Amlodipine benazepril impulse tablet and preparation method thereof
A technology of benazepril hydrochloride and dipinbei, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of increasing the incidence of adverse reactions, and achieve a suitable large-scale Effects of industrialized production, improvement of quality of life, improvement of drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
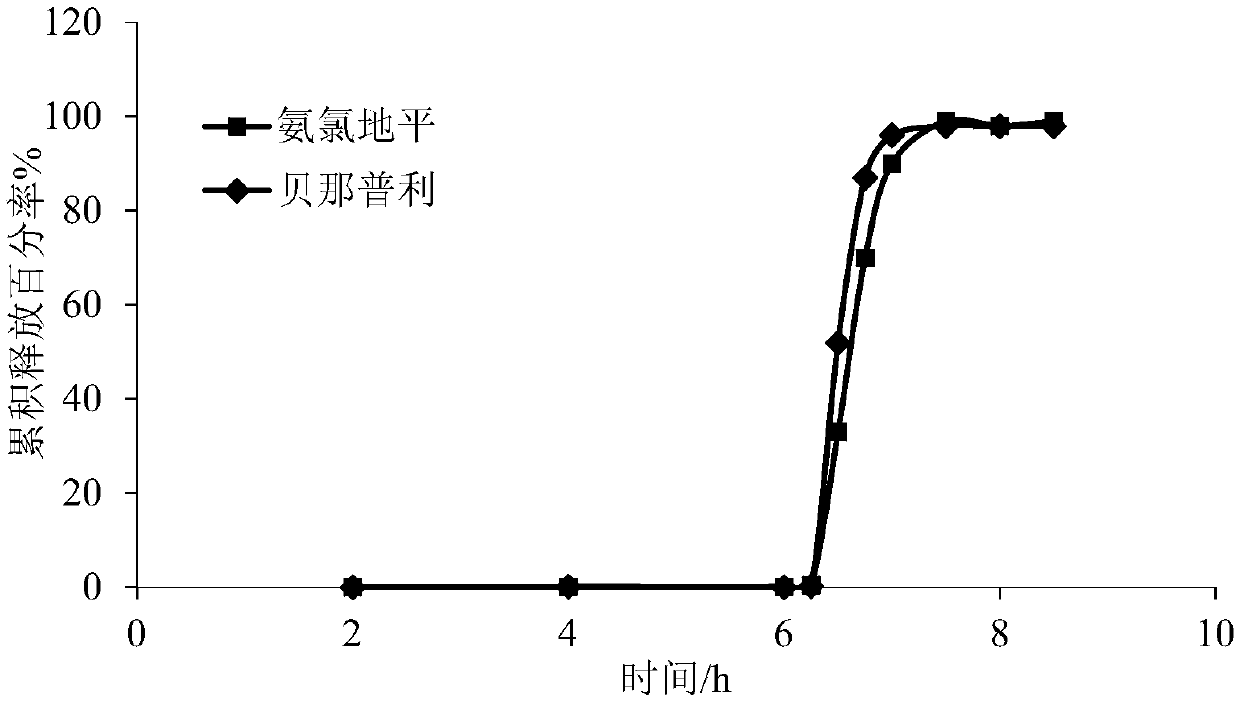
Image
Examples
Embodiment 1
[0071] Immediate-release tablet core prescription:
[0072]
[0073]
[0074] Preparation process: After fully mixing the components, immediate-release tablet cores were prepared by powder direct compression method, and a total of 2,500 immediate-release tablet cores were prepared.
[0075] Swelling Coating Recipe:
[0076] HPMC E5 130g
[0077] Absolute ethanol 2470g
[0078] Swelling coating layer coating process: prepare coating liquid according to prescription, and use pan coating method for coating. The material temperature of the coating pan is 38-45° C., the flow rate of the coating liquid is 15 g / min, the rotational speed of the coating pan is 12 rpm, and the weight gain of the coating is 10%.
[0079] Controlled release coating formulation:
[0080] Eudragit RS 100 25g
[0081] Eudragit RL 100 5g
[0082] Absolute ethanol 100g
[0083] Coating process of the controlled-release coating layer: Prepare the coating solution according to the prescription, and ...
Embodiment 2
[0086] In this example, the same prescription and process conditions as in Example 1 were used to prepare the immediate-release tablet core and the swelling coating layer, and then form the controlled-release coating layer.
[0087] Controlled release coating formulation:
[0088] Eudragit RS 100 150g
[0089] Eudragit RL 100 30g
[0090] Absolute ethanol 600g
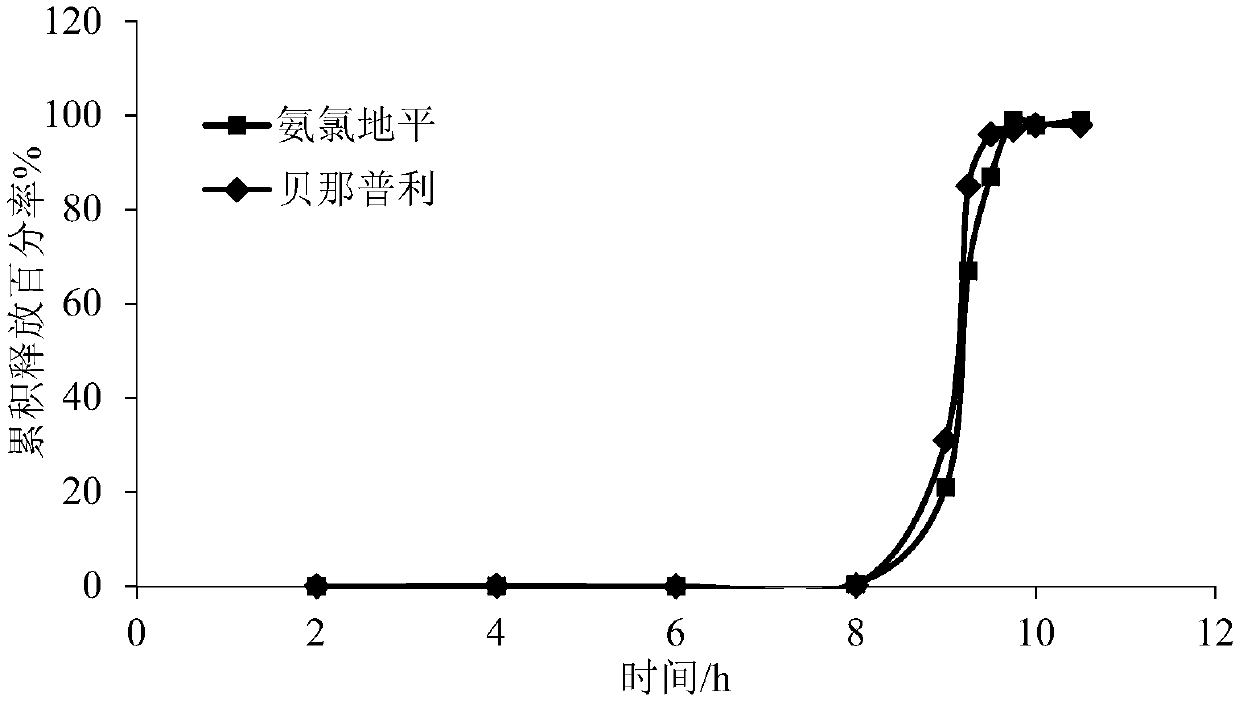
[0091] Coating process of the controlled-release coating layer: Prepare the coating solution according to the prescription, and use the pan coating method for coating. The material temperature of the coating pan is 38-45° C., the flow rate of the coating solution is 15 g / min, the rotational speed of the coating pan is 10 rpm, and the weight gain of the coating is 12%. The drug release time lag of the prepared pulse tablet is about 9h, and the time lag is reached, and the dissolution rate of the drug is greater than 80% in 1 hour. The drug release curve in vitro is shown in figure 2 .
[0092] from figure 2 It c...
Embodiment 3
[0094] In this example, the same prescription and process conditions as in Example 1 were used to prepare the immediate-release tablet core and the swelling coating layer, and then form the controlled-release coating layer.
[0095] Controlled release coating formulation:
[0096] Eudragit RS 100 80g
[0097] Eudragit RL 100 100g
[0098] Absolute ethanol 600g
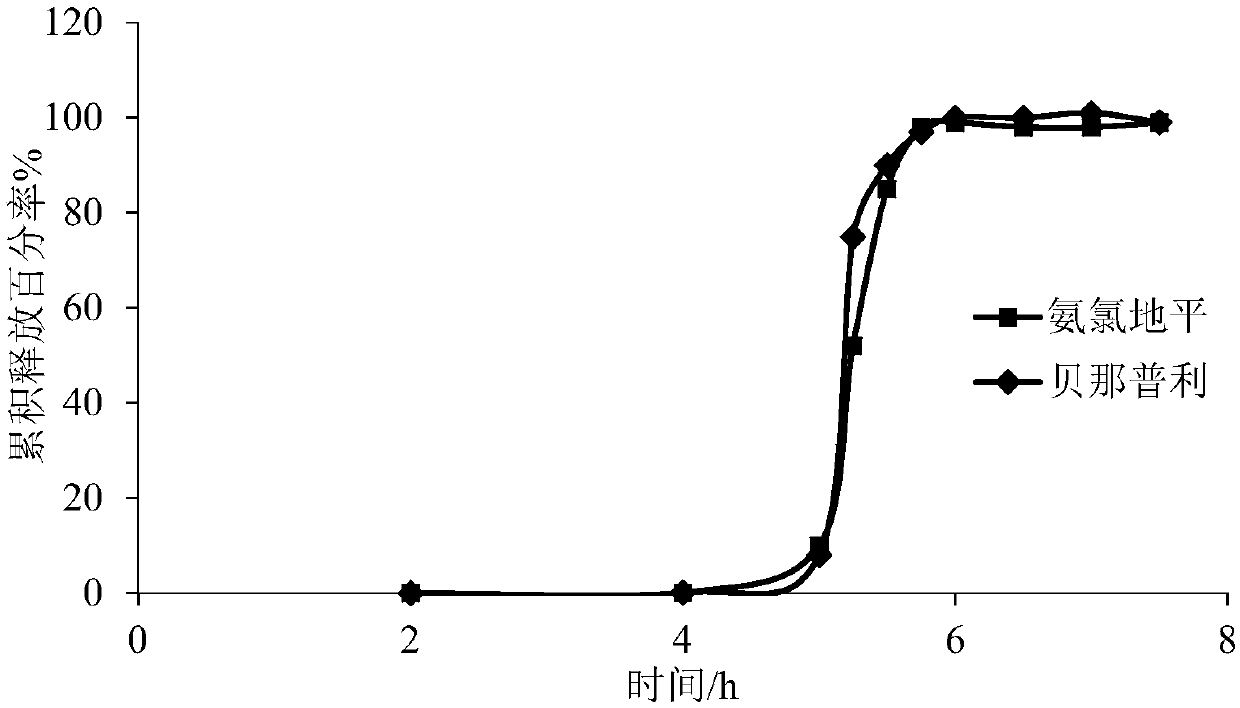
[0099] Coating process of the controlled-release coating layer: the coating solution was prepared according to the prescription, and the same coating conditions as in Example 2 were used to prepare the controlled-release coating layer. The drug release time lag of the prepared pulse tablet is about 6h, and the time lag is reached, and the dissolution rate of the drug is greater than 80% in 1 hour. The drug release curve in vitro is shown in image 3 .
[0100] from image 3 It can be seen from the figure that the prepared pulsating tablets began to exhibit obvious drug release behavior after the 6th hour, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com