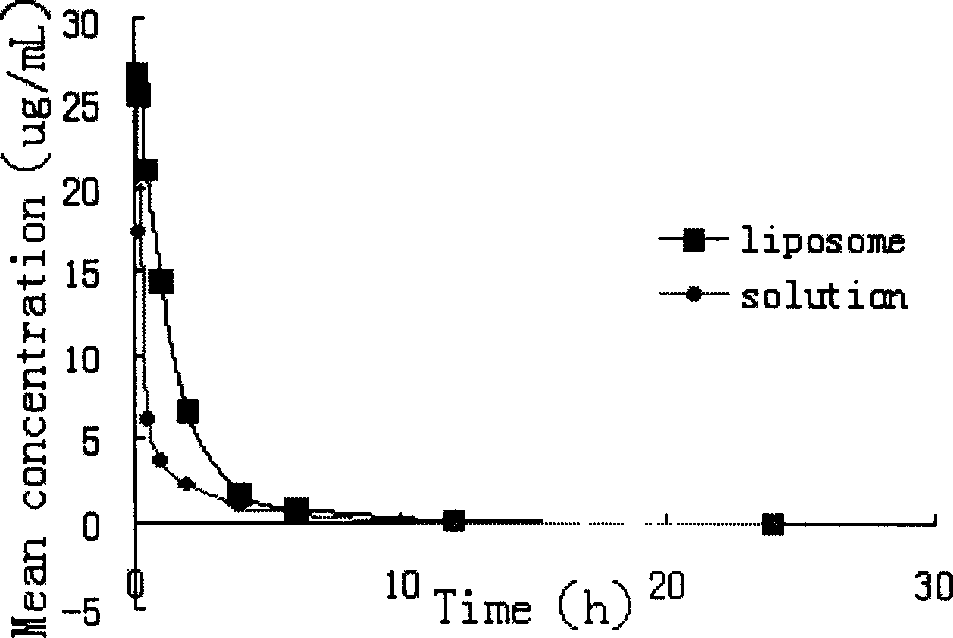
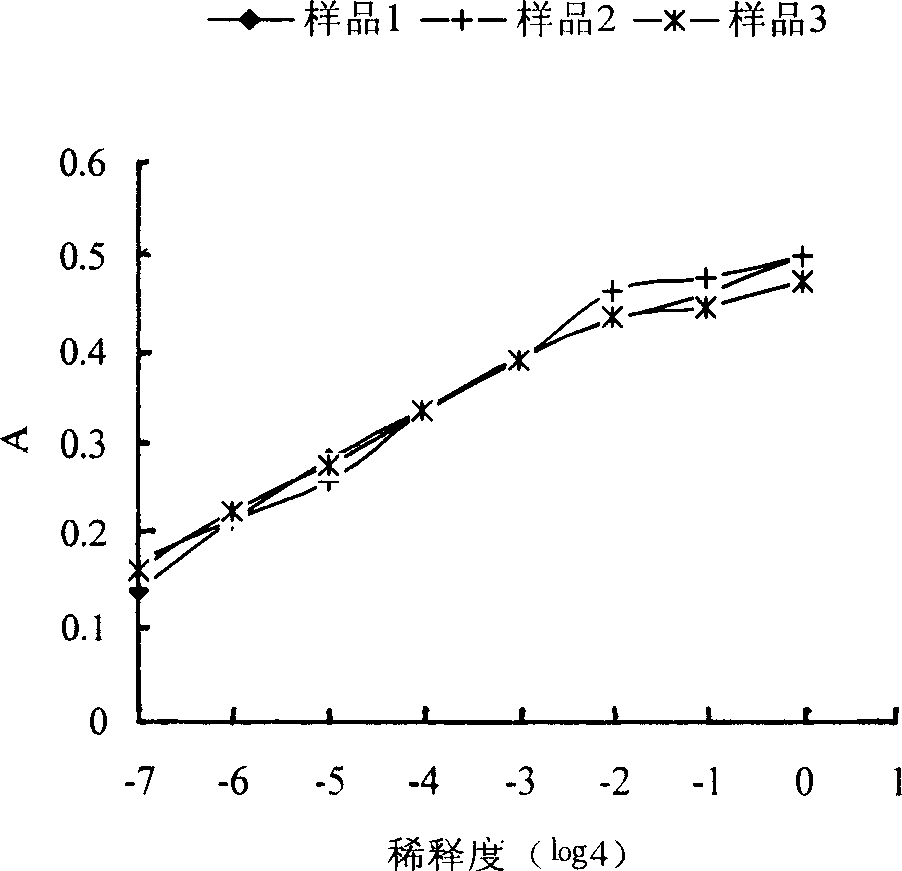
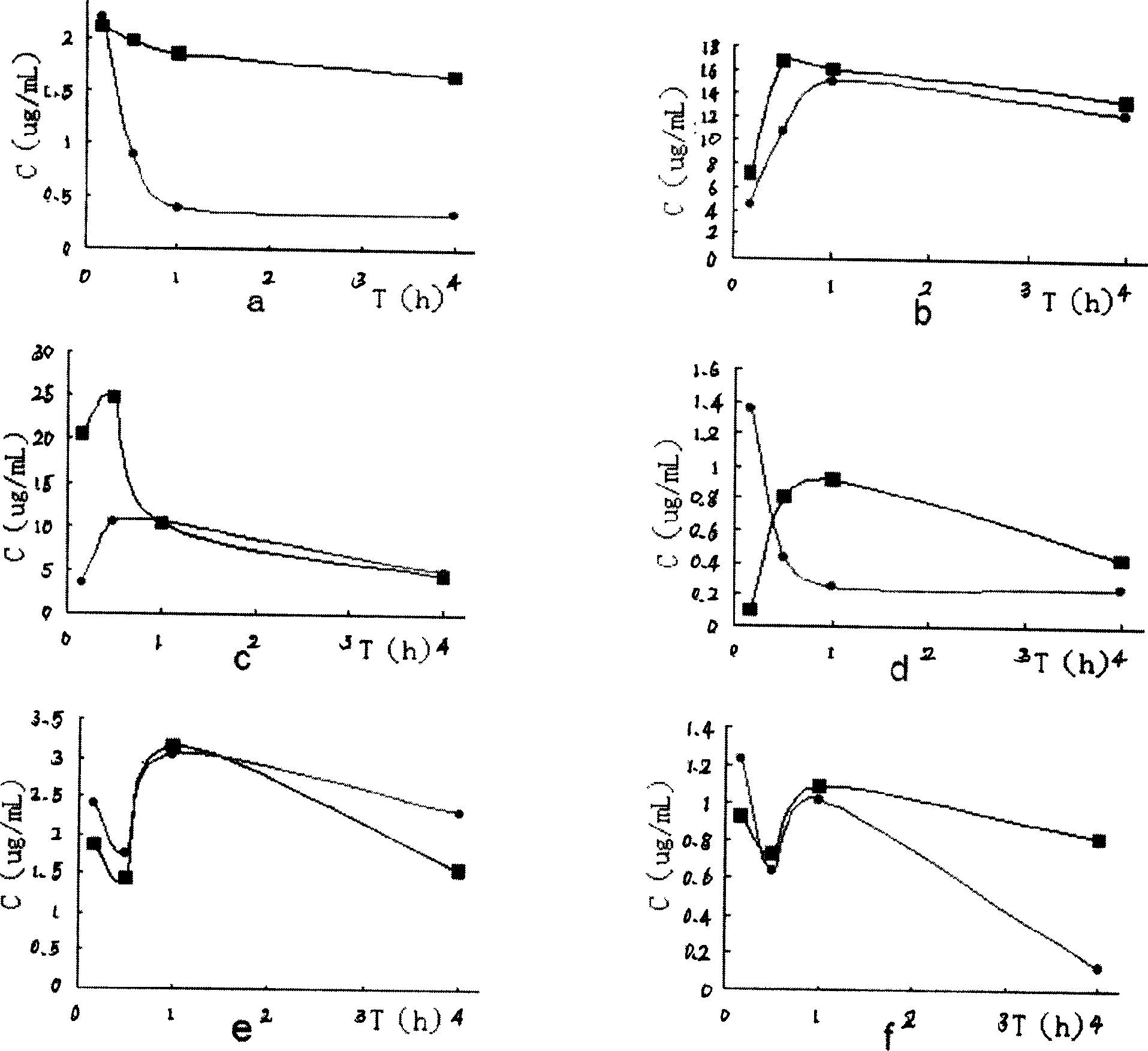
Medicinal preparation of coenzyme Q10 liposome and its preparation technology
A technology of pharmaceutical preparations and liposomes, applied in the field of medicine, can solve problems such as low bioavailability and inconvenience for patients to swallow, and achieve the effects of improving bioavailability, improving passive targeting, and improving therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] coenzyme Q 10 50mg, soybean lecithin 300mg, cholesterol 300mg, pH6.5 phosphate buffer 10mL.
[0059] Coenzyme Q 10 , soybean lecithin, and cholesterol in a pear-shaped bottle, add 30 mL of dichloromethane, heat to dissolve, put it on a rotary evaporator, remove the dichloromethane under reduced pressure in a water bath at 60°C, add phosphate buffer, continue to rotate for 1 hour, and Extrude through 0.8m filter membrane to get coenzyme Q 10 Liposomal oral solution.
Embodiment 2
[0061] coenzyme Q 10 10mg, egg yolk lecithin 60mg, cholesterol 30mg, Tris buffer solution of pH 7.5 10mL.
[0062] Coenzyme Q 10 , egg yolk lecithin, cholesterol, add 2mL ethanol to dissolve, put the Tris buffer solution in a three-necked bottle, inject the lipid solution into the buffer solution at 50°C in a water bath, and squeeze out through a 0.2m filter membrane to obtain coenzyme Q 10 Liposomal injections.
Embodiment 3
[0064] coenzyme Q 10 15mg, soybean lecithin 100mg, cholesterol 20mg, pH6.7 phosphate buffer 10mL.
[0065] Coenzyme Q 10 , soybean lecithin, and cholesterol were placed in a pear-shaped bottle, added 10 mL of chloroform to dissolve, placed on a rotary evaporator, and decompressed in a water bath at 40°C for 5 minutes until gelatinous, added 10 mL of phosphate buffer, continued to rotate for 1 hour, and Extrude through a 0.45m filter membrane to obtain coenzyme Q 10 Liposome injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com