Fixed dose combination therapy of parkinson's disease
A fixed-dose, dose-based technology for neurodegenerative diseases that addresses issues such as reduced side effects, impulse control disorders, and limited utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation of these capsules or tablets may be carried out using any suitable technique known in the art.
[0052] Examples of suitable excipients that may be used in the preparation of oral pharmaceutical compositions include, without limitation, silicon dioxide and other glidants known in the art as defined above.
[0053] Tablet fillers fill the dimensions of a tablet or capsule, making them practical for manufacture and convenient for the consumer. Fillers make it possible for the final product to have the proper volume for the patient to hold by increasing the overall volume. A good filler must be inert, compatible with the other components of the formulation, non-hygroscopic, relatively inexpensive, compactable, and preferably odorless or have a pleasant taste. Vegetable cellulose (pure vegetable filler) is a common filler in tablets or hard gelatin capsules. Dicalcium phosphate is another generic tablet filler. A variety of vegetable fats and oils can be ...
Embodiment 1
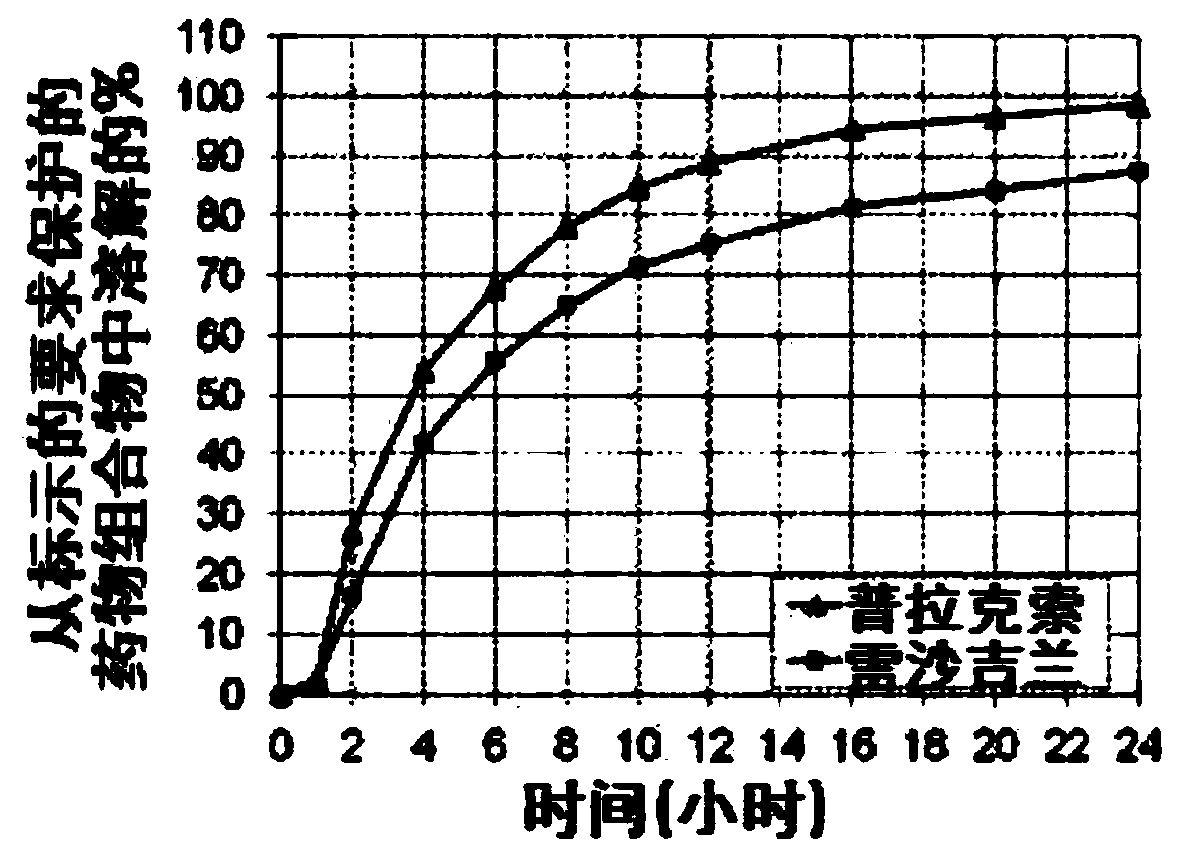
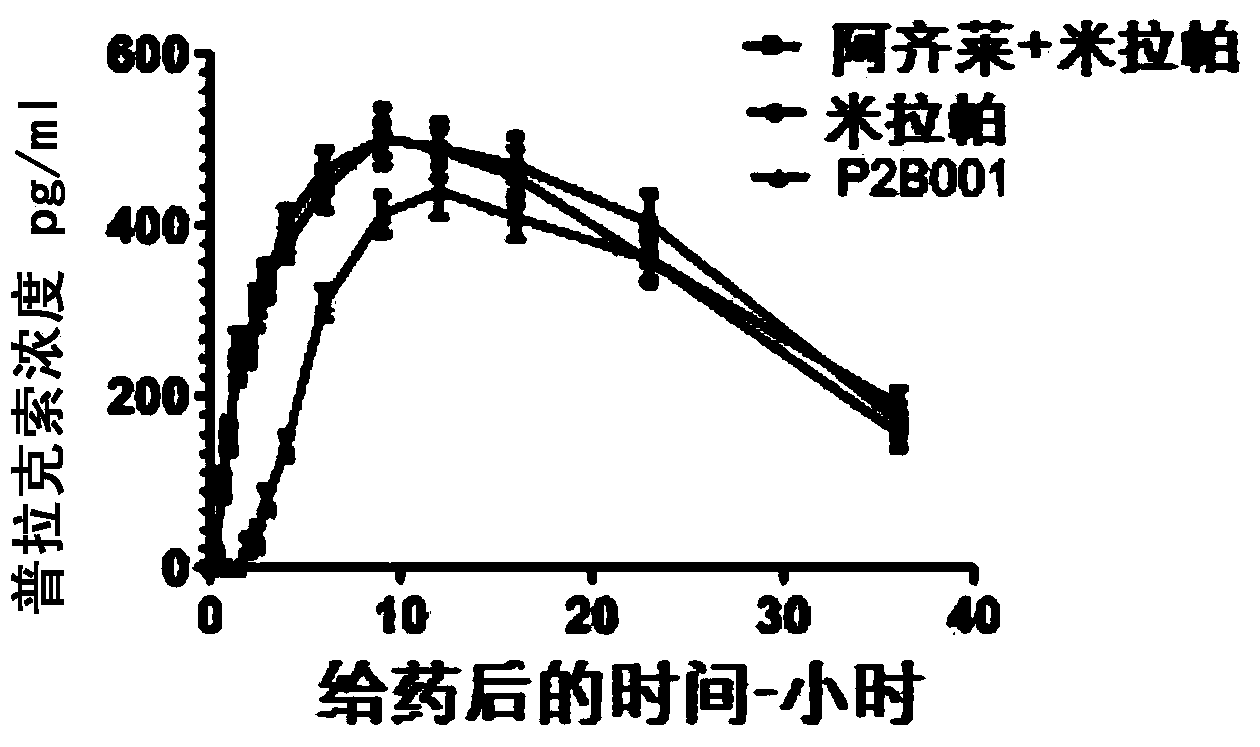
[0063] Example 1: Formulation and dissolution profile of the combination product.
[0064] Each component (Tables 1 and 2) was formulated separately and the beads were packaged in respective weights to give doses of 0.6 mg pramipexole and 0.75 mg rasagiline.
[0065] Analytical Method - Dissolution Test on the Combination Product
[0066] The method quantifies the active pharmaceutical ingredient (API), pramipexole ( PPX) and the dissolution profiles of rasagiline (RAS) were evaluated.
[0067] A sample of the contents of a capsule or a dose of beads (pellets) is placed in a basket which is rotated inside a vessel containing the medium at a constant specified rate and temperature. The sample dissolves in the vehicle solution over time at a rate that reflects the release profile of the formulation, so the solution contains different concentrations of API at different time points. At designated time points, samples were taken automatically or manually at designated time int...
Embodiment 2
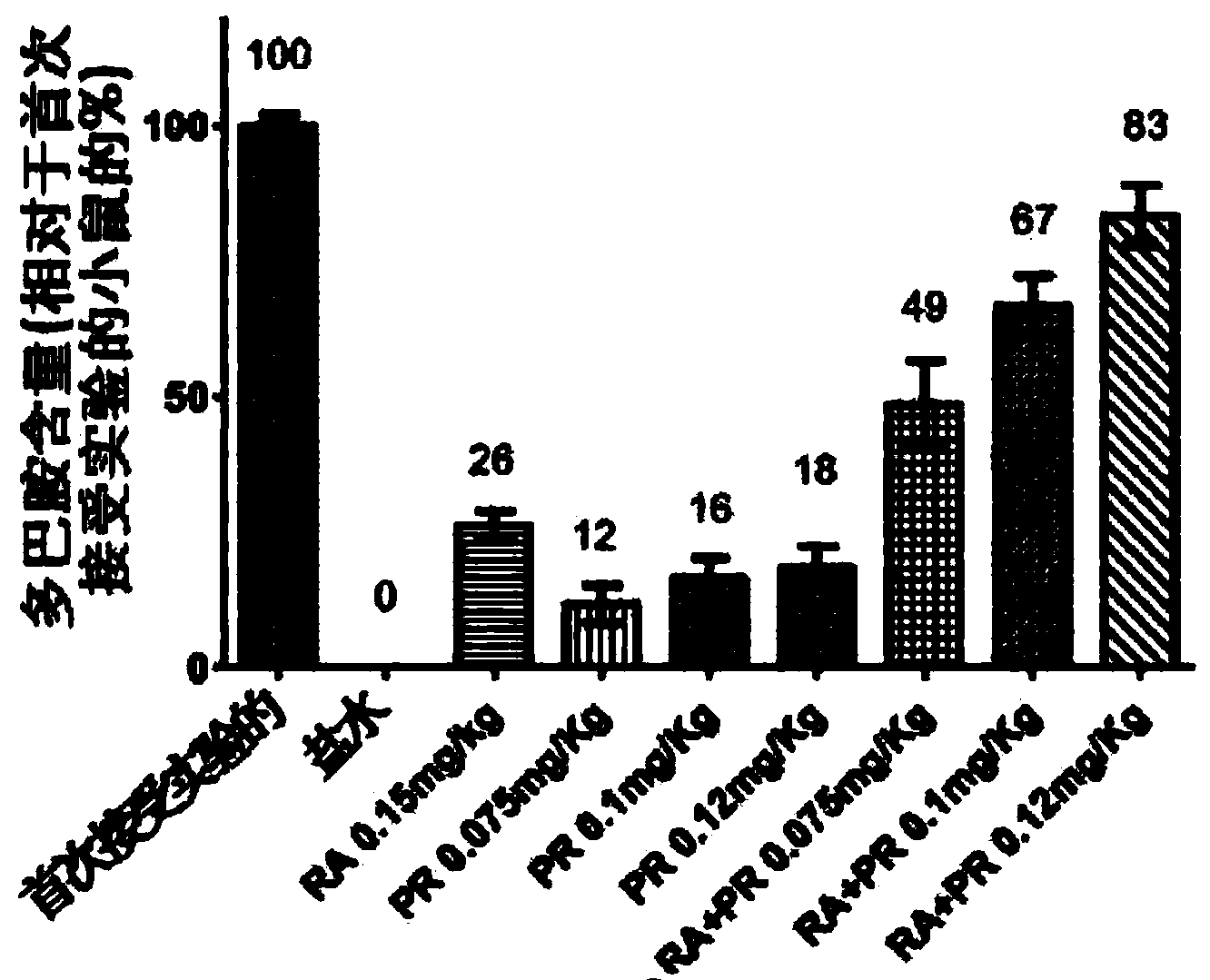
[0084] Example 2: Drug in vivo studies in the MPTP model of Parkinson's disease.
[0085] Materials and Methods
[0086] Models: Experimental models of Parkinson's disease (PD) are needed to gain insight into possible pathological mechanisms of the disease. In addition to this function, they are also essential in the development and testing of new therapeutic strategies, whether pharmacological or otherwise.
[0087] MPTP mouse model: Extensive biochemical data from anatomical studies of the human brain and from animal models suggest ongoing oxidative stress processes in the substantia nigra that trigger dopaminergic neurodegeneration. It is unclear whether oxidative stress is a primary or secondary event. However, oxidative stress induced by the neurotoxin MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) has been used in animal models to study neurodegenerative processes for the purpose of developing Antioxidant neuroprotective drugs.
[0088] Table 4: Group assign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com