New crystal form of benazepril hydrochloride and preparation method thereof
A kind of technology of bena hydrochloride and ethyl homophenylalanine carboxylate, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] THF (2300mL) was used as a solvent, and NaOH (33g) was used as a base, and (S,S)-homophenylalanine carboxylate ethyl ester-benzazepine (ethyl (S)-2-(((S)- 2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-
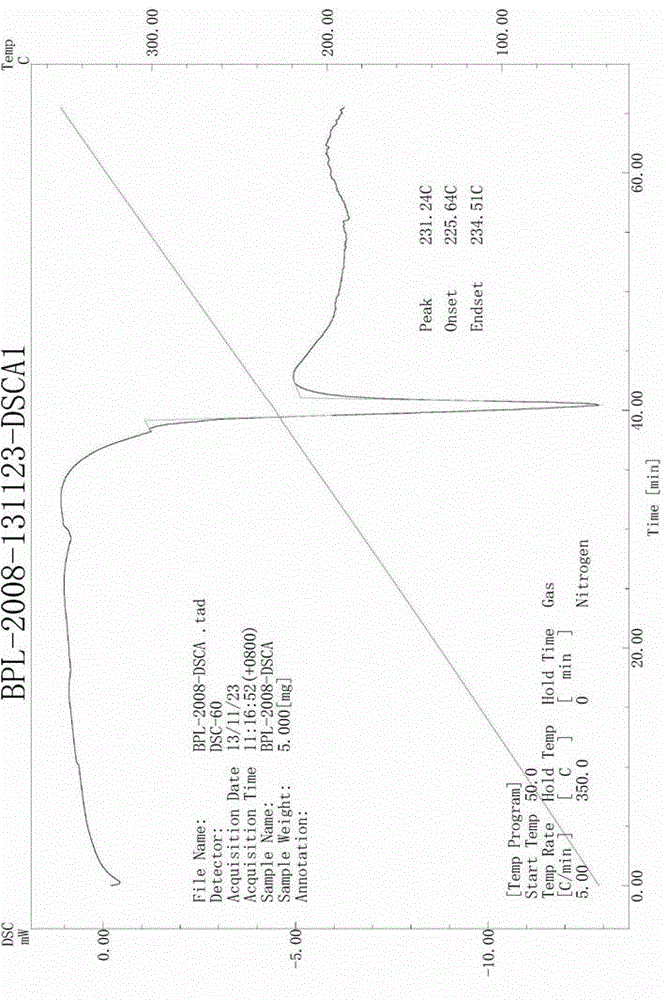
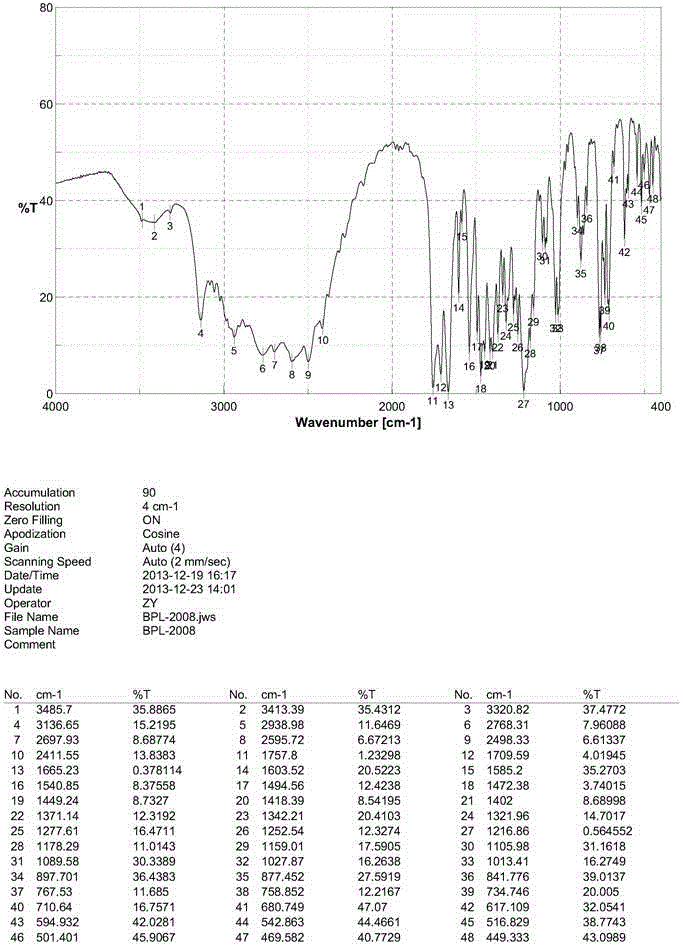
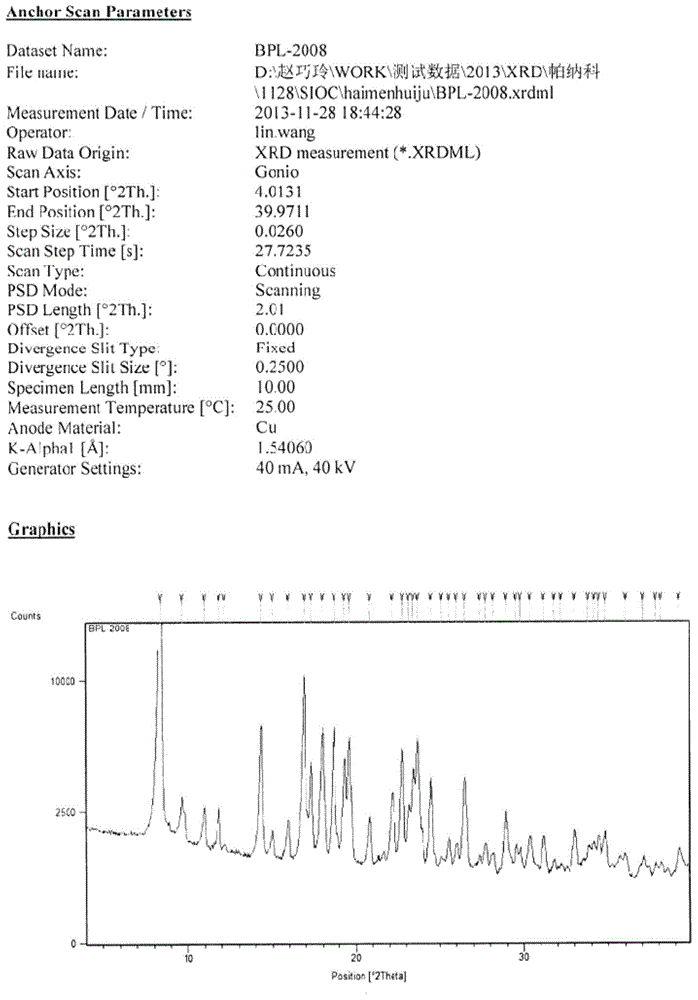
[0031] yl)amino)-4-phenylbutanoate), see patent CN101830850B) (150g) and tetrabutylammonium halide (12g) for specific structure, add tert-butyl chloroacetate (95g) dropwise, stir until the reaction is complete, then filter, and the filtrate is precipitated Then add toluene (400mL), heat and stir to dissolve, and then pass in hydrogen chloride gas until the reaction of removing the tert-butyl protecting group is complete and a solid is produced. After the solid is filtered and dried, acetonitrile is directly added and heated, and the solid obtained after hot filtration is dried to obtain a white crystalline powder, which is the new crystal form of benazepril hydrochloride. Its X-ray diffraction diagram, infrared absorption spectrum diagram and differential thermal analys...
Embodiment 2
[0033] THF (3000mL) was used as solvent, KOH (50g) was used as base, (S,S)-homophenylalanine carboxylate ethyl ester-benzazepine (see patent CN101830850B for specific structure) (150g) and tetrabutyl ammonium halide (5g), dropwise added tert-butyl chloroacetate (95g), stirred until the reaction was complete, then filtered, added toluene (360mL) after the filtrate was desolvated, heated and stirred to dissolve, and then introduced hydrogen chloride gas to remove the tert-butyl protection The base reaction is complete and a solid is produced. After the solid is filtered and dried, acetonitrile is directly added and heated, and the solid obtained after hot filtration is dried to obtain a white crystalline powder, which is the new crystal form of benazepril hydrochloride of the present invention.
Embodiment 3
[0035] Dioxane (2000mL) as solvent, KOH (50g) as base, add (S,S)-homophenylalanine carboxylate ethyl ester-benzazepine (see patent CN101830850B for specific structure) (150g) and tetrabutylammonium halide (15g), add tert-butyl bromoacetate (125g) dropwise, stir until the reaction is complete, then filter, add toluene (450mL) after the filtrate is desolvated, heat and stir until dissolved, then pass in hydrogen chloride gas to remove The tert-butyl protecting group reacted completely and a solid was produced. After the solid is filtered and dried, acetonitrile is directly added and heated, and the solid obtained after hot filtration is dried to obtain a white crystalline powder, which is the new crystal form of benazepril hydrochloride of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com