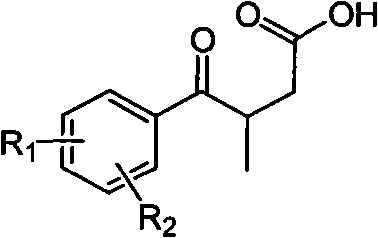
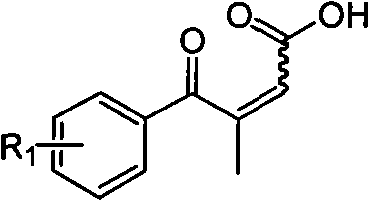
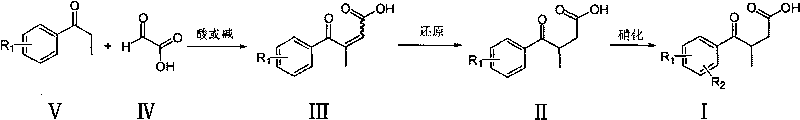
Novel process for preparing 3-(substituted benzoyl) butyric acid
A benzoyl and new process technology, applied in the field of preparation of pimobendan intermediate 3-butyric acid, can solve the problems of difficult waste disposal, long route, high cost, etc., and achieve easy separation and purification, high yield and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] 50ml round bottom flask, add 5ml of dioxane, 1.00g (5.9mmol) of p-chloropropiophenone, 0.82g (8.9mmol) of oxoacetic acid, and stir until all the solids are dissolved. Add dropwise 0.70g (7.1mmol) of 98% concentrated sulfuric acid ). Heated to reflux. After 20h, the reaction was complete. Cooled to room temperature, evaporated the solvent under reduced pressure, poured the mixture into water, a solid precipitated out, filtered to obtain 1.22g of the yellow solid product 3-p-chlorobenzoyl-2-butenoic acid , yield 71%, melting point 130~132℃.
Embodiment 2
[0032]
[0033] 100ml there-necked flask, add 5.00g (22.26mmol) of 3-p-chlorobenzoyl-2-butenoic acid, 27ml of glacial acetic acid, 1ml of water, stir and add 2.90g (44.62mmol) of Zn powder. Heat to reflux. The reaction was complete after 1 h. Cool to room temperature, filter, pour the reaction solution into 250ml of water, extract with dichloromethane (100ml×3), combine the organic phases, evaporate the solvent under reduced pressure, and dry to obtain a light yellow solid product 3-p-chlorobenzoylbutanoic acid 4.20 g, yield 83%, melting point 84-85°C.
Embodiment 3
[0035]
[0036] Add 17ml of fuming nitric acid to a 50ml three-neck flask, cool down to -15°C, add 7.00g (30.88mmol) of 3-p-chlorobenzoylbutyric acid in batches, and stir at -15 to -10°C for reaction. After 4h the reaction was complete. The reaction solution was poured into 200ml of crushed ice, stirred thoroughly, and filtered to obtain 6.30g of white solid product 3-(3-nitro-4-chlorobenzoyl)butanoic acid with a yield of 75.1% and a melting point of 118-121°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com