A kind of improved preparation method of benazepril hydrochloride and pharmaceutical composition containing the benazepril hydrochloride
A technology of benazepril hydrochloride and organic phase, applied in the field of pharmaceutical invention, can solve the problem of low total yield, and achieve the effects of simplified operation, reduced types and dosage, and reduced amount of waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
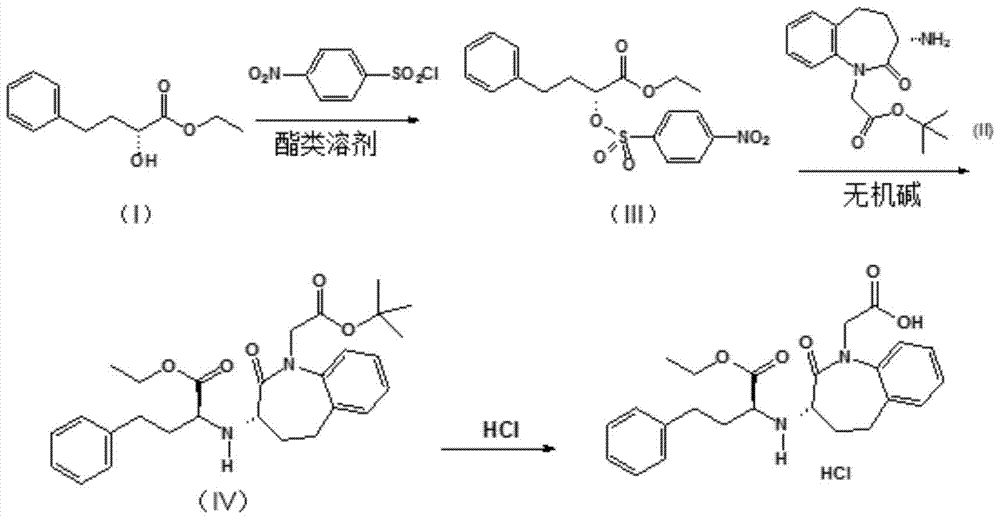
[0031] The preparation of embodiment 1 benazepril hydrochloride
[0032] Add 105g (0.50mol) of ethyl R-α-hydroxyphenylbutyrate (I), 122g (0.55mol) of 4-nitrobenzenesulfonyl chloride and 600ml of ethyl acetate into the reaction flask, stir to dissolve and cool to 0-5 ℃. 71 g (0.70 mol) of triethylamine was added dropwise for about 1 h. After the dropwise addition, the temperature was raised to 25° C. and stirring was continued for 2 h. The crystals of triethylamine hydrochloride were filtered out, washed with an appropriate amount of ethyl acetate and combined with the filtrate. Add 100 ml of water to the filtrate, stir for 0.5 h, and then adjust the pH of the aqueous phase to about 6 with 10% hydrochloric acid. The phases were separated after standing, and the organic phase was washed twice with water. The phases were separated after standing, and the organic phase was dried with 50 g of anhydrous sodium sulfate and then filtered. The filter cake was washed with an appropr...
Embodiment 2
[0035] The preparation of embodiment 2 benazepril hydrochloride
[0036] Add 105g (0.50mol) of R-α-hydroxyphenylbutyrate ethyl ester (I), 122g (0.55mol) of 4-nitrobenzenesulfonyl chloride and 600ml of isopropyl acetate into the reaction flask, stir and dissolve, then cool to 0- 5°C. 71 g (0.70 mol) of triethylamine was added dropwise for about 1 h. After the dropwise addition, the temperature was raised to 25° C. and stirring was continued for 2 h. The triethylamine hydrochloride crystals were filtered out, washed with an appropriate amount of isopropyl acetate and combined with the filtrate. Add 100 ml of water to the filtrate, stir for 0.5 h, and then adjust the pH of the aqueous phase to about 6 with 10% hydrochloric acid. The phases were separated after standing, and the organic phase was washed twice with water. Stand still and separate the phases, the organic phase is dried with 50 g of anhydrous potassium sulfate and filtered, and the filter cake is washed with an a...
Embodiment 3
[0039] Embodiment 3 preparation method improvement comparative experiment
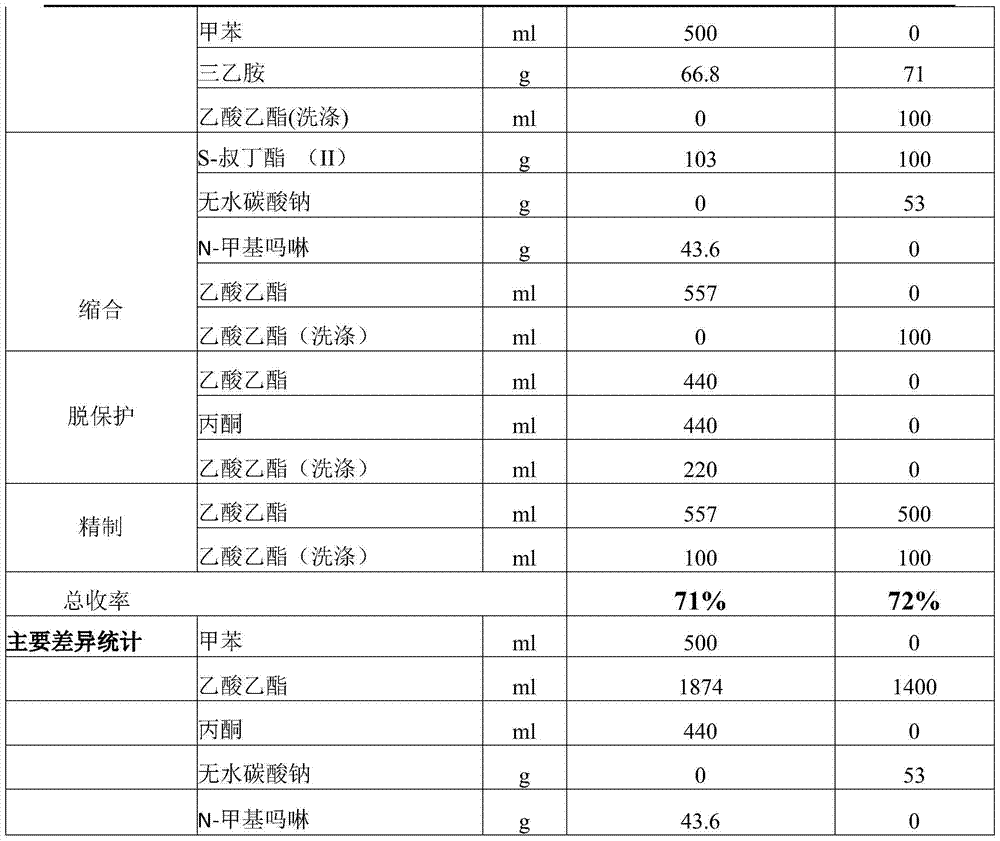
[0040] Under the same feeding scale, compare the consumption of main organic material with US4785089 embodiment 6 and 16, the result is shown in the table below.
[0041]
[0042]
[0043] As can be seen from the comparative data in the above table, the present invention does not use toxic toluene and N-methylmorpholine, and the organic solvent types are reduced from three to one, and the use of a single organic solvent greatly facilitates the recovery of solvents, and the acetic acid used Ethyl ester is significantly reduced, and the amount of waste liquid produced is significantly reduced, which has good clean production value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com