Synthesis method of (S)-amino compound
A technology of amino compounds and compounds, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of expensive chiral starting materials, unfavorable industrial production, and increased synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
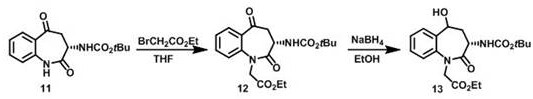
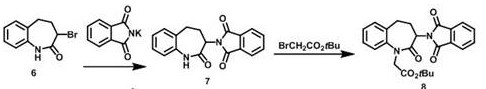
Image
Examples
Embodiment 1
[0070] Preparation of Compound 16
[0071] Take 1000 ml of three bottles, add o-amino-aminoceal (100.23 g, 825 mmol), pyruvate (87.23 g, 991 mmol) and drinking water (500.00 g, 5 times the mass of o-amino), temperature control 20 ~ 30 o C, then add potassium hydroxide (111.16 g, 1.98 mol) in batch to the reaction system, temperature control temperature 20 to 30 during hydrogenated potassium hydroxide o C; after the addition, the temperature control temperature is 20 ~ 30 o C reaction 2H, after the reaction, the pH of the reaction system is adjusted to 4 to 5 after the reaction is completed, and there will be a large amount of solid formation during the tuning pH; filter, filter cake with 100.00 g Drinking water and 1155 ~ 65 o C Heaves dry 10 h. Compound 16, mass of 155.46 g, HPLC purity: 99.34%, yield: 98.5% ,.
[0072] Preparation of Compound 17
[0073] The 2 L high pressure reactor was taken into a compound 16 (140.00 g, 732 mmol), methanol (700.00 g, the compound 16 mass of t...
Embodiment 2
[0081] Preparation of Compound 16
[0082] Take 1000 ml of three bottles, add o-amino-aminoceraldehyde (100.00 g, 825 mmol), pyruvate (72.69 g, 825 mmol) and drinking water (500.00 g, 5 times the mass of o-amino), temperature control 20 ~ 30 o C, then add potassium hydroxide (111.16 g, 1.98 mol) in batch to the reaction system, temperature control temperature 20 to 30 during hydrogenated potassium hydroxide o C; after the addition, the temperature control temperature is 20 ~ 30 o C reaction 2H, after the reaction, the pH of the reaction system is adjusted to 4 to 5 after the reaction is completed, and there will be a large amount of solid formation during the tuning pH; filter, filter cake with 100.00 g Drinking water and 1155 ~ 65 o C Heaves dry 10 h. The compound 16 was obtained as 142.99 g, yield: 90.6%, HPLC purity: 99.08%.
[0083] Preparation of Compound 17
[0084] A 2 L high pressure reactor was added to the compound 16 (140.00 g, 732 mmol), methanol (700.00 g, the compoun...
Embodiment 3
[0092] Preparation of Compound 16
[0093] Take 1000 ml of three bottles, incorporate an o-amino-aminoceal (100.00 g, 825 mmol), pyruvate (87.23 g, 991 mmol) and drinking water (500.00 g, 5 times the compound of the compound), temperature control 20 to 30 o C, then add sodium hydroxide (79.25 g, 1.98 mol) in a reaction system, temperature control temperature in the process of hydroxide, 20 ~ 30 o C; after the addition, the temperature control temperature is 20 ~ 30 o C reaction 2H, after the reaction, the pH of the reaction system is adjusted to 4 to 5 after the reaction is completed, and there will be a large amount of solid formation during the tuning pH; filter, filter cake with 100.00 g Drinking water and 1155 ~ 65 o C Heaves dry 10 h. The compound 16 was obtained as 148.83 g, yield: 94.3%, HPLC purity: 99.12%.
[0094] Preparation of Compound 17
[0095] A 2 L high pressure reactor was added to the compound 16 (140.00 g, 732 mmol), methanol (700.00 g, 5.00 g / g) and PD / C (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com