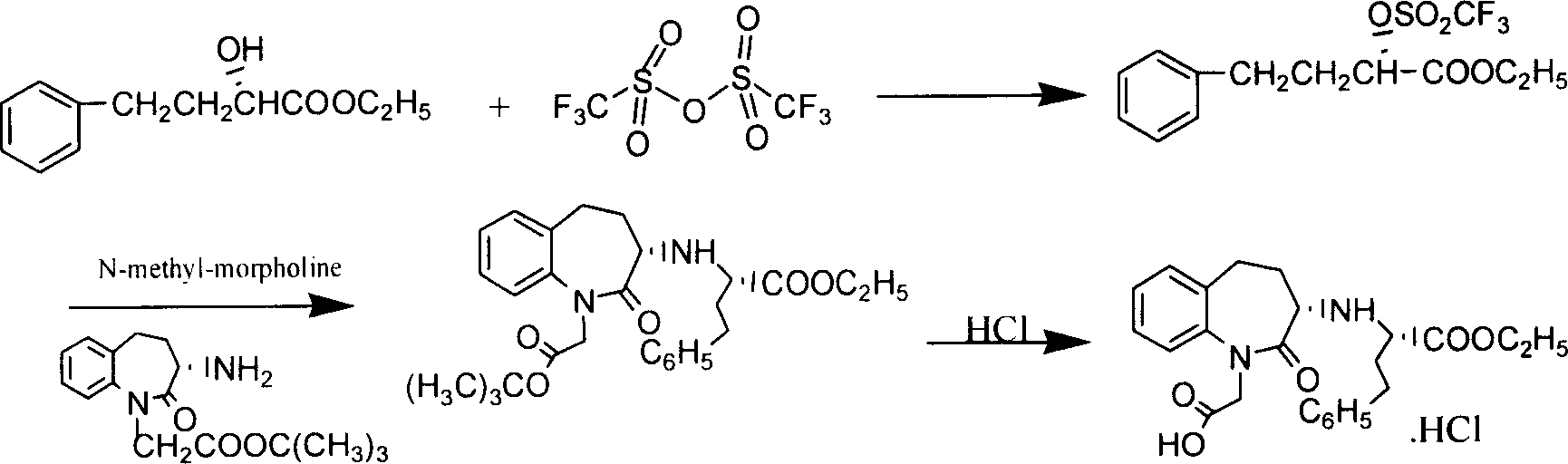
Process for the preparation of benazepril hydrochloride
A process for the preparation of benazepril hydrochloride (2) starting from the Michael adduct (14), obtained reacting compounds of formula (11) and (13), as defined in the disclosure
Owner:DIPHARMA SPA
Benazepril hydrochloride composition with effect of reducing blood pressure and preparation method thereof
The invention discloses a benazepril hydrochloride composition with effect of reducing blood pressure and a preparation method thereof. The preparation process comprises a solid fermentation method and a liquid fermentation method. Aspergillus CICC 2436 produces enzyme to hydrolyze benazepril hydrochloride, wherein the structure of part of benazepril hydrochloride is changed, namely an analog of benazepril hydrochloride. The original benazepril hydrochloride forms a composition containing the benazepril hydrochloride and the analog of the benazepril hydrochloride under the action of the enzyme, and the level of reducing the blood pressure by using the composition is superior to that of the benazepril hydrochloride with the same concentration.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Preparation process of benazepril hydrochloride tablets
InactiveCN104873472AReduce financial burdenNo side effectsPill deliveryCardiovascular disorderRHODIOLA ROSEA ROOTAchyranthes
The invention relates to a preparation process of benazepril hydrochloride tablets which are traditional Chinese and western medicine composited preparations. The benazepril hydrochloride tablets are prepared from the following raw materials: 1 part of benazepril hydrochloride, 35 parts of curcuma aromatic, 35 parts of the root of red-rooted salvia, 35 parts of rhizoma corydalis, 30 parts of semen cassiae, 30 parts of the root of kudzu vine, 30 parts of burdock root, 25 parts of rhodiola rosea, 25 parts of honeysuckle, 25 parts of dandelion, 25 parts of uncaria rhynchophylla, 20 parts of ligusticum wallichii, 20 parts of polygala tenuifolia, 20 parts of eucommia ulmoides, 20 parts of cassia twig, 15 parts of hawthorn, 15 parts of liquorice, 15 parts of semen plantaginis, 10 parts of folium mori, 10 parts of the fruit of Chinese wolfberry, 8 parts of herba lycopi, 8 parts of root of common peony, 5 parts of the root of bidentate achyranthes, and 5 parts of peach kernel. The composition medicine can treat both symptoms and root causes of hypertension, is small in side effect, small in price, and simple and convenient to prepare, and has a wide application prospect.
Owner:XIANGYU PHARMA
Traditional Chinese medicine for treating diabetic nephropathy and preparation method thereof
ActiveCN103316101BReduce contentImprove lipid metabolism disorder in the bodyMetabolism disorderUrinary disorderCoptisTubulointerstitial fibrosis
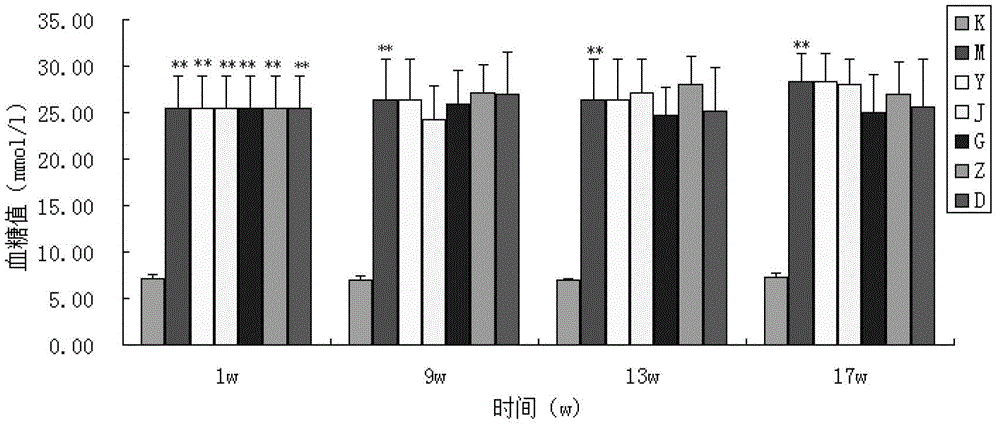
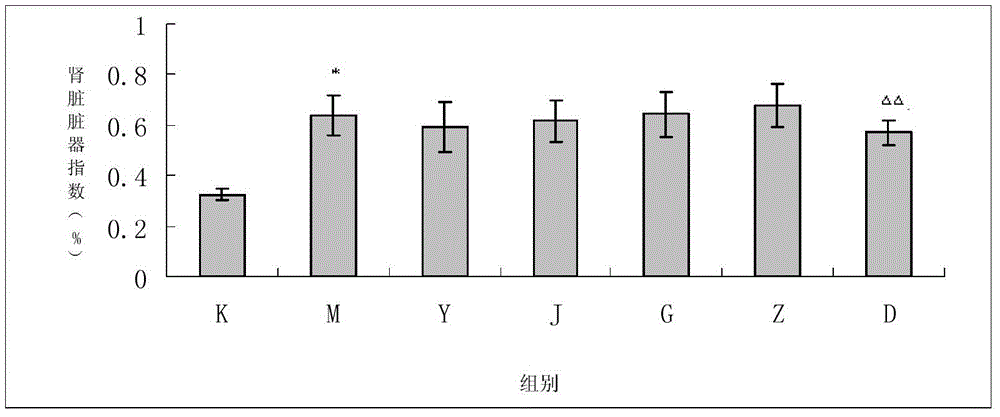
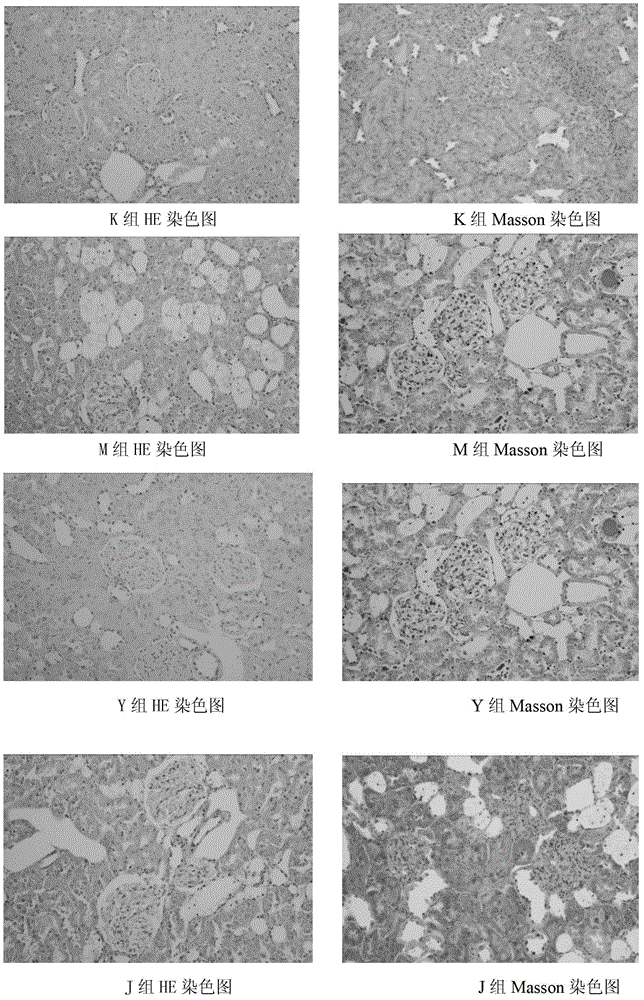
The invention provides a traditional Chinese medicine for treating diabetic nephropathy and a preparation method thereof. The traditional Chinese medicine for treating diabetic nephropathy is prepared from the following traditional Chinese medicinal materials by weight: 6 to 15 parts of common fenugreek seed, 6 to 15 parts of milkvetch root, 3 to 8 parts of epimedium herb, 3 to 8 parts of malaytea scurfpea fruit, 3 to 8 parts of Asiatic cornelian cherry fruit, 1 to 5 parts of rhubarb root and rhizome, 3 to 8 parts of Chinese cassia tree and 2 to 6 parts of coptis. The invention further discloses the preparation method for the traditional Chinese medicine. According to the invention, renal tubulointerstitial fibrosis is dose-dependently inhibited through reduction of urine protein of rats with diabetic nephropathy, so a certain treatment effect is exerted on type 1 diabetic nephropathy and the treatment effect is equal to that of a benazepril hydrochloride tablet but better than that of a kidney-qi-tonifying pill. The traditional Chinese medicine also exerts a certain treatment effect on rats with type 2 diabetic nephropathy through approaches like protection of a kidney structure, reduction of the content of urine protein and improvement of in-vivo lipid metabolism disorder.
Owner:WUHAN JIANHENG PHARMA
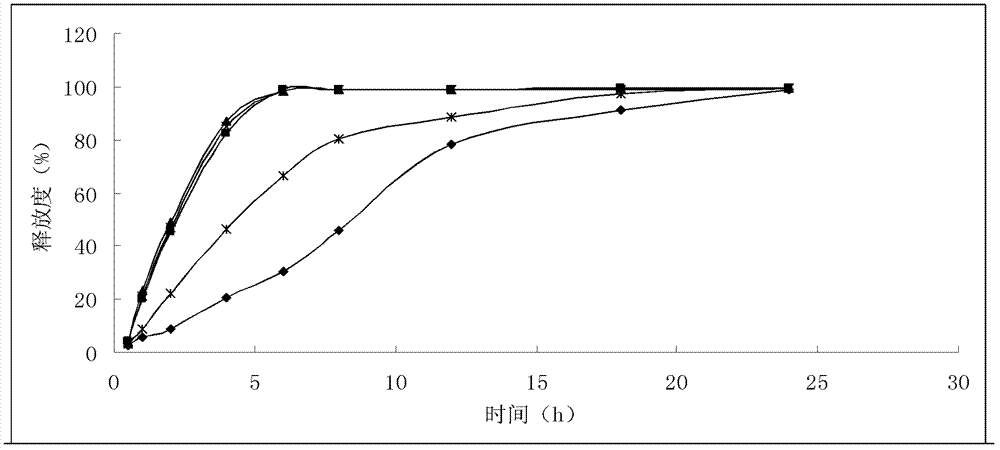
Process for Crystallization of Benazepril Hydrochloride
An improved process for the crystallization of benazepril hydrochloride to obtain in at least 99.8% diastereomeric purity. The process comprises making a concentrated solution of benazepril hydrochloride in ethanol and adding the resulting solution to a non-solvent diisopropyl ether.
Owner:LUPIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/49dbea35-a765-4586-b04b-349146c33d00/GSA00000122969300011.PNG)
![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/49dbea35-a765-4586-b04b-349146c33d00/GSA00000122969300021.PNG)
![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/49dbea35-a765-4586-b04b-349146c33d00/GSA00000122969300022.PNG)


![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/367ea7eb-6328-461c-97cf-6d263cdc0870/FSB0000118133590000011.PNG)
![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/367ea7eb-6328-461c-97cf-6d263cdc0870/GSA00000122969300011.PNG)
![Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine Preparation method of 3-[(1-(1S)-phenylethyl) amino]-2,3,4,5-tetrahydro-2-oxo-1H-(3S)-benzazepine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/367ea7eb-6328-461c-97cf-6d263cdc0870/GSA00000122969300021.PNG)