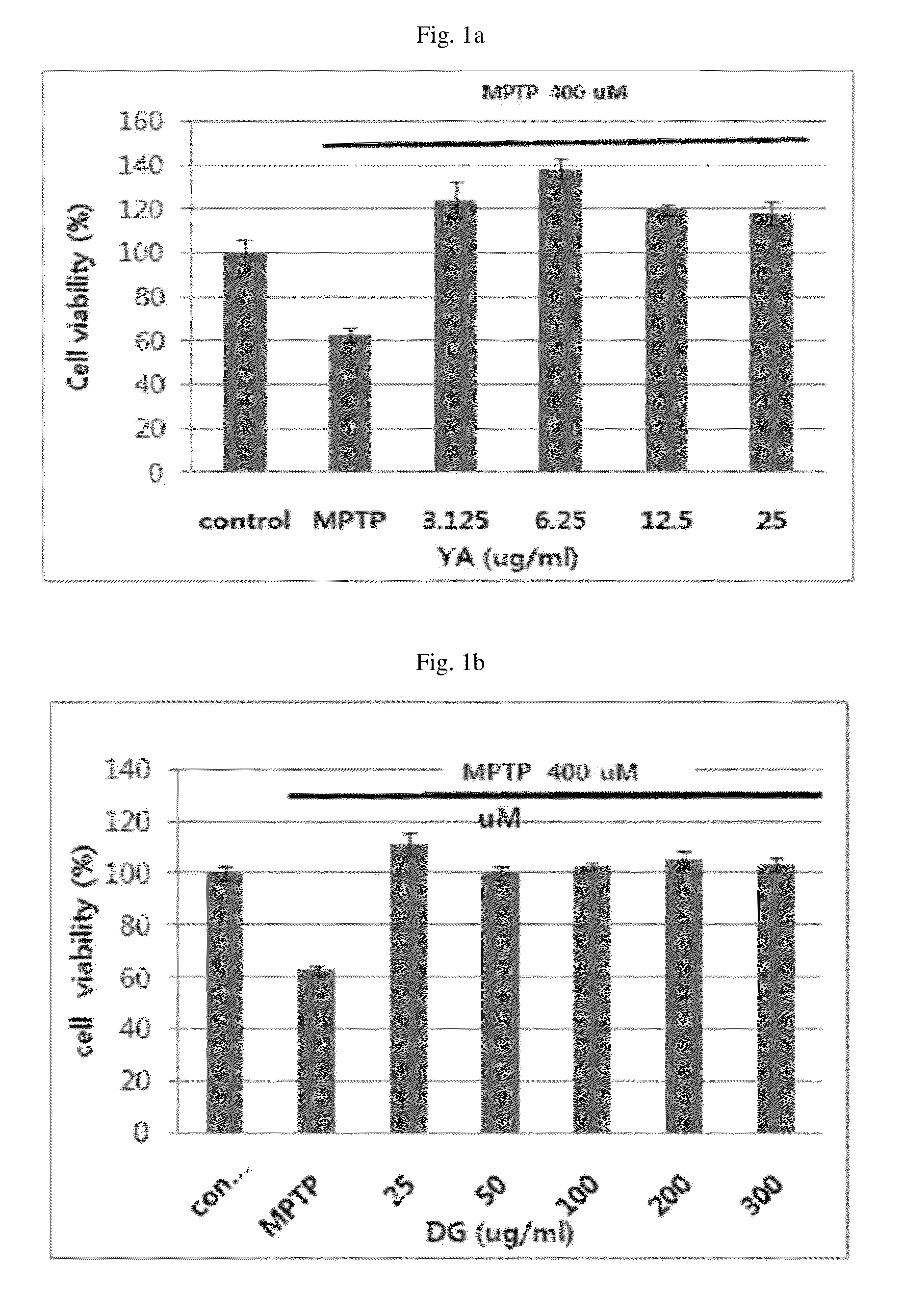
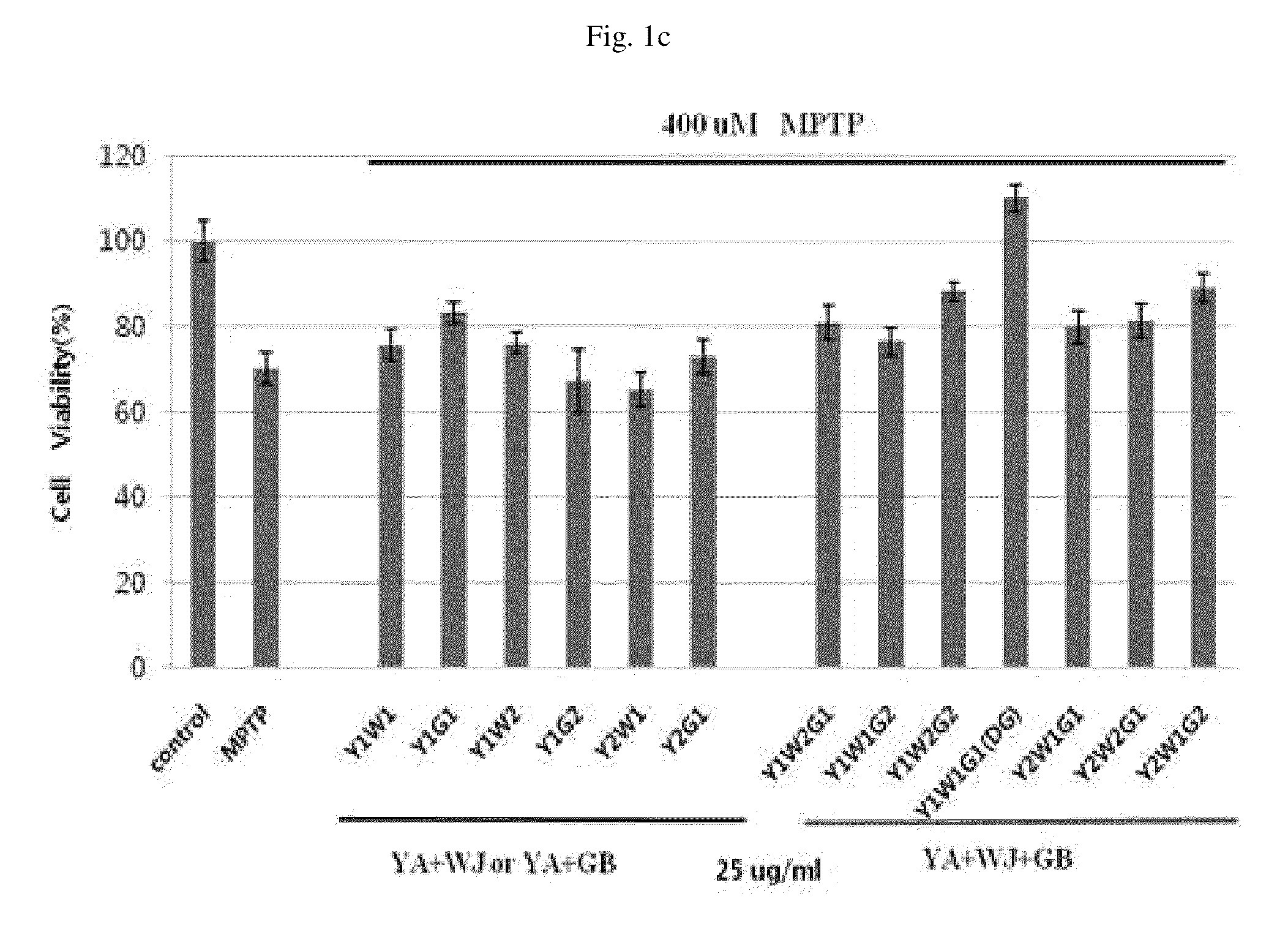
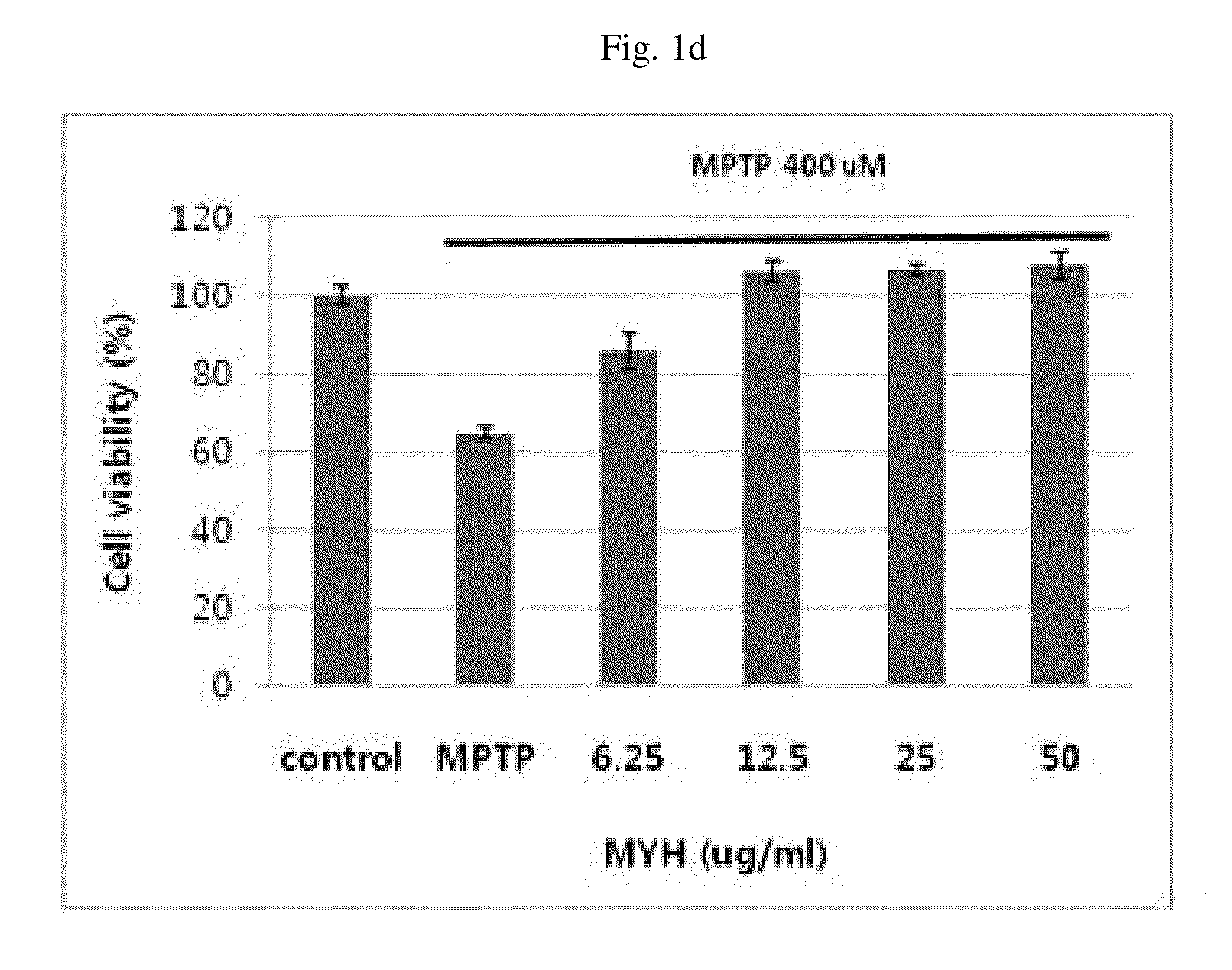
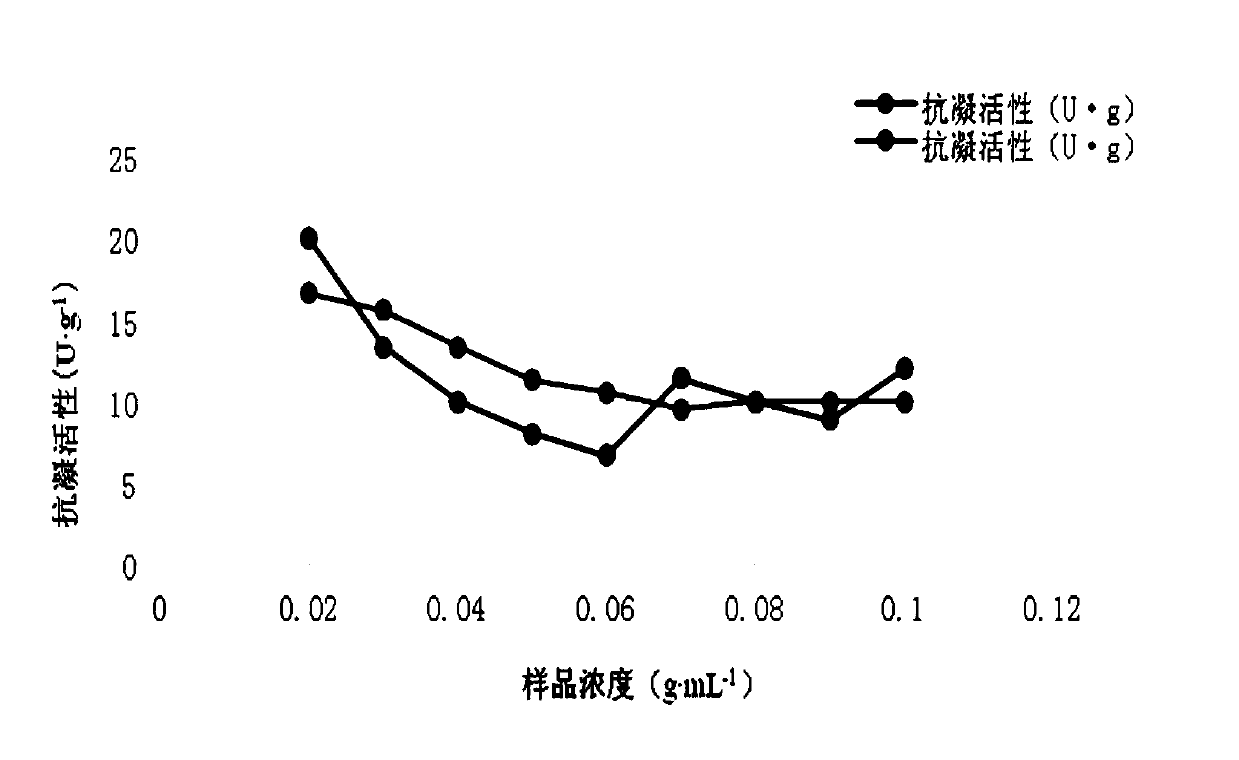
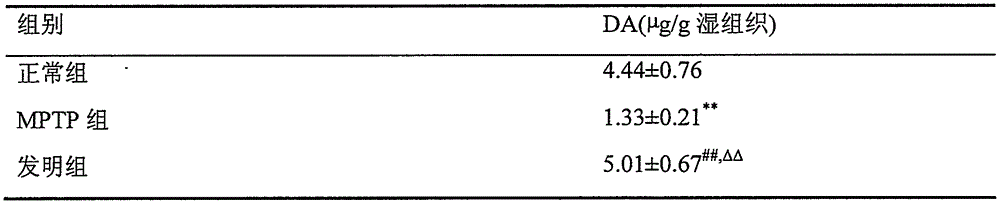
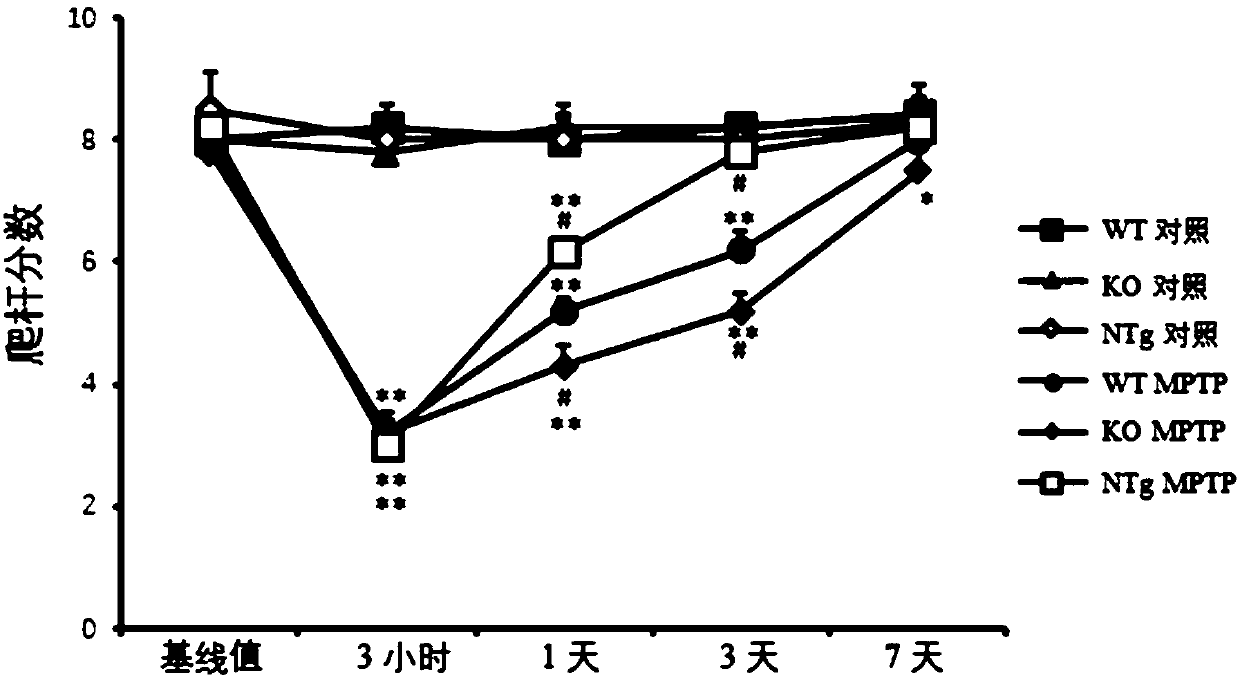
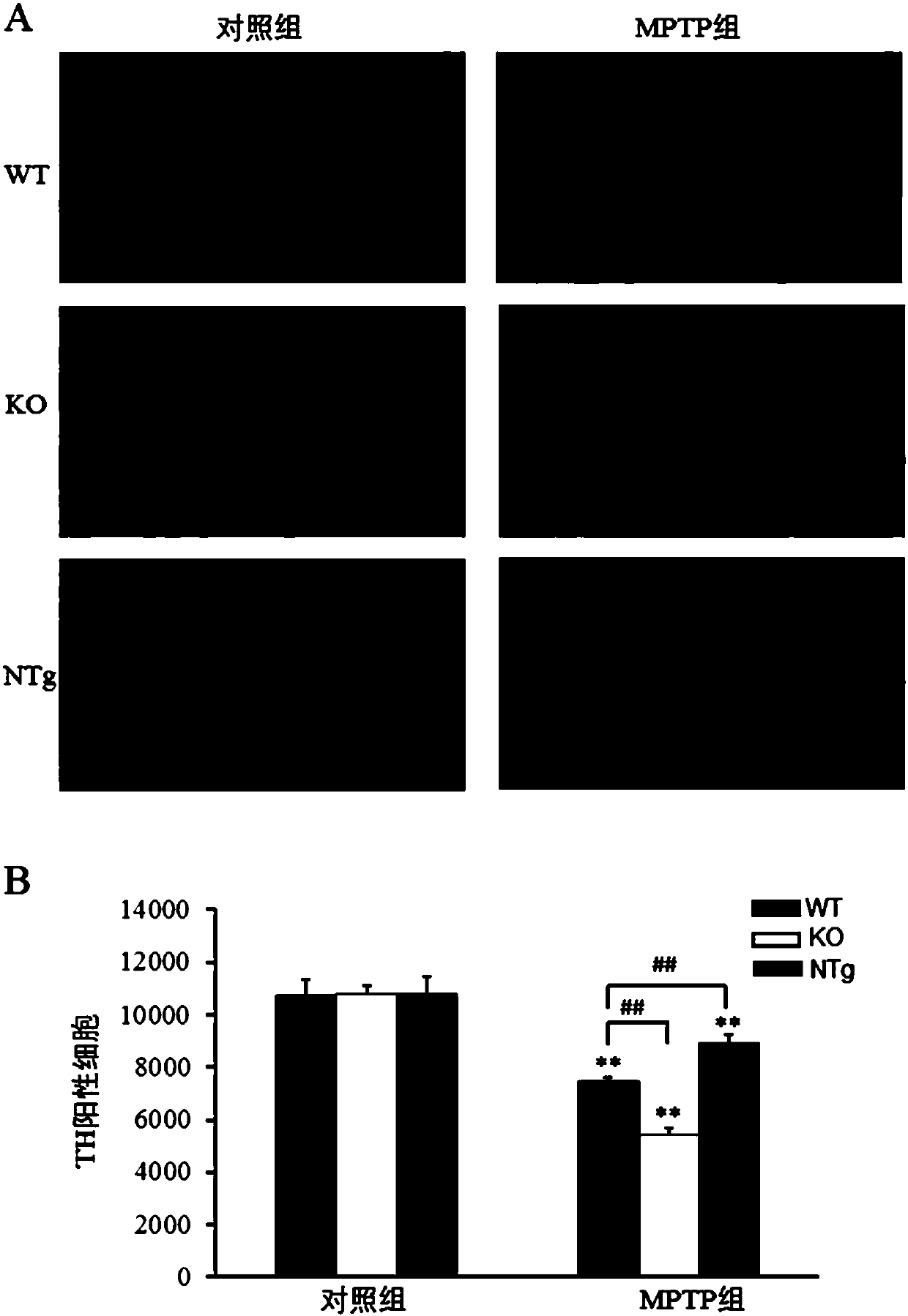
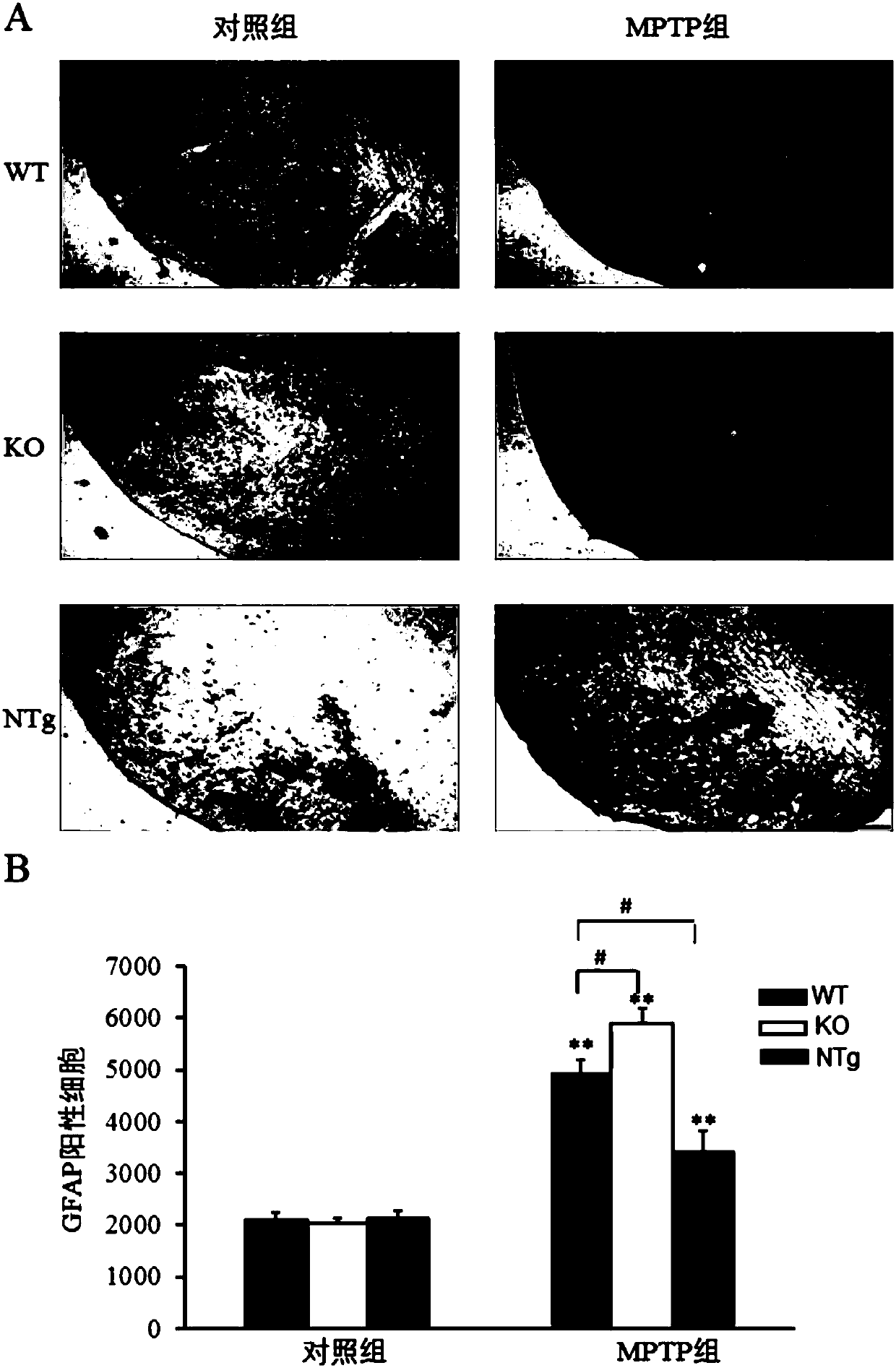
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "MPTP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
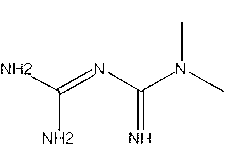
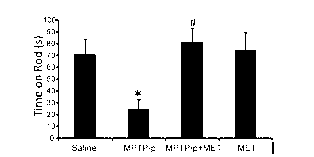
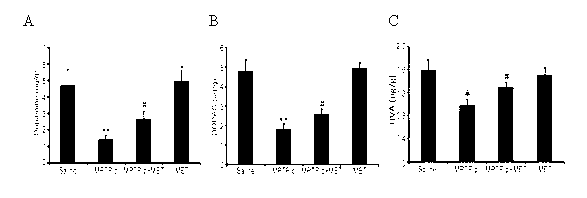
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a prodrug to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animal studies.
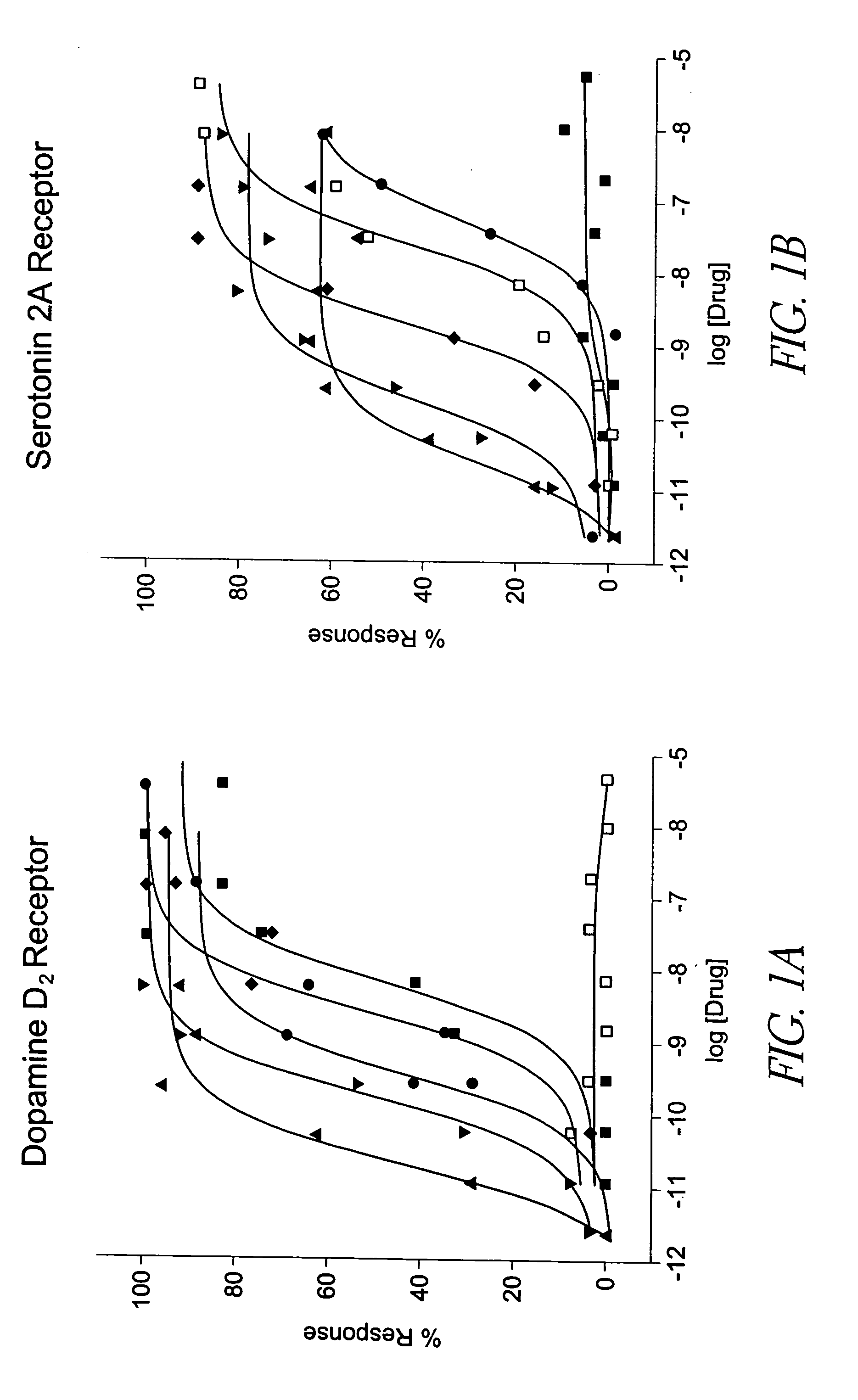
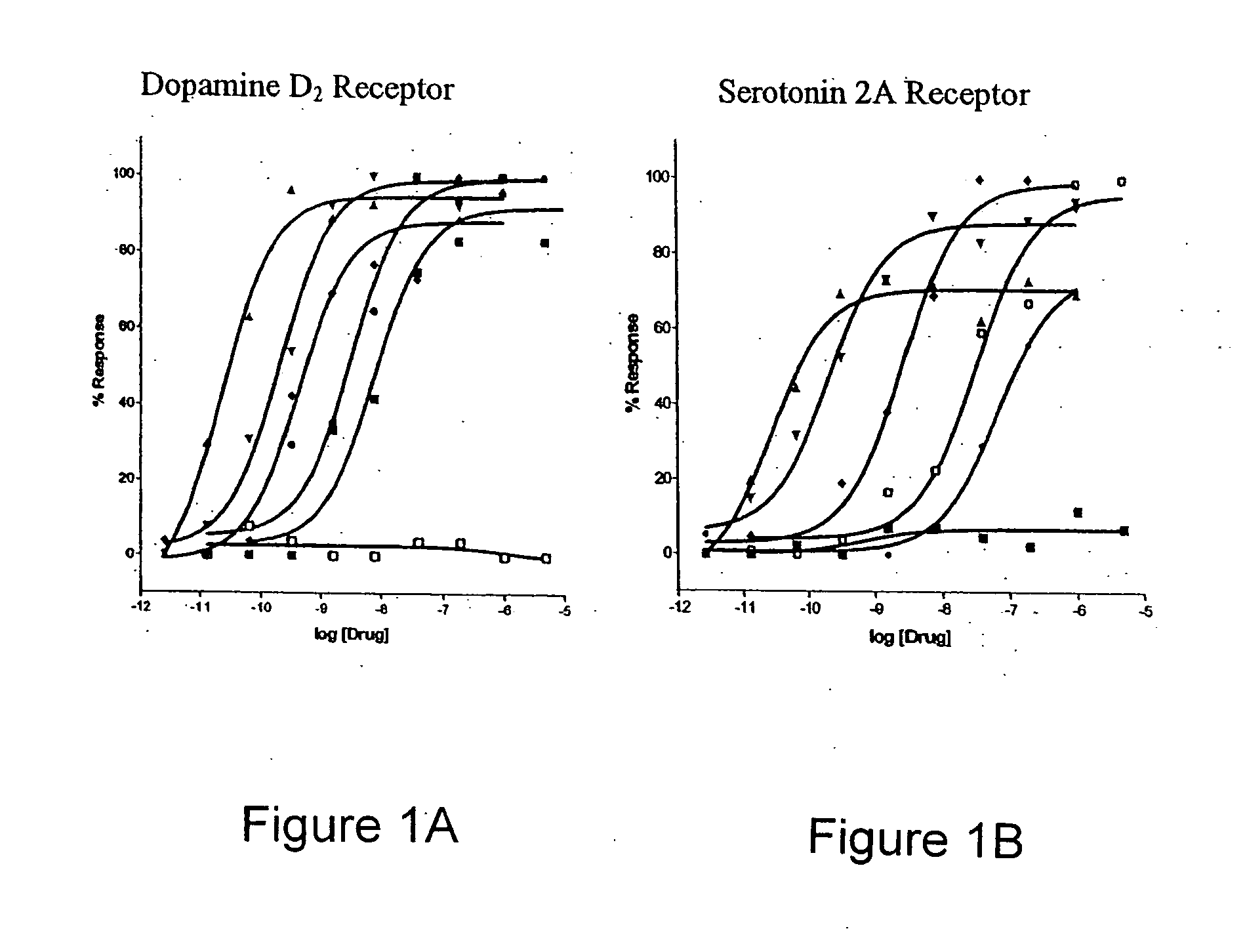
Selective serotonin 2A/2C receptor inverse agonists as therapeutics for neurodegenerative diseases
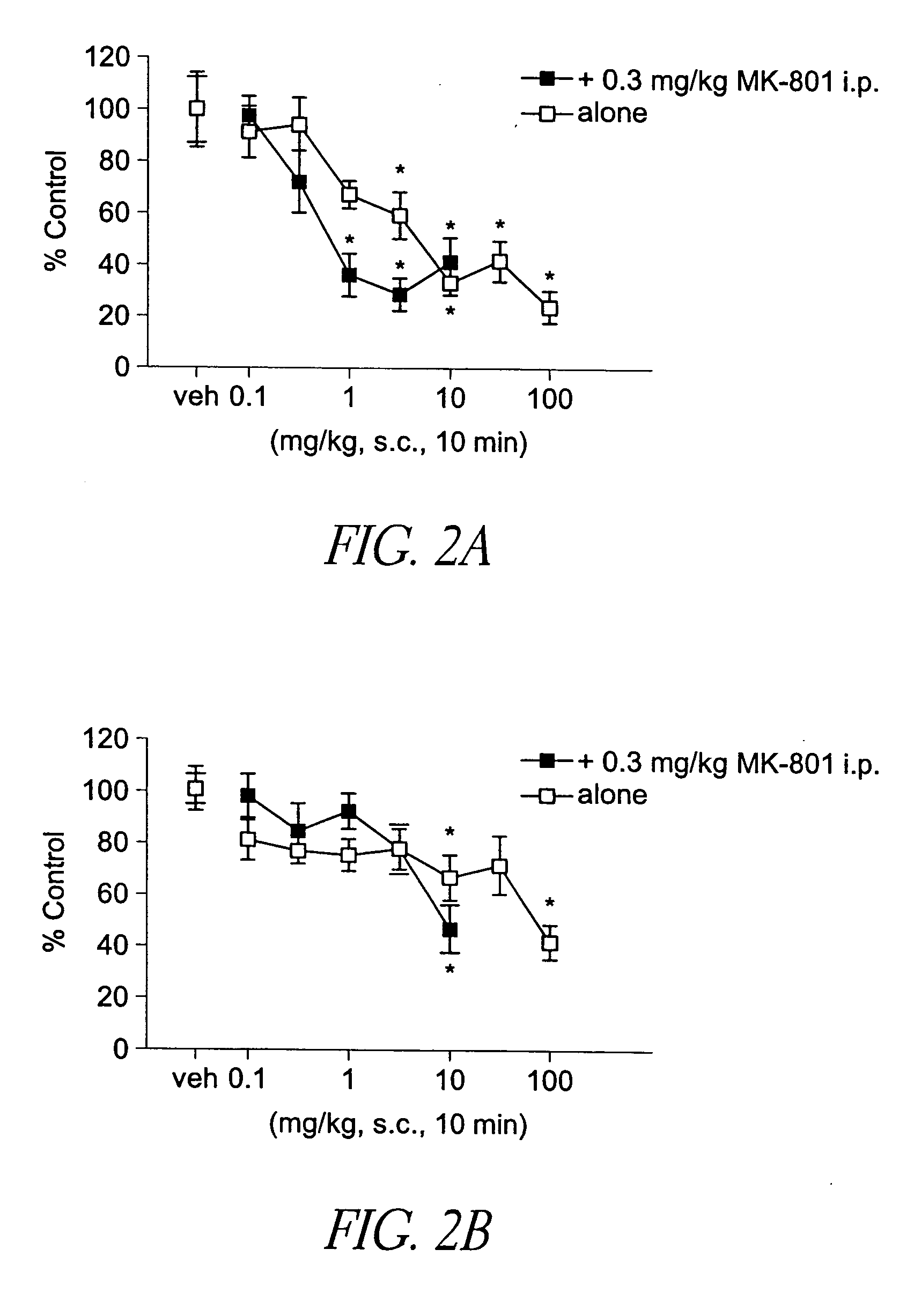
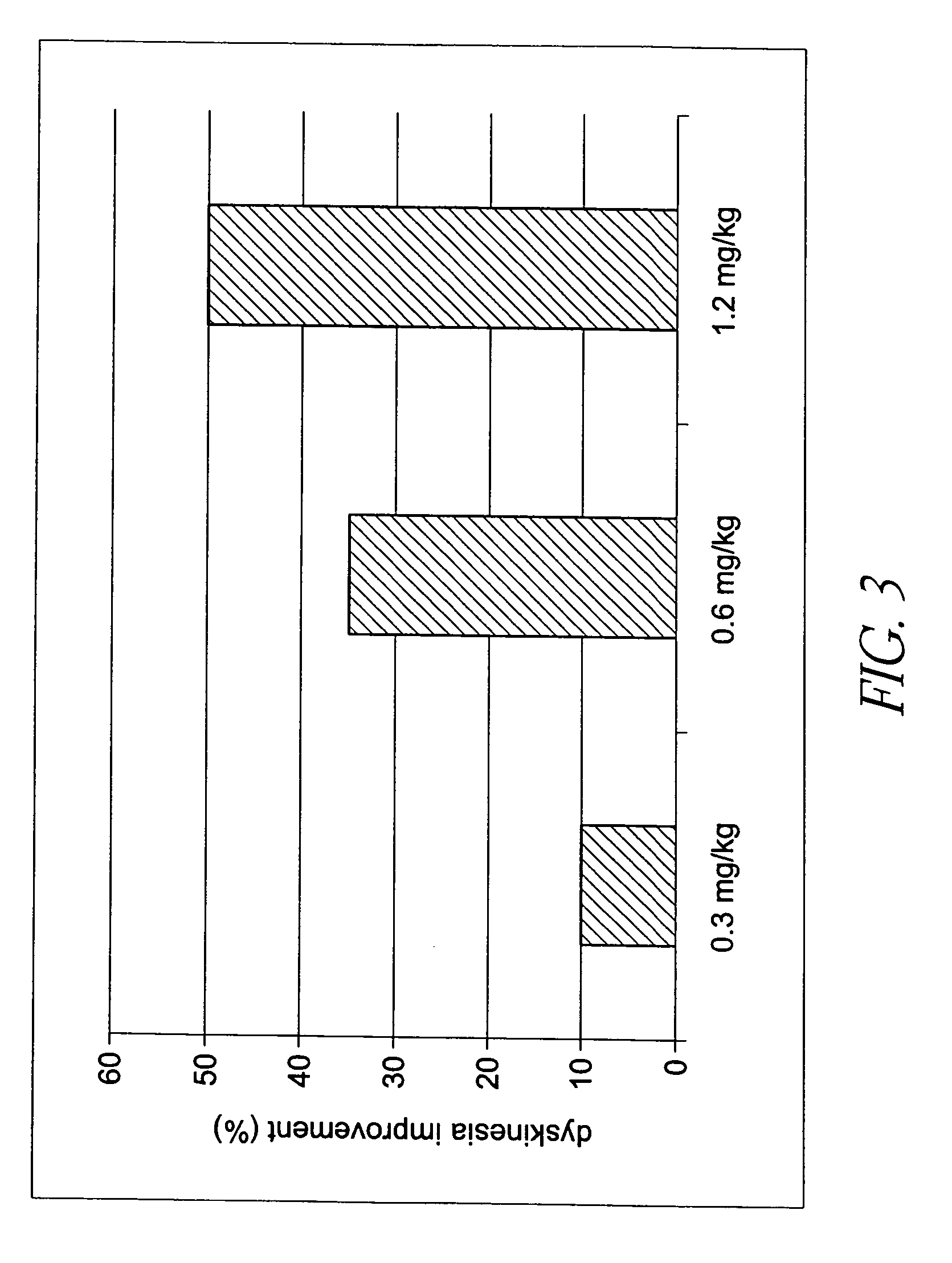
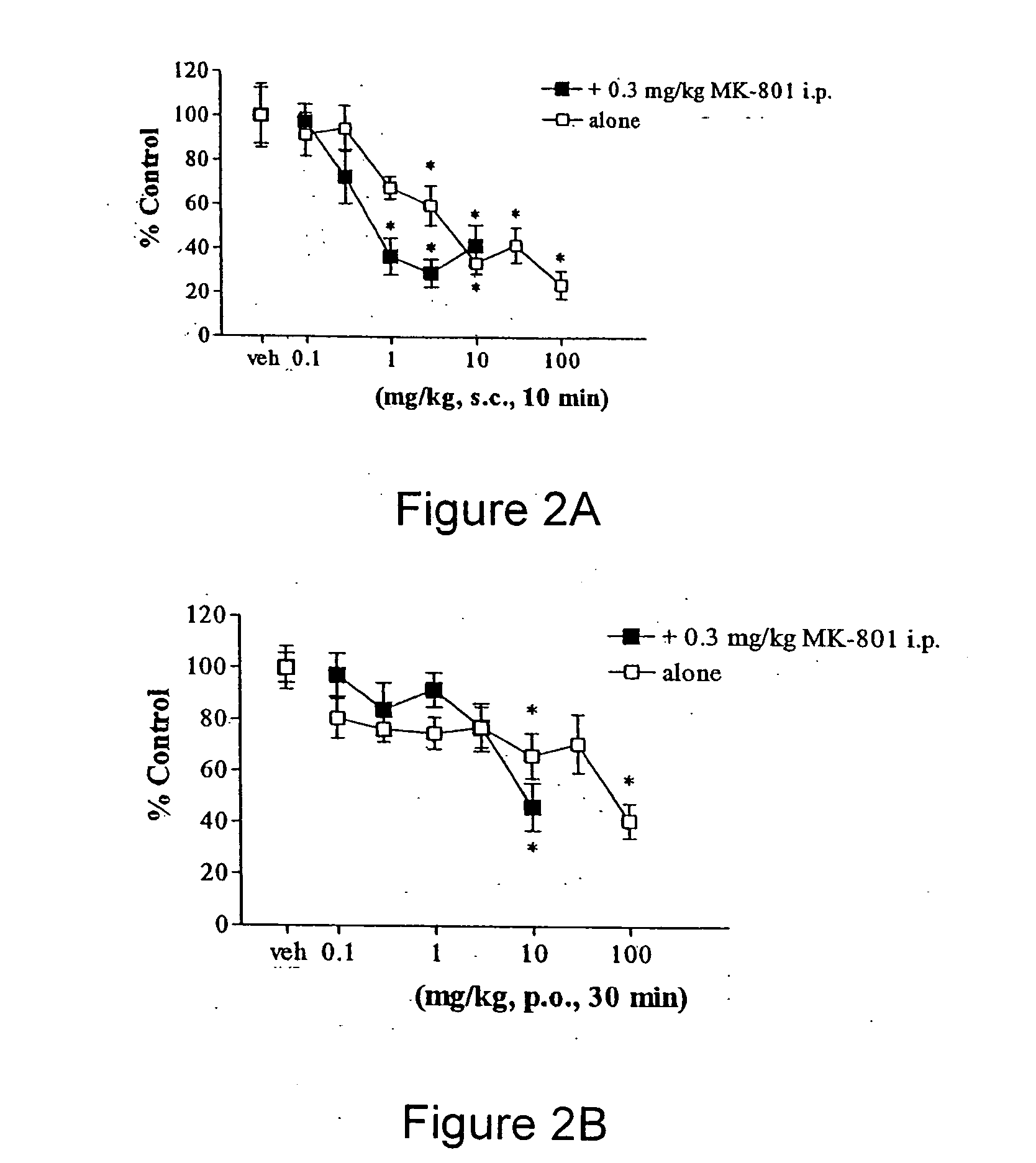
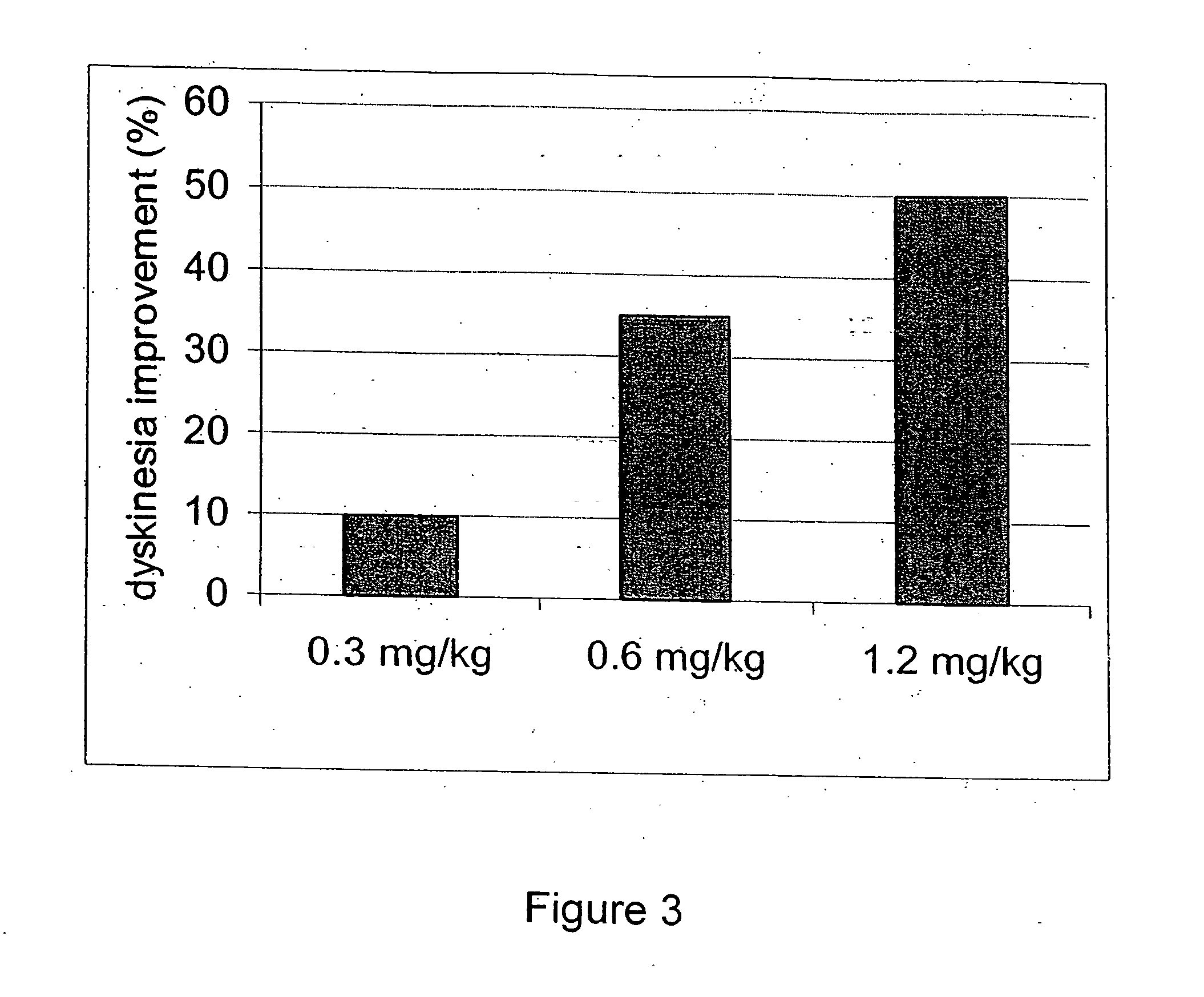
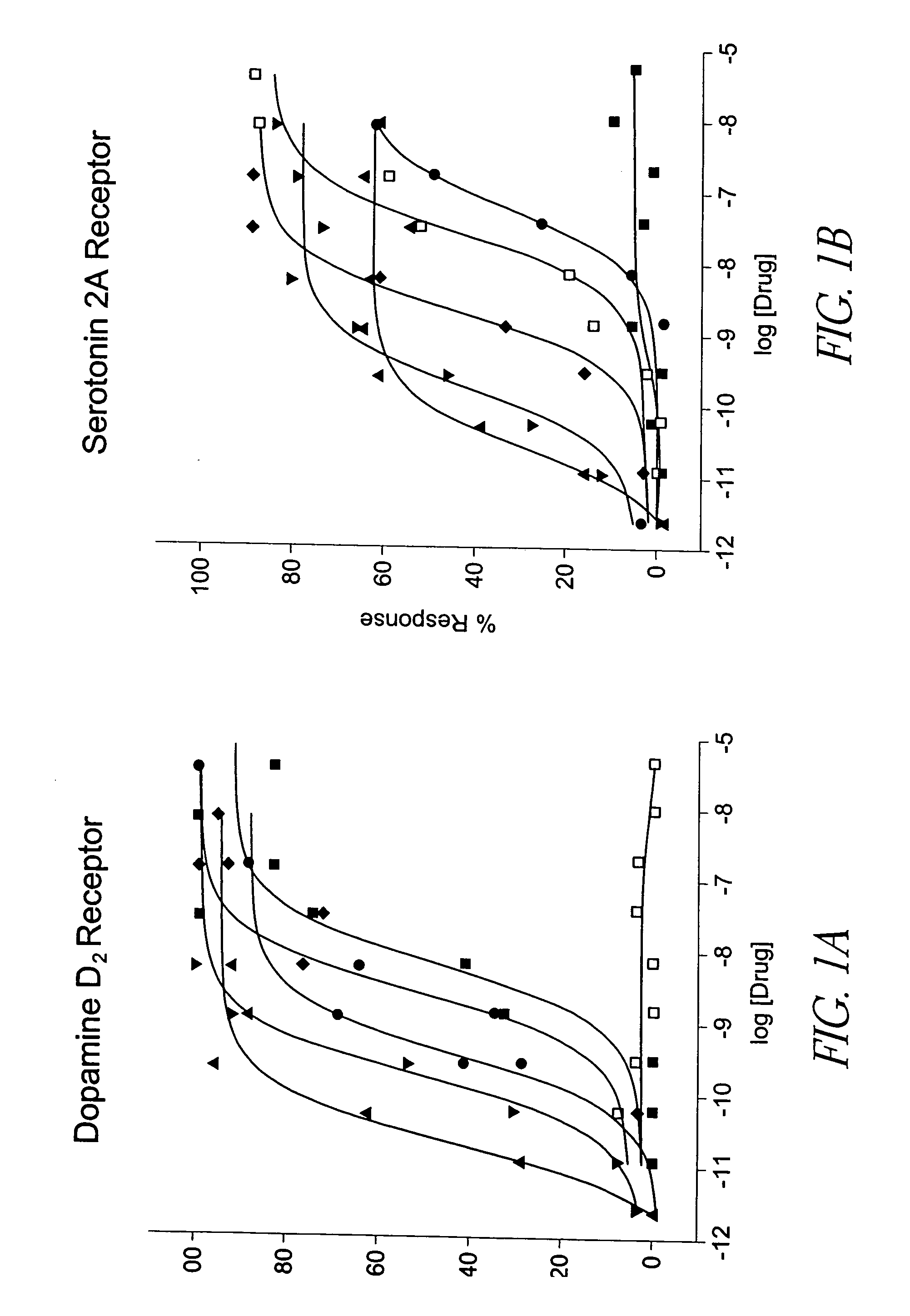
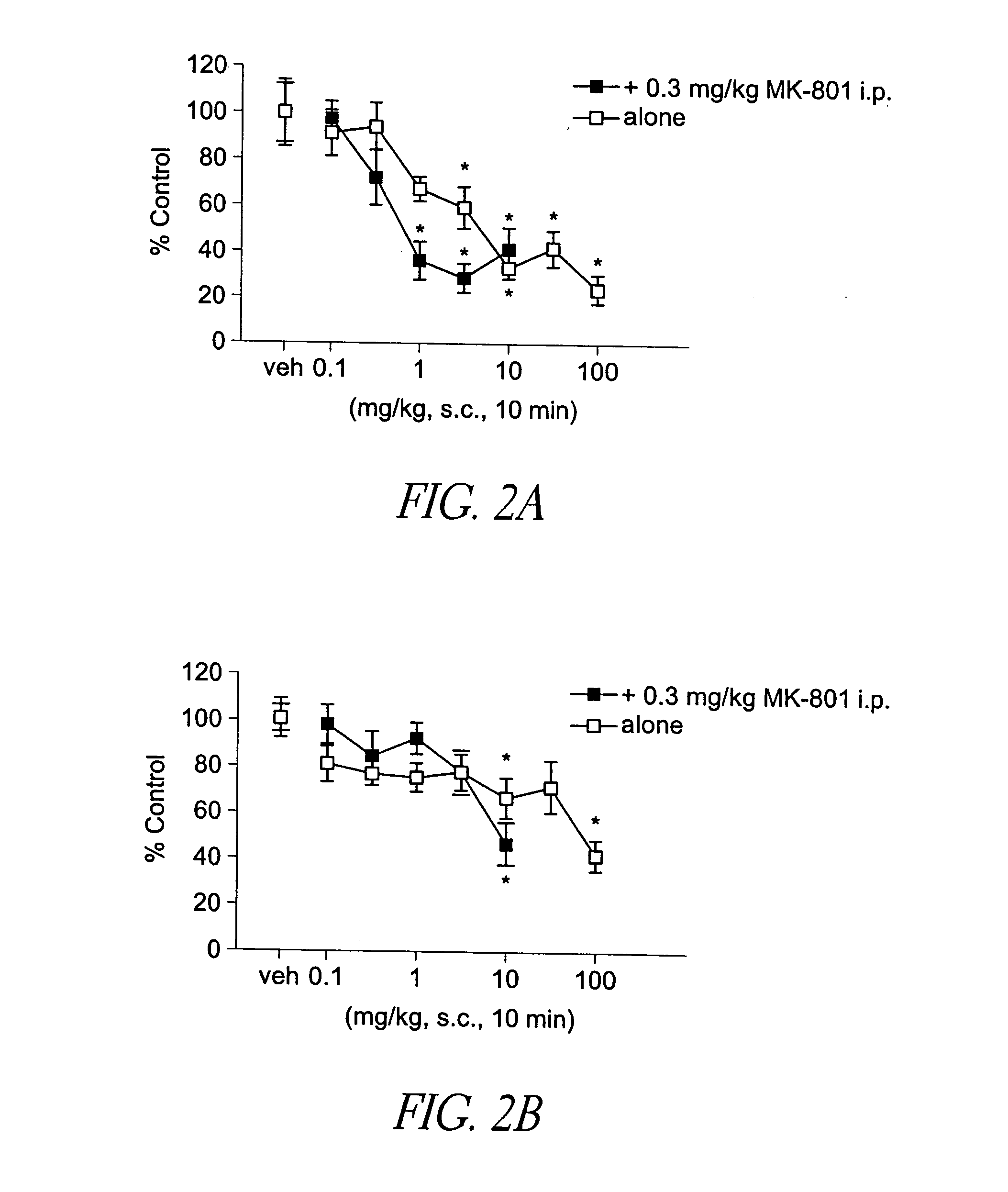
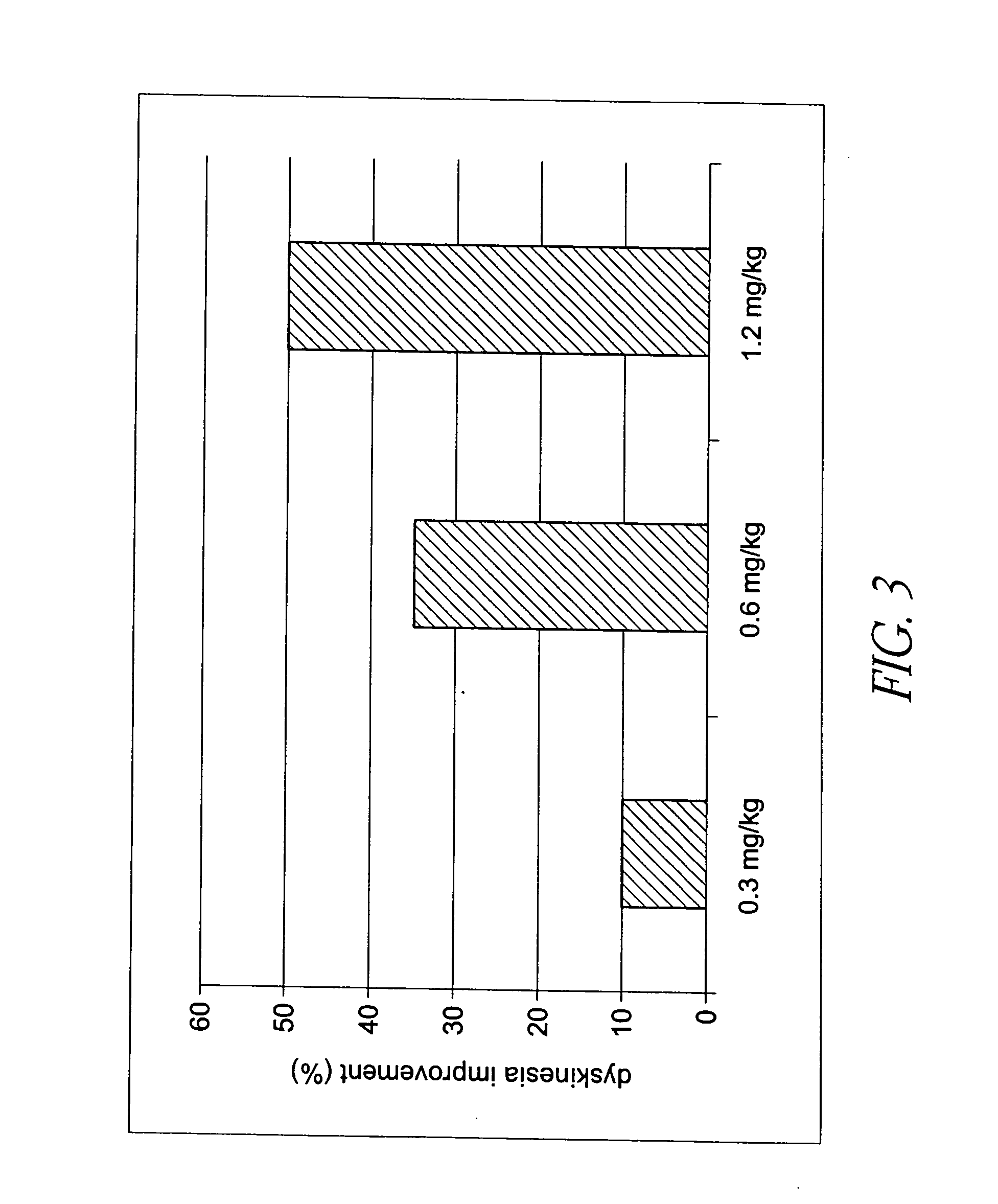
Behavioral pharmacological data with the compound of formula (I), a novel and selective 5HT2A / 2C receptor inverse agonist, demonstrate in vivo efficacy in models of psychosis and dyskinesias. This includes activity in reversing MK-801 induced locomotor behaviors, suggesting that this compound may be an efficacious anti-psychotic, and activity in an MPTP primate model of dyskinesias, suggesting efficacy as an anti-dyskinesia agent. These data support the hypothesis that 5HT2A / 2C receptor inverse agonism may confer antipsychotic and anti-dyskinetic efficacy in humans, and indicate a use of the compound of formula (I) and related agents as novel therapeutics for Parkinson's Disease, related human neurodegenerative diseases, and psychosis.
Owner:ACADIA PHARMA INC
Selective serotonin 2A/2C receptor inverse agonists as therapeutics for neurodegenerative diseases
ActiveUS20060199842A1Inhibition of activationInhibition is effectiveBiocideNervous disorderMPTPNeuro-degenerative disease
Behavioral pharmacological data with the compound of formula (I), a novel and selective 5HT2A / 2C receptor inverse agonist, demonstrate in vivo efficacy in models of psychosis and dyskinesias. This includes activity in reversing MK-801 induced locomotor behaviors, suggesting that this compound may be an efficacious anti-psychotic, and activity in an MPTP primate model of dyskinesias, suggesting efficacy as an anti-dyskinesia agent. These data support the hypothesis that 5HT2A / 2C receptor inverse agonism may confer antipsychotic and anti-dyskinetic efficacy in humans, and indicate a use of the compound of formula (I) and related agents as novel therapeutics for Parkinson's Disease, related human neurodegenerative diseases, and psychosis.
Owner:ACADIA PHARMA INC
Selective serotonin 2A/2C receptor inverse agonists as therapeutics for neurodegenerative diseases
Behavioral pharmacological data with the compound of formula (I), a novel and selective 5HT2A / 2C receptor inverse agonist, demonstrate in vivo efficacy in models of psychosis and dyskinesias. This includes activity in reversing MK-801 induced locomotor behaviors, suggesting that this compound may be an efficacious anti-psychotic, and activity in an MPTP primate model of dyskinesias, suggesting efficacy as an anti-dyskinesia agent. These data support the hypothesis that 5HT2A / 2C receptor inverse agonism may confer antipsychotic and anti-dyskinetic efficacy in humans, and indicate a use of the compound of formula (I) and related agents as novel therapeutics for Parkinson's Disease, related human neurodegenerative diseases, and psychosis.
Owner:ACADIA PHARMA INC
Acrylamido derivatives useful as inhibitors of the mitochondrial permeability transition
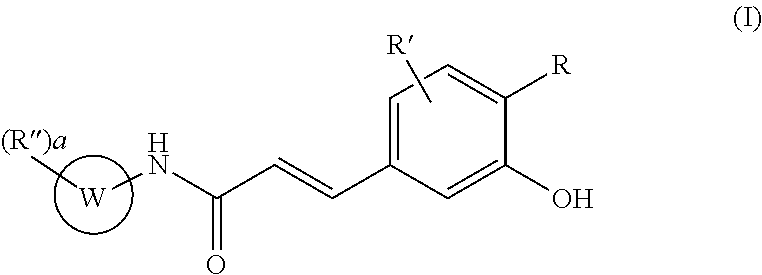
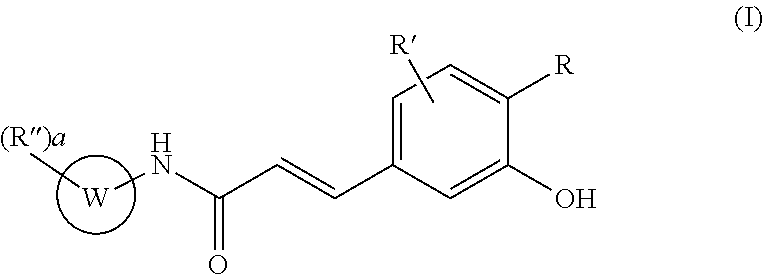
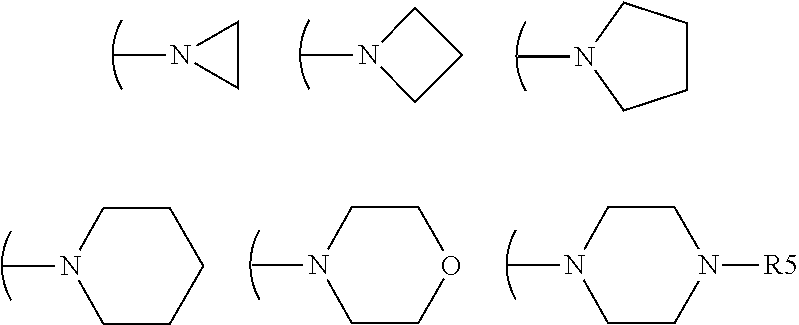
Acrylamido derivatives useful as therapeutic agents, particularly for the prevention and / or treatment of diseases and conditions associated with the activity of the mitochondrial permeability transition pore (MPTP), such as the diseases characterized by ischemia / reperfusion, oxidative or degenerative tissue damage, are herein described. These compounds belong to the structural formula (I) wherein R, R′, R″, W and a are as defined in the specification. The invention also relates to the preparation of these compounds, as well as to pharmaceutical compositions comprising them.
Owner:CONGENIA
Application of turmeric native
InactiveCN101366709AAvoid damageNervous disorderKetone active ingredientsDiseaseDopamine biosynthesis
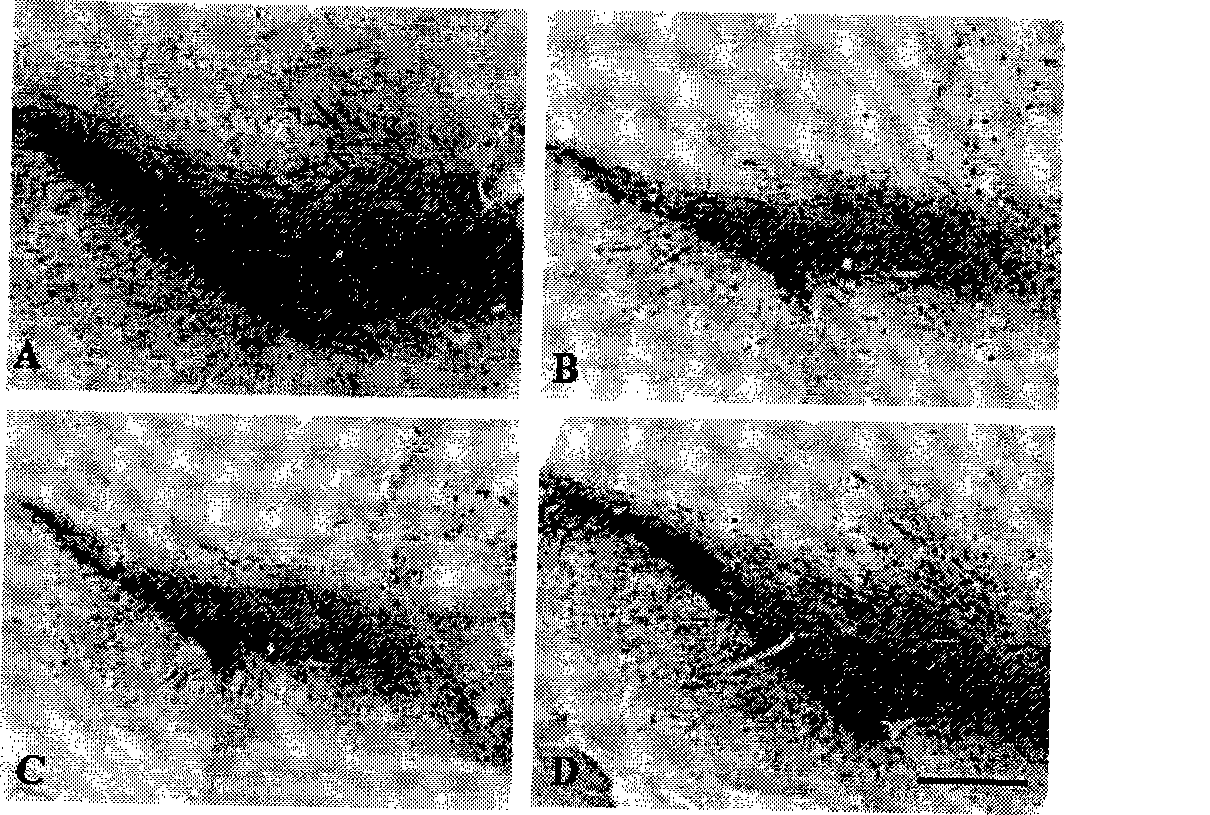
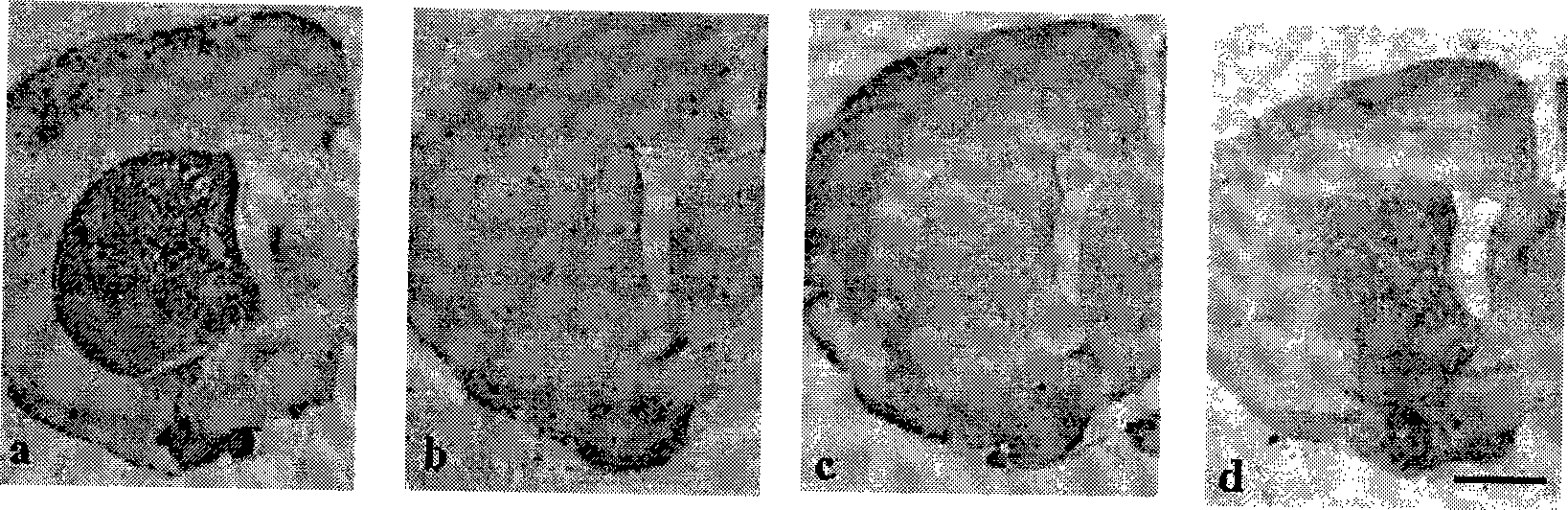
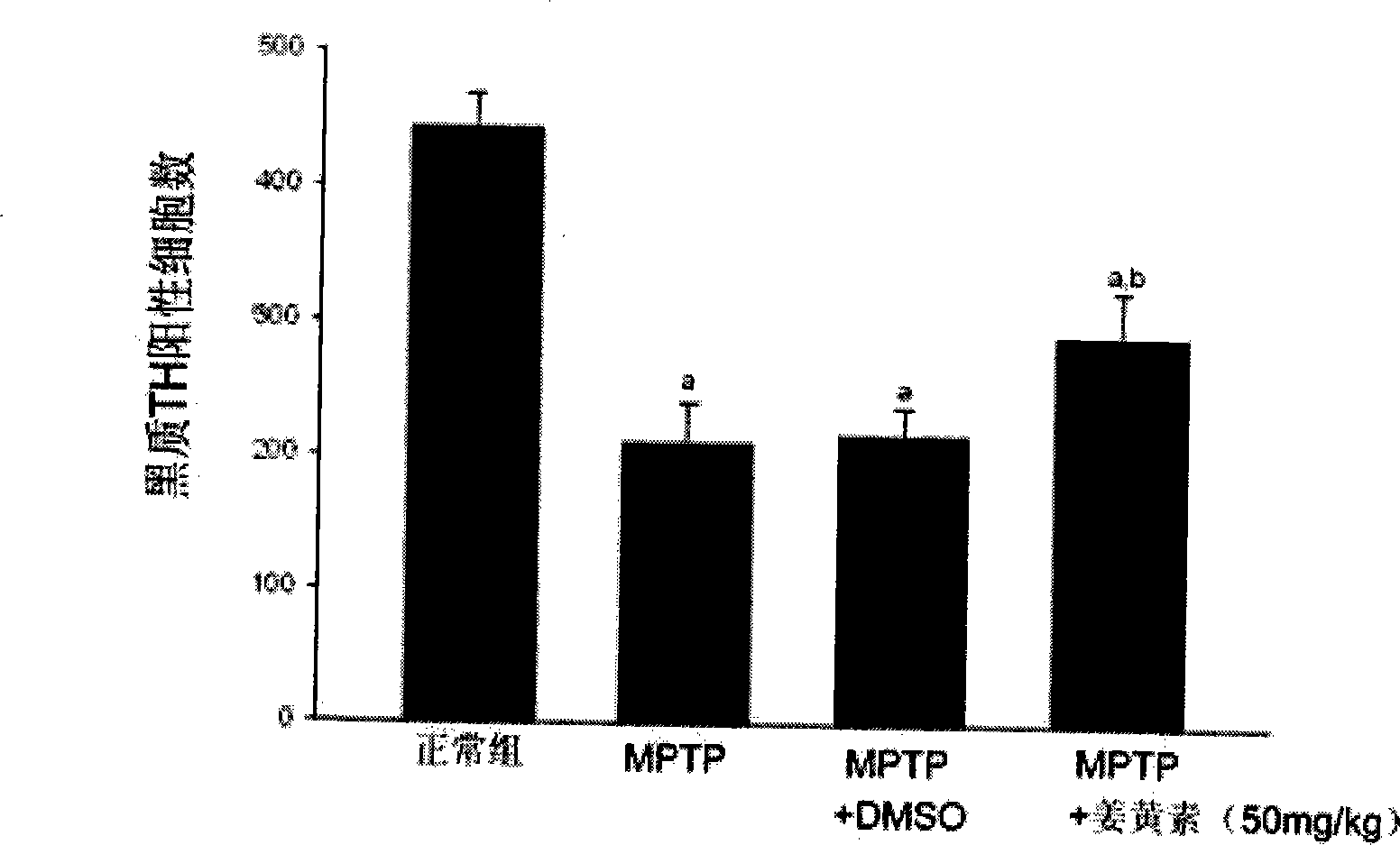
The invention discloses novel application of a medicine - curcumin, namely application of the curcumin in preventing and treating or treating the Parkinson disease. After the curcumin acts on an MPTP-constructed Parkinson disease mouse model, the curcumin can effectively promote expression of tyrosine hydroxylase in substantia nigra, lighten damage of sneurotoxin on the tyrosine hydroxylase in substantia nigra, reduces damage of dopaminergic neurons, promote biosynthesis of damaged dopamine and promote restoration and growth of damaged dopaminergic neurons; the curcumin can also inhibit astrocytes to secrete gelatinous fiber proteins, reduce stimulus of the neurotoxin on the astrocytes and lighten phlegmonosis of a brain tissue; moreover, the curcumin can inhibit expression of induction type nitrous oxide synthase, lighten induction of the neurotoxin on the induction type nitrous oxide synthase and inhibit reinforcement of intracephalic oxidative stress reaction. Therefore, the curcumin can effectively reduce damage of the dopaminergic neurons, thereby having significant potential value in developing medicines for preventing and treating and / or treating the Parkinson disease.
Owner:RUIJIN HOSPITAL ATTACHED TO SHANGHAI NO 2 MEDICALUNIV +1
Metal-organic framework-superoxide dismutase assembly, preparation method and application thereof in preparation of drugs for treating Parkinson
InactiveCN109833468AProtection stabilityEasy to synthesizeNervous disorderPeptide/protein ingredientsSuper oxide dismutaseTreatment effect
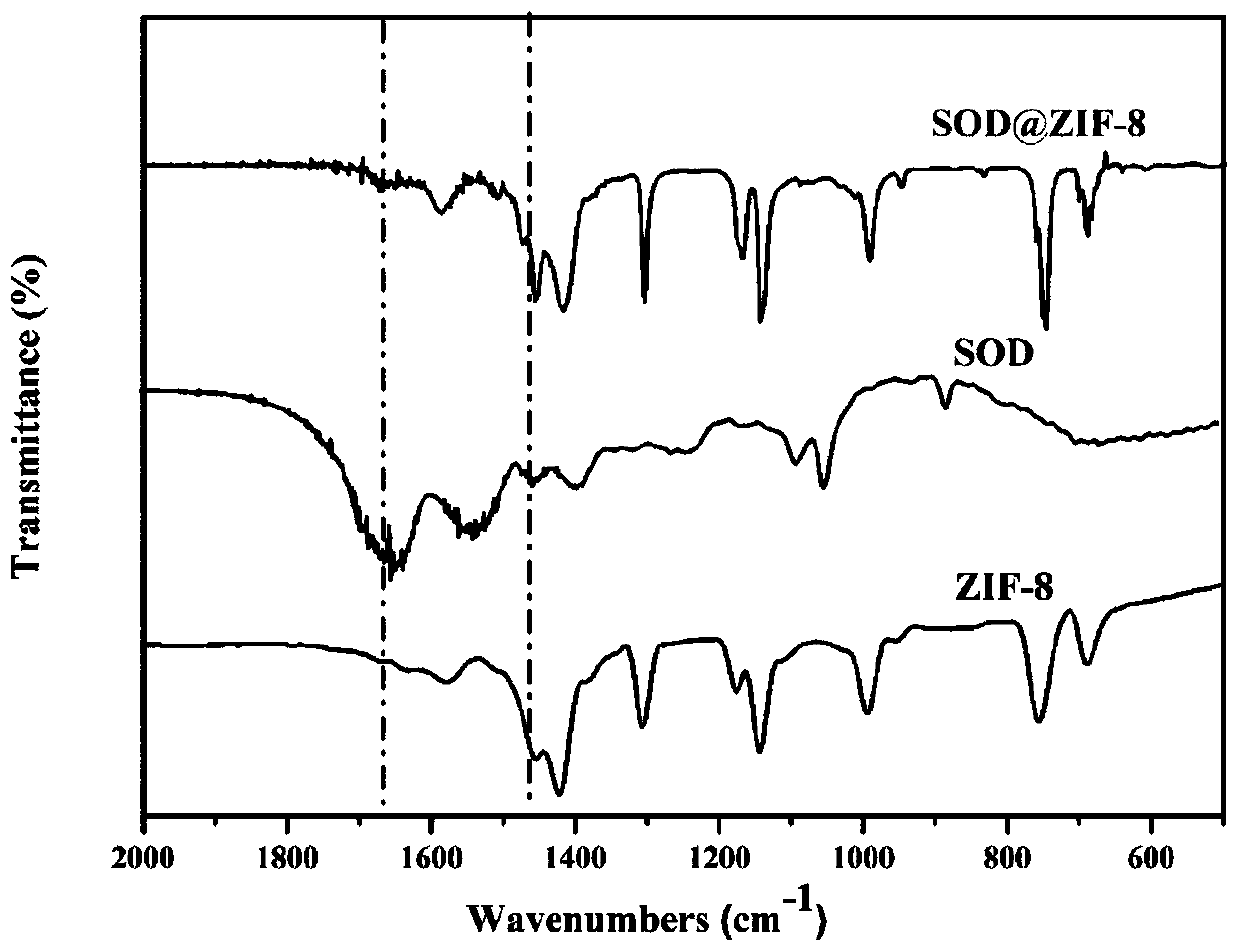
The invention provides a metal-organic framework-superoxide dismutase assembly, a preparation method and an application thereof in preparation of drugs for treating Parkinson, and belongs to the technical field of biology. The framework makes use of biomimetic mineralization assembly strategy to immobilize SOD enzyme molecules into a metal-organic framework ZIF-8, coordination bonds are formed between SOD amino and zinc ions of ZIF-8, so that metal organic framework-SOD enzyme assembly (SOD@ZIF-8) are constructed based on enzymes. The temperature stability of SOD enzyme molecules and pH tolerance are improved through the frame work, by treating the SOD@ZIF-8 assembly as a therapeutic drug, at the cellular level, active oxygen produced by stimulation of MPP+ can be removed effectively, so as to relieve apoptosis caused by active oxygen; at the animal level, after intravenous chemotherapy by the assembly, the barrier in sports ability of Parkinson model mouse constructed by MPTP can be relieved, the expression level of tyrosine hydroxylase in substantia nigra is improved, the therapeutic effect is good.
Owner:JILIN UNIV
Composition comprising longan arillus extract or combined extract comprising the same for treating neurodegenerative disease
ActiveUS20110318435A1Effective preventionEffective treatmentBiocideNervous disorderNervous systemTreatment effect
This disclosure relates to a composition for prevention or treatment of neurodegenerative disease comprising longan arillus extract, or combined extract comprising longan arillus. The composition exhibits remarkably excellent prevention or treatment effect of neurodegenerative disease by containing longan arillus extract or combined extract comprising longan arillus, and particularly, it may effectively prevent or treat neurodegenerative disease by significantly protecting dopaminergic neurons from neurotoxicity due to MPTP selectively acting on dopaminergic nervous system and neurotoxicity due to aggregation of alpha-synuclein proteins.
Owner:MEDI HELP LINE CO LTD
Application of imatinib mesylate in preparation of drugs for resisting Parkinson's disease (PD)
InactiveCN102406648AImprove motor dysfunctionImprove cognitive impairmentOrganic active ingredientsNervous disorderPhosphorylationApoptosis
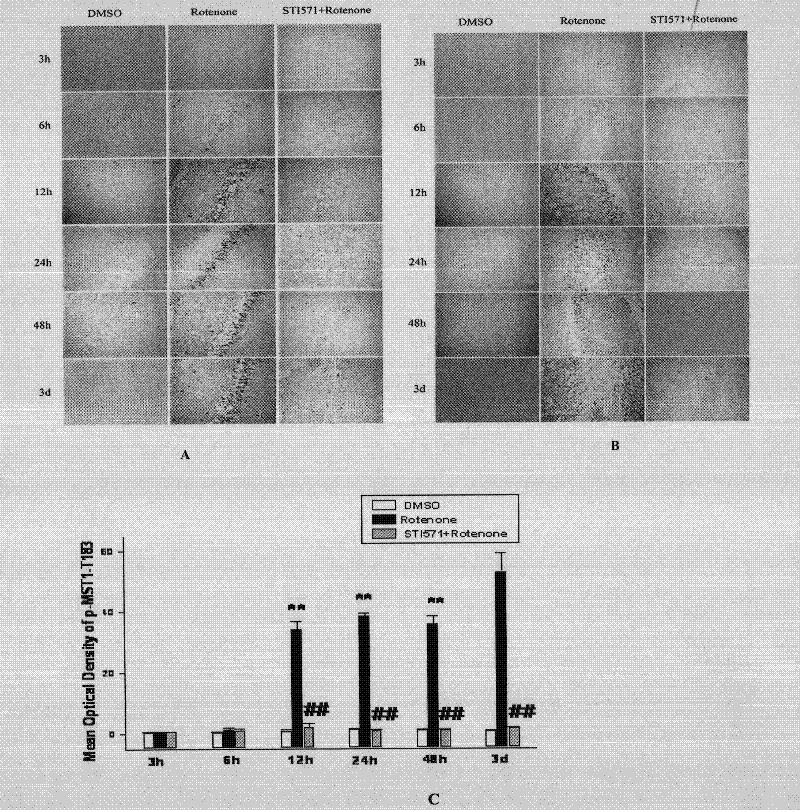
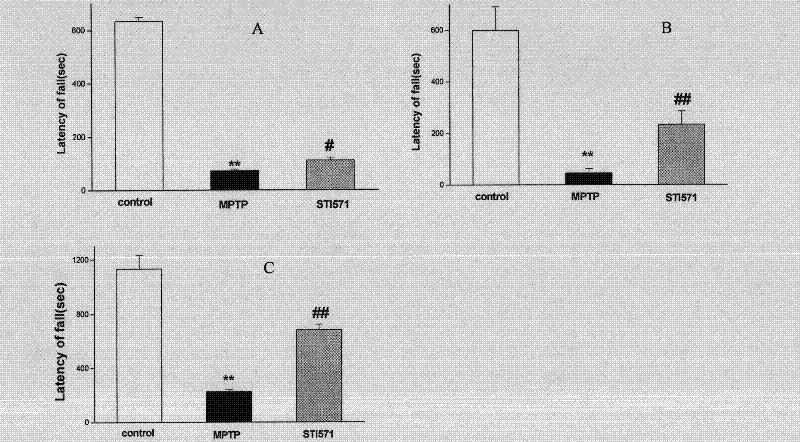
The invention discloses an application of imatinib mesylate in preparation of drugs for resisting Parkinson's disease (PD). The application of imatinib mesylate (STI571) in preparation of drugs for treating neurodegenerative diseases belongs to the protective range of the invention, and the neurodegenerative diseases can be PD. An experiment proves that by continuous administration of the STI571, the dyskinesia induced by MPTP can be obviously improved, and the cognitive dysfunction of mice can be improved. The STI571 can obviously improve the dopaminergic neuron loss induced by the MPTP, can inhibit the rat CA1 and DG (diacylglycerol) region nerve cell apoptosis reaction induced by rotenone, can obviously inhibit the primary substantia nigra neuron apoptosis and Lewy protein expression induced by rotenone, and can inhibit the phosphorylation activation of PKC (Protein Kinase C) and Akt. The STI571 used as a specific inhibitor of c-Ab1 can resist the formation of PD, can improve the behavioral abnormity and cognitive dysfunction of PD, can be used as a new candidate drug for resisting neurodegenerative diseases such as PD and the like, and has favorable application prospects; and the clinical indications of the STI571 are greatly expanded.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Application of isorhynchophylline to preparation of medicine with nerve protection effect
InactiveCN108186637ARescue Toxic EffectsSlow down heart rateOrganic active ingredientsNervous disorderDiseasePoisonous effects
The invention provides application of isorhynchophylline to preparation of a medicine with a nerve protection effect. The medicine with the nerve protection effect, provided by the invention, can effectively relieve the toxic effect of the neurotoxin MPTP to the dopaminergic neuron and has a protection effect on the dopaminergic neuron. Aiming at the common effects of the isorhynchophylline on reducing blood pressure, expanding blood vessels and slowing down the heart rate, the invention develops new application of the isorhynchophylline and provides a theoretical foundation and an experimental basis for further researching the neuropharmacology of the traditional Chinese medicinal material uncaria; furthermore, the isorhynchophylline provides a new idea and possibility for development ofmedicines for treating Parkinson's disease, medicines for resisting depression and medicines for treating Alzheimer's disease; and particularly, the isorhynchophylline has especially prominent effectsby serving as a medicine for treating Parkinson's disease.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
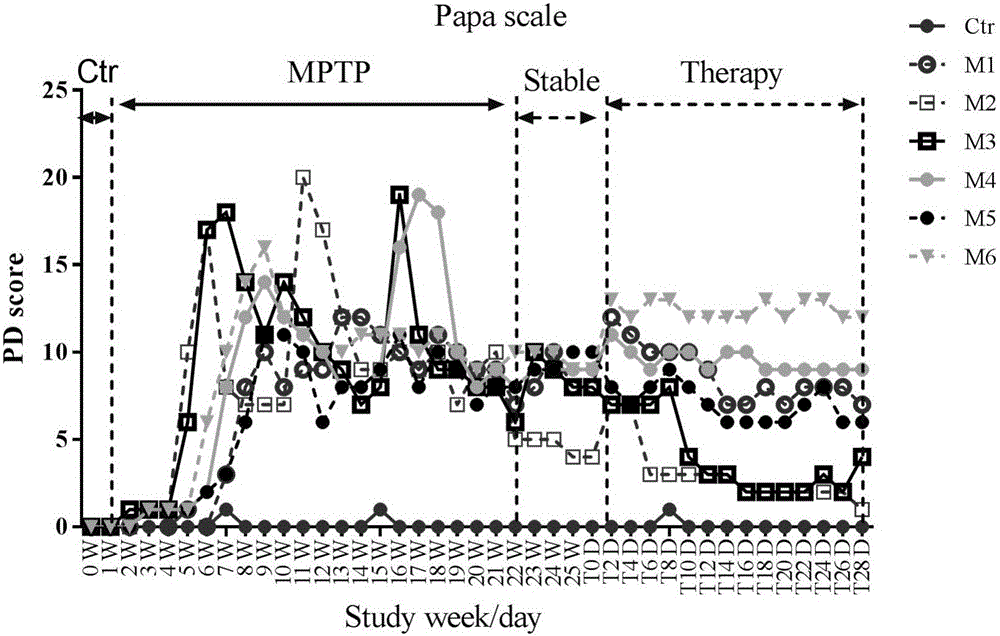
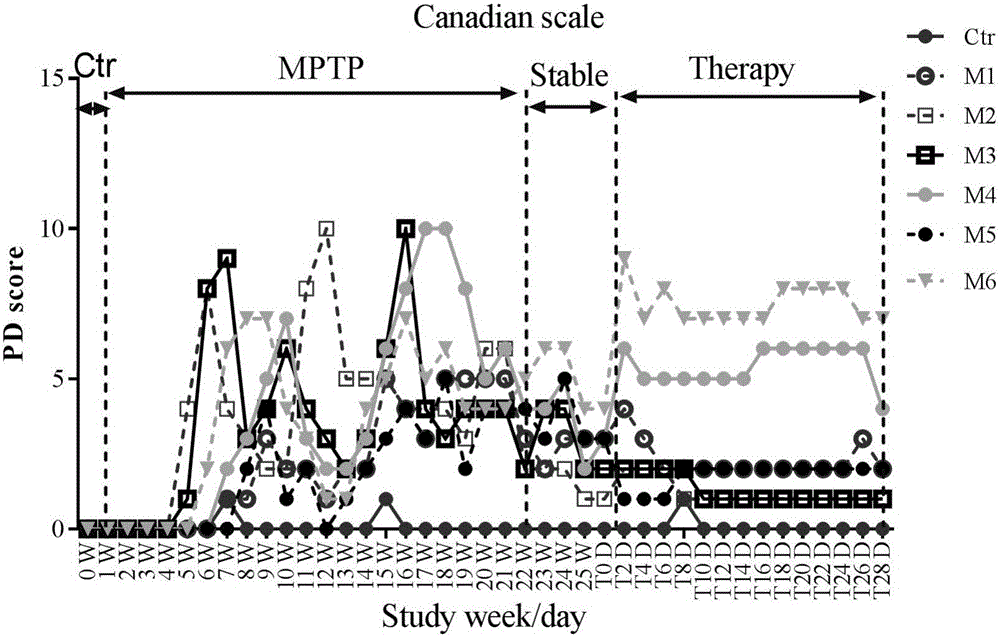
Model of monkey with chronic systemic Parkinson's disease and method for sieving medicament for treating chronic systemic Parkinson's disease
ActiveCN102526047AReduce the number of timesLong lastingOrganic active ingredientsNervous disorderMedicineMPTP
The invention provides a method for producing a model of monkey with chronic systemic Parkinson's disease. The MPTP with the dose of 0.2mg / kg is fed to the monkey for the first time, then the MPTP with the same dose is fed to the monkey every 2 days for 7 continuous times, and in the following days, the MPTP is fed to the monkey every 3 days for 7 continuous times. The invention further provides a method for sieving a medicament for treating the chronic systemic Parkinson's disease. The modeling method has the advantages that: the modeling operation is simple and is easy to implement, the modeling cost is low, the times of injecting the medicament is few, the model maintaining time is long, the symptom of the model of the monkey is typical, the severity of the symptom of the model of the monkey is moderate, and the monkey is easy to feed.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
Method for establishing monkey systematic Parkinson's disease model
The invention discloses a method for establishing a monkey systematic Parkinson's disease model. 0.2mg / kg of MPTP is slowly injected into the vein of lower extremity of a monkey at a flow rate of 1ml / min once daily and is continuously injected for 10-13 days, and the injection is stopped when the Kurlan clinical score is more than or equal to 10; at 3-12 days after the injection is stopped, if the Kurlan scale clinical behavior score is more than or equal to 15 and is stable for at least three months, a stable monkey systematic Parkinson's disease model is obtained; the monkey is a middle-aged and elderly cynomolgus monkey. The cynomolgus monkeys with individual differences and big individual differences can be established into a stable Parkinson's disease model; the obtained monkey systematic Parkinson's disease model can not spontaneously recover; the modeling process and characteristics are similar to the course of primary Parkinson's disease, so a stable model carrier can be provided for the Parkinson's disease pathogenesis study, the pharmacodynamics study and other translational medicine preclinical studies.
Owner:广西南宁灵康赛诺科生物科技有限公司
Application of metformin in preparations of drugs for treatment of Parkinson's disease
InactiveCN102743364AImprove behavioral disordersAvoid damageOrganic active ingredientsNervous disorderMetaboliteMPTP
The present invention provides an application of metformin in preparations of drugs for treatment of Parkinson's disease (PD). Experiment results show that: with the metformin, behavioral disorders of PD model mice are significantly improved; dopaminergic neuron loss of mice midbrain substantia nigra compact part (SNc) and ventral tegmental area (VTA) due to MPTP / p is reduced; levels of dopamine (DA) and metabolites thereof in corpus striatum are improved; excessive proliferation activation of astrocyte is inhibited; and reduction of dopaminergic neuron cell line SH-SY5Y cell viability, and increases of lactate dehydrogenase (LDH) generation and reactive oxygen species (ROS) generation are inhibited, wherein the reason of the reduction of dopaminergic neuron cell line SH-SY5Y cell viability, and the increases of lactate dehydrogenase (LDH) generation and reactive oxygen species (ROS) generation is MPP<+>. In addition, the results show that the metformin provides pharmacological effects for treatment of PD. According to the application of the present invention, the metformin is adopted as an active ingredient to prepare drugs for treatment of PD.
Owner:NANJING MEDICAL UNIV
Acute general Parkinson's disease monkey model and medicament screening method thereof
The invention provides a preparation method for an acute general Parkinson's disease monkey model. The preparation method comprises the following step of: continuously applying 0.36 mg / kg of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to a monkey for five times once every two days in an intravenous injection way. The invention provides a method for screening a medicament for treating the acute Parkinson's disease. By performing continuous peripheral vein injection of MPTP, an acute general Parkinson's disease monkey model with symptoms similar to those of an advanced-stage Parkinson's disease patient can be established. The model has stable symptoms within 1-4 months after establishment, and recovers rapidly later.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
Composition comprising longan arillus extract or combined extract comprising the same for treating neurodegenerative disease
ActiveUS9040102B2Prevention and treatmentEffective preventionBiocideNervous disorderNervous systemTherapeutic effect
This disclosure relates to a composition for prevention or treatment of neurodegenerative disease comprising longan arillus extract, or combined extract comprising longan arillus. The composition exhibits remarkably excellent prevention or treatment effect of neurodegenerative disease by containing longan arillus extract or combined extract comprising longan arillus, and particularly, it may effectively prevent or treat neurodegenerative disease by significantly protecting dopaminergic neurons from neurotoxicity due to MPTP selectively acting on dopaminergic nervous system and neurotoxicity due to aggregation of alpha-synuclein proteins.
Owner:MEDI HELP LINE CO LTD
Substance content determination method and application thereof
ActiveCN111366680AExpanding the scope of clinical medicine to treat diseasesGood treatment effectNervous disorderComponent separationDiseaseAngelica Sinensis Root
The invention relates to a substance content determination method and an application thereof. The substance is prepared from 225 parts of astragalus membranaceus, 100 parts of leech, 90 parts of ligusticum wallichii, 90 parts of angelica sinensis, 90 parts of safflower carthamus, 113 parts of peach kernel, 90 parts of radix paeoniae rubra, 90 parts of elecampane, 90 parts of rhizoma acori graminei, 60 parts of lumbricus, 90 parts of parasitic loranthus and 35 parts of acanthopanax extract. The content detection method has characteristics of simple and rapid operation and good repeatability andaccuracy. The method is advantaged in that pharmacological experiments of the substance show that the substance can significantly improve the mouse behavior coordination ability, significantly shorten the mouse pole climbing time, enhance the mouse grabbing force and the mouse swimming time, and significantly relieve MPTP-induced neuron damage, the substance content determination method can effectively control the inherent quality of the drug, and the new treatment application of the substance enlarges the clinical medication disease treatment range of the drug.
Owner:SHAANXI BUCHANG PHARMA
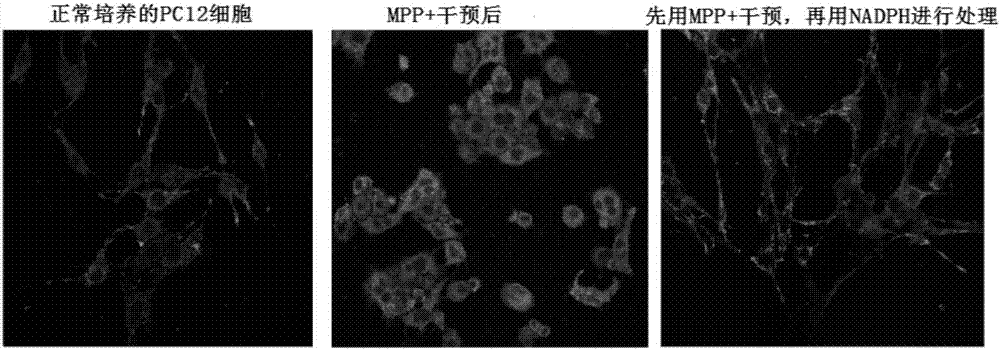
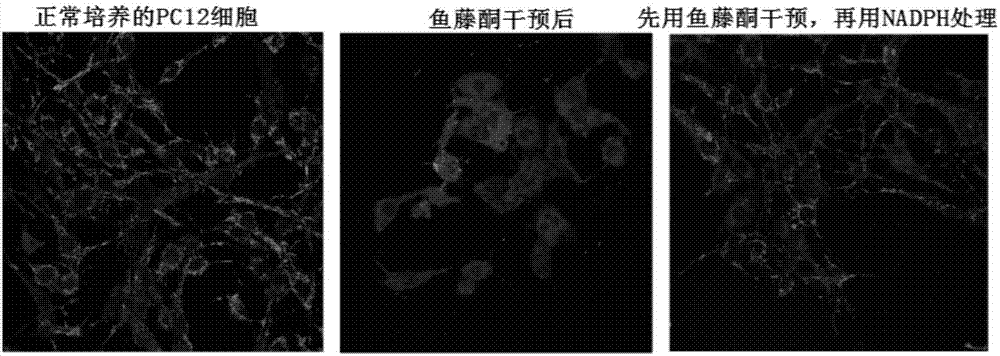
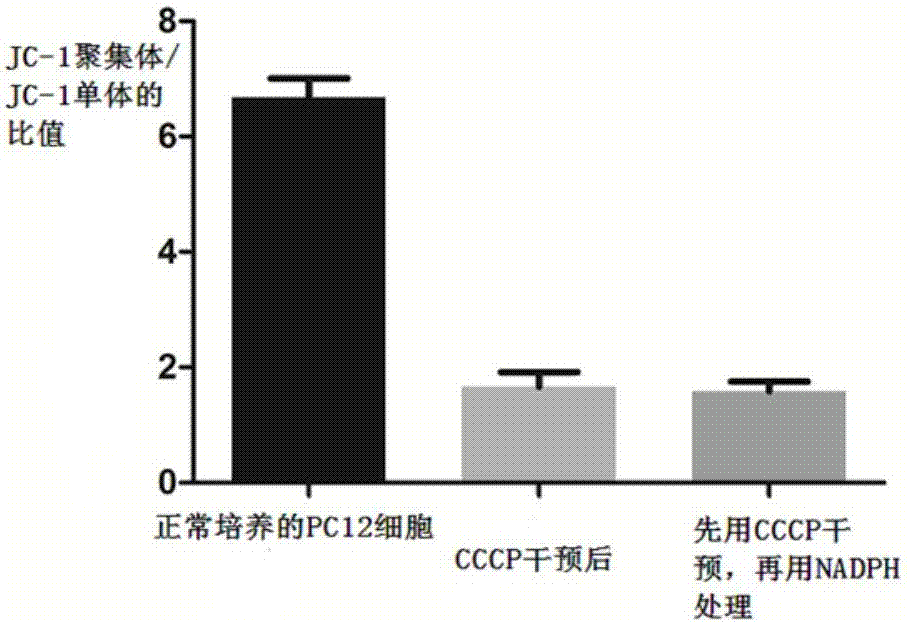
Application of NADPH in positional drug-induced mitochondrial toxicity
ActiveCN107213160AReduce free radicalsReduce mortalityOrganic active ingredientsNervous disorderMPTPMitophagy
The invention belongs to the field of drugs or health products and particularly relates to an application of NADPH in positional drug-induced mitochondrial toxicity. The research finds that the NADPH has a protective effect on mitochondria damage caused by MPTP and rotenone, plays a neuroprotective effect through the protective effect, but has no protective effect on CCCP-caused mitochondria damage, so that the NADPH can be applied to control of neurodegenerative diseases, such as ageing, sub-health and huntington's chorea caused by mitochondrial dysfunction, vascular dementia, Lewy body dementia with frontal dementia, amyotrophic lateral sclerosis and multiple sclerosis.
Owner:山东蓝康药业有限公司
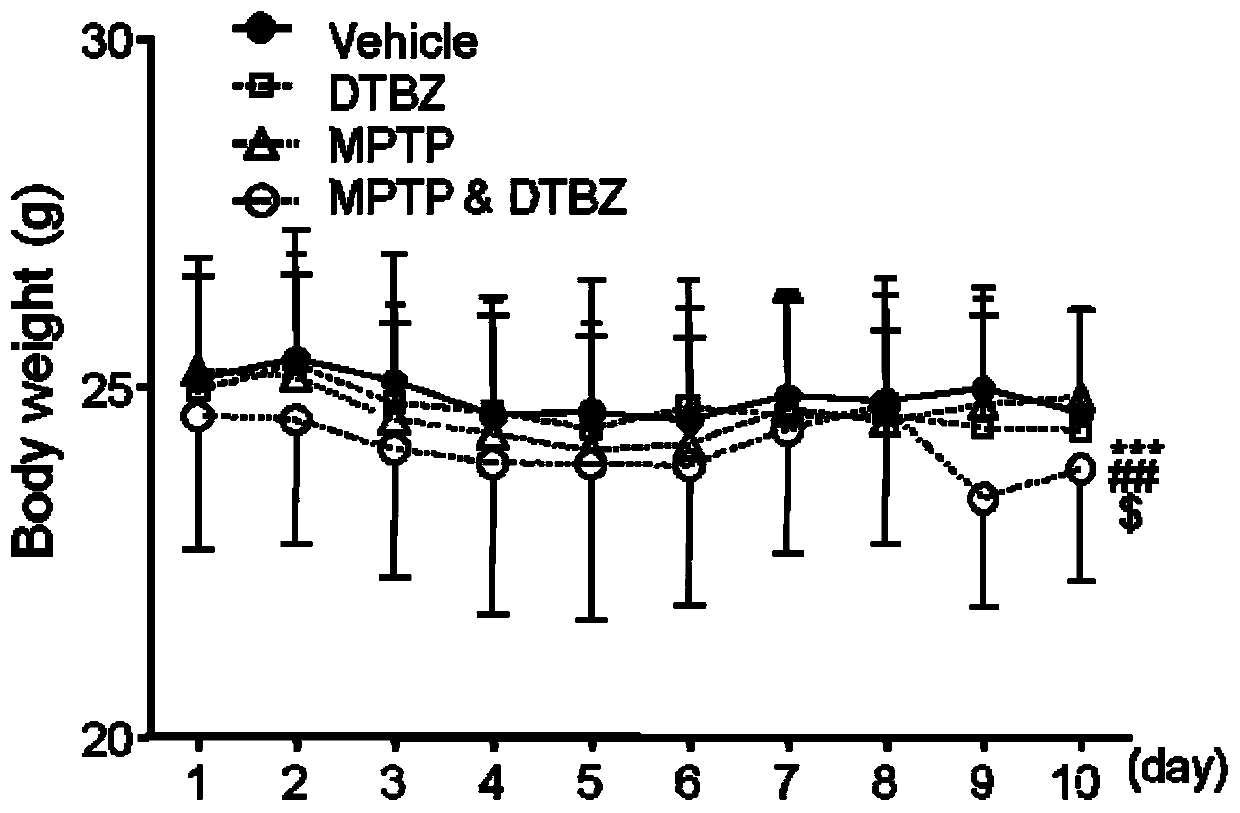
Construction method of Parkinson's disease animal model
InactiveCN110840889AHigh simulationInhibitory activityHeterocyclic compound active ingredientsAnimal husbandrySide effectPhysiology
The invention belongs to the technical field of animal model construction, and relates to a construction method of a Parkinson's disease animal model. The construction method includes the step: integrally applying an inhibitor of a vesicular monoamine transporter II and an environmental neurotoxin to an animal. According to the construction method, the inhibitor of the vesicular monoamine transporter II and the environmental neurotoxin are targeted to the VMAT2 (vesicular monoamine transporter II) and a mitochondrial complex I, the PD (Parkinson's disease) animal model induced by non-single factors is coincident with an etiological idea that a Parkinson's disease is induced by multi-factors, and characteristics of human Parkinson's diseases can be more effectively simulated. According to the construction method, DTBZ and MPTP are combined to construct the Parkinson's disease animal model, dosage of the MPTP is remarkably lower than that of the MPTP in a classical Parkinson's disease model induced by the MPTP, on one hand, slow progressing characteristics of the human Parkinson's diseases can be effectively simulated, on the other hand, experiment operators are obviously protected,and side effects are reduced.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Hemi-parkinsonism monkey model and medicament screening method thereof
InactiveCN102380106AAvoid enteringAvoid lossIn-vivo testing preparationsAnimal husbandryScreening methodExperimental animal
The invention provides a preparation method for a hemi-parkinsonism monkey model. The method comprises the following steps of: applying 20 ml of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) of which the dosage is 0.45-0.9 mg / kg to a monkey at the uniform speed 3ml / min. The invention further provides application of an animal model to screening of a medicament for treating the hemi-parkinsonism. The invention further provides a method for screening a medicament for treating the hemi-parkinsonism. The hemi-parkinsonism monkey model established with the method has the advantages of remarkable and typical symptoms and high stability after modeling; and an experimental animal survives easily, and is easy to breed. The stable time of the model is relatively long, and multiple stages of medicament treatment evaluation can be performed. Moreover, the model can undergo own control in the entire disease process, the using quantity of the model is saved, the requirement of the animal experiment ethnics is met, and the model is more economical and reasonable.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
Method for establishing rhesus monkey model with chronic process Parkinson's disease
The invention belongs to the technical field of life science and particularly relates to a method for establishing a rhesus monkey model with a chronic process Parkinson's disease (PD). By using the interventional mode that MPTP is injected for a long time starting from a smallest dose which is gradually increased according to the difference in animal sensitivity mainly by means of intramuscular injection assisted with intravenous injection, a rhesus monkey is slowly stimulated to generate all clinical symptoms of the PD. The success rate of the animal model established through the method for establishing the rhesus monkey model with the chronic process PD is 100%, lewy bodies are observed in the made animal model, and the method is used for evaluating the effect of new PD drugs and screening the new PD drugs.
Owner:SICHUAN AGRI UNIV
Traditional Chinese medicine preparation for treating Parkinson's disease
InactiveCN104922406AInhibition of motor responsesCompatibilityNervous disorderPlant ingredientsMPTPChinese drug
The invention relates to a traditional Chinese medicine preparation for treating Parkinson's disease, and belongs to the traditional Chinese medicine pharmaceutical technical field. The traditional Chinese medicine preparation is composed of uncaria rhynchophylla, prepared rehmannia root, angelica sinensis, radix paeoniae alba, rhizoma anemarrhenae and the like; the raw material drugs of the traditional Chinese medicine preparation are added with regular auxiliary materials and can be made into a decoction, granules, tablets, capsules or other traditional Chinese medicine oral preparations according to a conventional method. The traditional Chinese medicine preparation has a significant effect on stick rotation and pole climbing ethology of an MPTP induced subacute PD mouse model, can improve the content of dopamine in corpora striatum of model mice, and can be used in treatment of Parkinson's disease.
Owner:SHANGHAI RUIYANG BIOTECH CO LTD
Compound with anti-parkinson pharmacological activity
InactiveCN101434626AImprove neurobehavioral symptomsPrevent content reductionNervous disorderSugar derivativesMPTPPharmacology
The invention relates to a compound with anti-parkinsonism pharmacological activity, which is called baicalin, with a systematic name that: the anti-PD pharmacological activity of baicalin acting on two kinds of animals with parkinsonism disease shows that the baicalin can protect the nigrostriatal dopaminergic neurons of animals of a PD model. The research results comprise that: (1) researches are carried out to check whether the baicalin can protect mice with parkinsonism disease induced by 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine (MPTP); and (2) researches are carried out to check whether the baicalin can protect rats with parkinsonism disease induced by rotenone; and the results of the researches shows that the baicalin can protect the nigrostriatal dopaminergic neurons of animals of the PD model, can reduce the loss of the nigrostriatal dopaminergic neurons, promote the growth of dopaminergic neuron protuberances, prevent the reduction of striatum dopamine, decrease the percentage of praxeological change occurring on animals, reduce total praxeological credits and improve the praxeological symptom of PD rats. The pharmacological activity of the baicalin suggests that the baicalin has great application significance in stopping the development of Parkinsonism disease.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
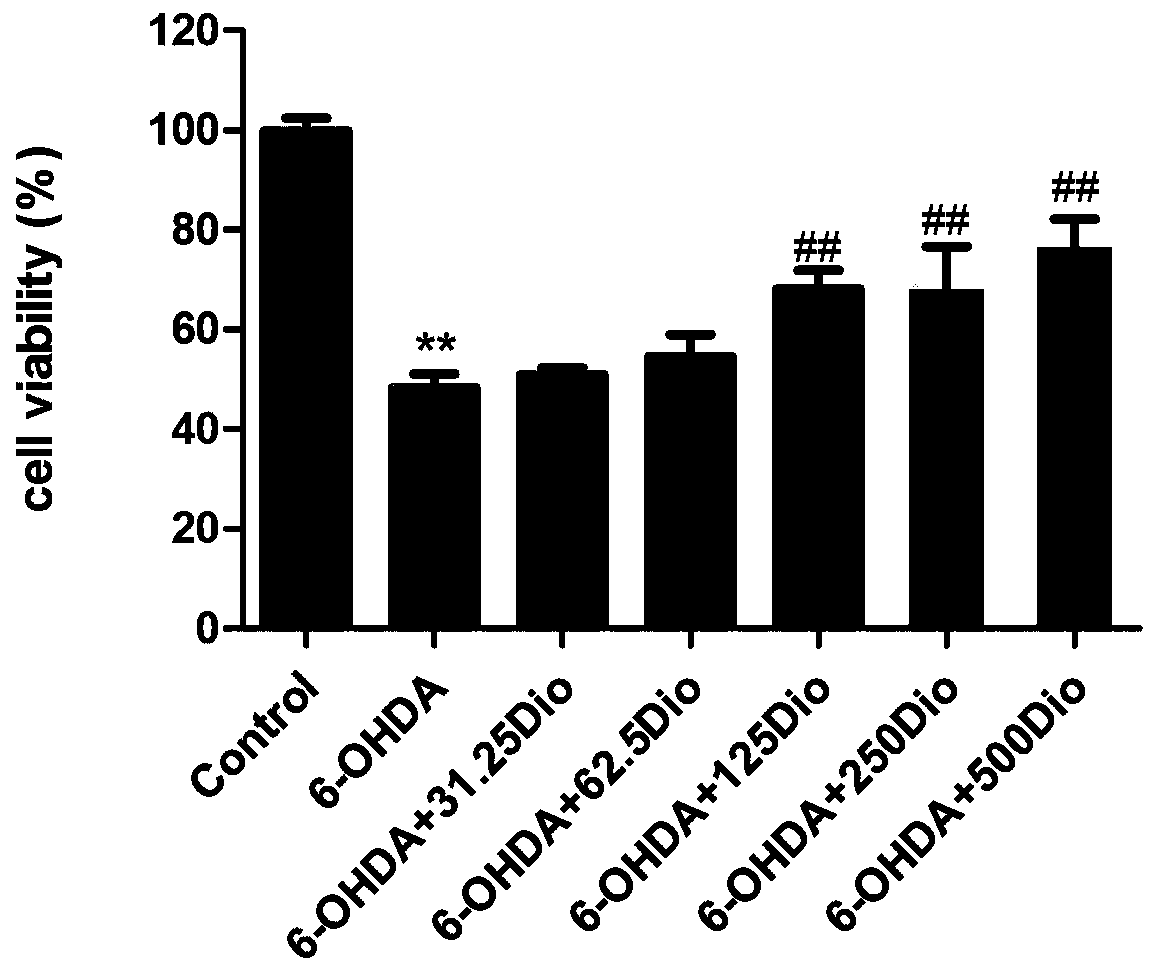
Application of dioscin in preparation of Parkinson's disease protection drug
InactiveCN110859849ANo side effectsPracticalOrganic active ingredientsNervous disorderSide effectPharmaceutical drug
The invention discloses an application of dioscin in preparation of a Parkinson's disease protection drug. MTT and morphological observation prove that the dioscin has an obvious protection effect onPC12 cells induced by 6-OHDA in vitro. Meanwhile, the dioscin has an obvious down-regulation effect on the remarkable increase of the expression level of GFAP and IBA-1 caused by MPTP induction of PDmouse, which indicates that the dioscin can inhibit the activation of microglial cells and astrocytes in brain tissues. According to clinical medication requirements, the dioscin can be used as a single effective component to be prepared into a medicine, and can also be combined with other medicines to be prepared into a compound preparation. The dioscin has the advantages of being safe, free of toxic or side effect, effective, economical, practical and the like.
Owner:DALIAN MEDICAL UNIVERSITY
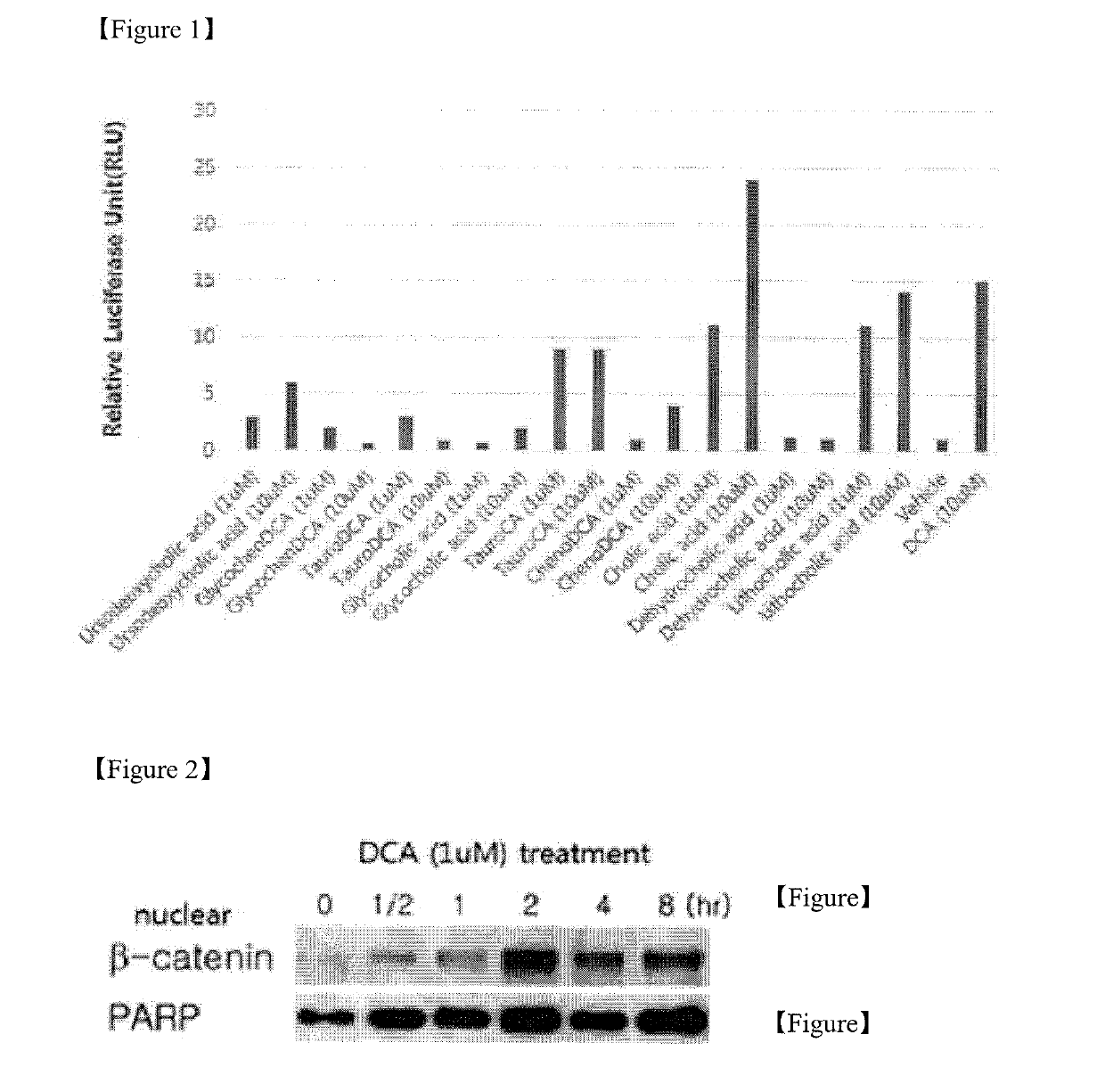
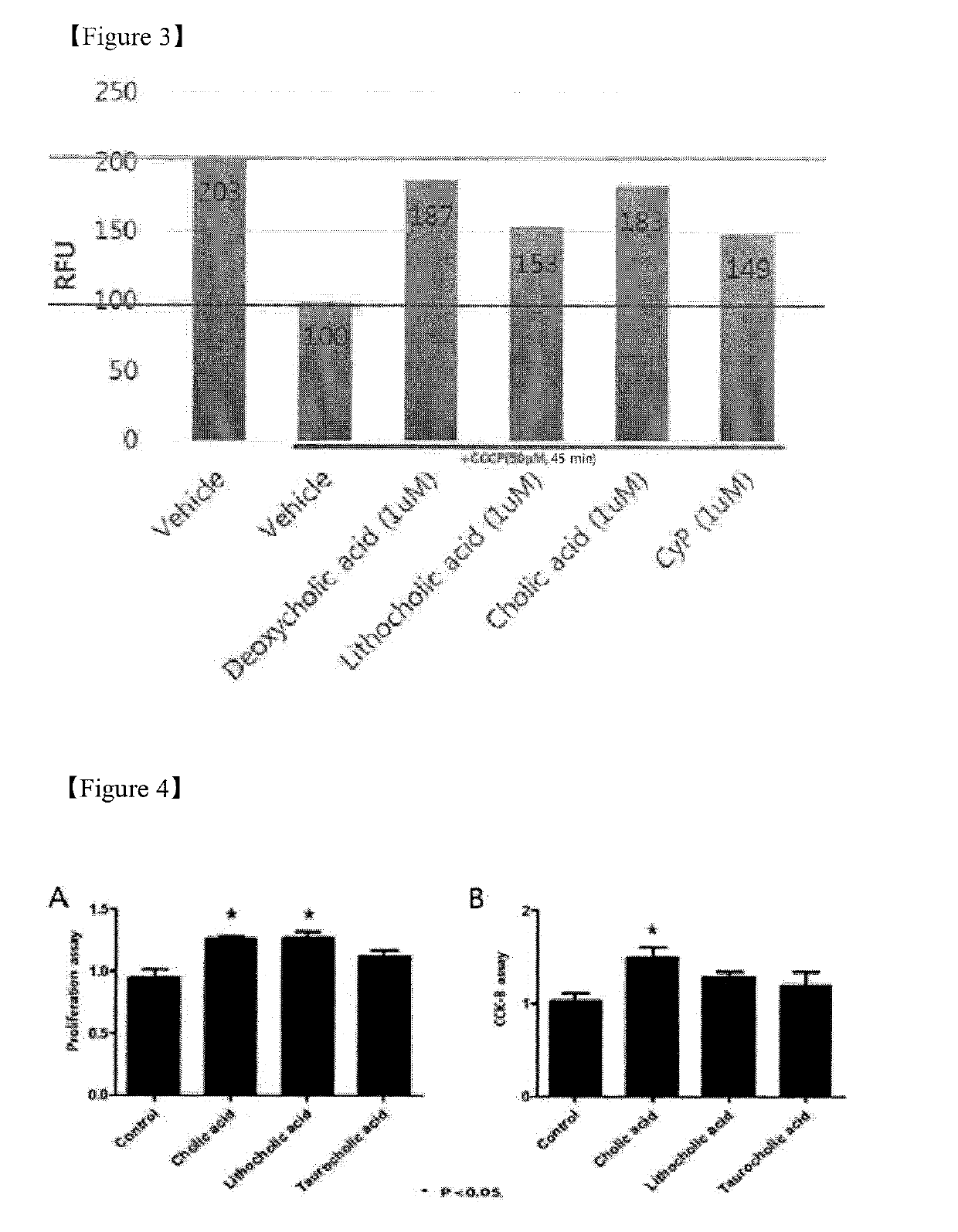
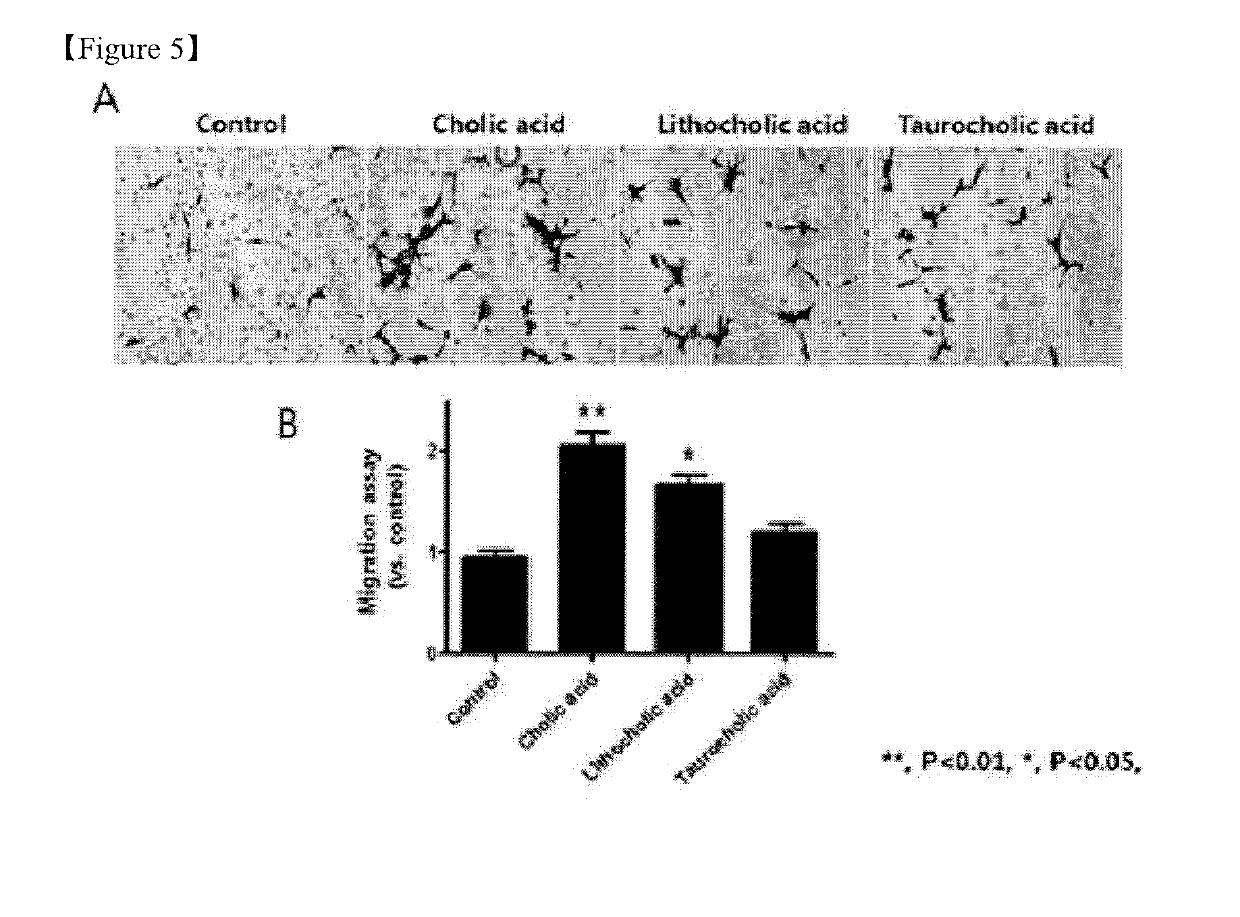
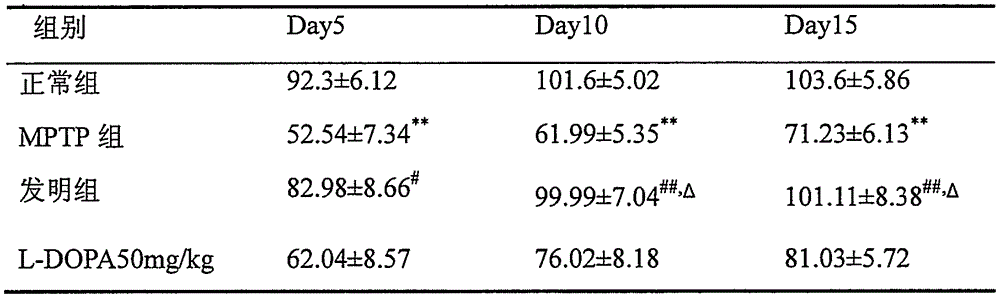
Pharmaceutical composition for preventing or treating ischemia-reperfusion injury, containing bile acid
ActiveUS20190247406A1Reduce tissue damageSmall sizeOrganic active ingredientsDispersion deliveryReperfusion injuryBeta-catenin
The present invention relates to a composition for preventing, treating, or alleviating ischemia-reperfusion injury, containing bile acid. According to the present invention, bile acid increases intranuclear beta-catenin levels, interferes with the opening of a mitochondria permeable transition pore (mPTP), and has excellent effects, in ischemia-reperfusion injury animal models, of alleviating tissue injury and reducing the size of infarcts, thereby being usable in the prevention, treatment, or alleviation of ischemia-reperfusion injury.
Owner:SAMSUNG LIFE PUBLIC WELFARE FOUND
Traditional Chinese medicine composition for treatment of Parkinson's disease
InactiveCN105267410AInhibition of motor responsesCompatibilityNervous disorderPlant ingredientsMPTPTraditional medicine
The invention discloses a traditional Chinese medicine composition for treatment of Parkinson's disease, and belongs to the field of traditional Chinese medicine. The traditional Chinese medicine composition for treatment of Parkinson's disease is prepared from radix bupleuri, prepared rehmannia root, radix paeoniae alba and liquorice according to a certain weight part proportion. An MPTP induced mice subacute Parkinson's disease model proves that the traditional Chinese medicine composition has the effect of improving symptoms of mice Parkinson's disease.
Owner:SHANGHAI ZICAO MEDICINE TECH CO LTD
Function application of SH2B adapter protein 1 in treating Parkinson's disease
PendingCN107802823AReduced behavioral impairmentLower levelNervous disorderPeptide/protein ingredientsMPTPTherapeutic effect
The invention discloses a function application of SH2B adapter protein 1 in treating Parkinson's disease. Through neuron-specific over-expression of the SH2B1, dopaminergic neuron loss of substantia nigra pars compacta in an MPTP-induced mouse can be obviously improved, and the level of dopamine in striatum can be increased; and the proliferation and the activation of astrocytes and microglia of the substantia nigra pars compacta can be inhibited. Based upon the research results, it is indicated that the SH2B1 can be used for preparing medicines for treating the Parkinson's disease. Accordingto the invention, the therapeutic effect of the SH2B1 in the Parkinson's disease is verified for the first time; therefore, the SH2B1 is huge in market value and social benefit.
Owner:胡军
Traditional Chinese medicine composition for treating neurodegenerative disease
InactiveCN107648482ABack to normalReduce deathNervous disorderPlant ingredientsGastrodiaNeuro-degenerative disease
The invention discloses a traditional Chinese medicine composition for treating neurodegenerative diseases, which consists of the following traditional Chinese medicines in parts by weight: 2-60 parts of Smilax smilax, 1-30 parts of Gastrodia elata, and 1-30 parts of Angelica sinensis. The traditional Chinese medicine composition can obviously promote the proliferation of nerve cells and inhibit the death of nerve cells, and the combination of traditional Chinese medicines can play a synergistic effect compared with a single traditional Chinese medicine; it can be used in the Parkinson's disease model caused by MPTP in vitro. Reduce nerve cell death and restore the normality of mice, and be used in the Parkinson's disease model caused by rat 6-hydroxydopa, which can restore function and reduce brain necrosis area; the traditional Chinese medicine composition of the present invention can regenerate and replace dead Nerve area, to treat or prevent neurodegenerative diseases, reduce the activation of microglial cells in diseased tissue, and then protect nerve tissue, thereby reducing or alleviating, or even improving neuromotor function and improving a series of symptoms .
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
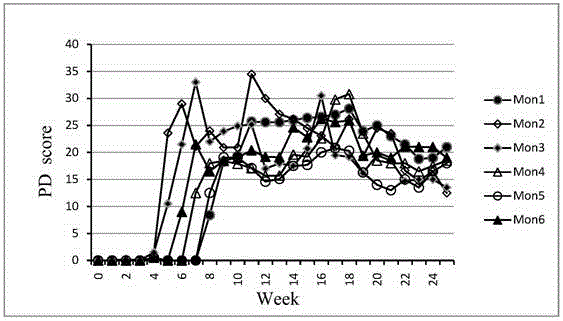
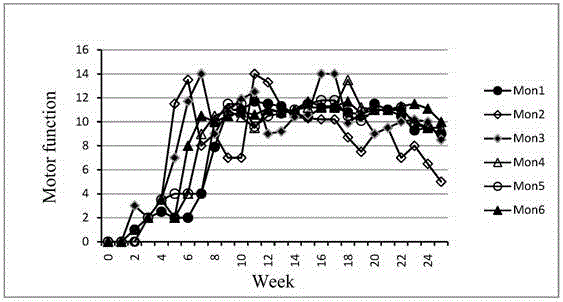
Primates Parkinson's disease model behavior assessment optimal method
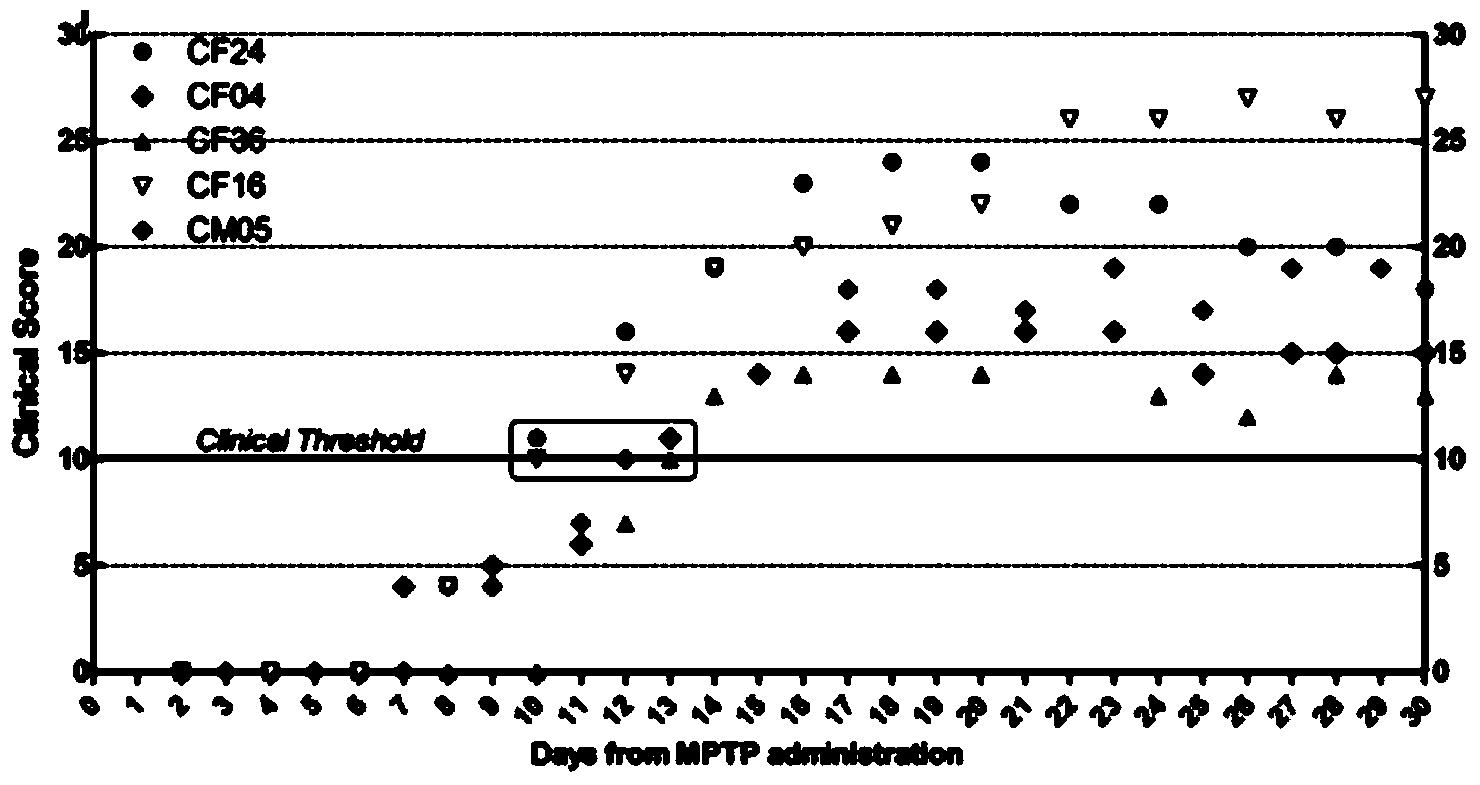
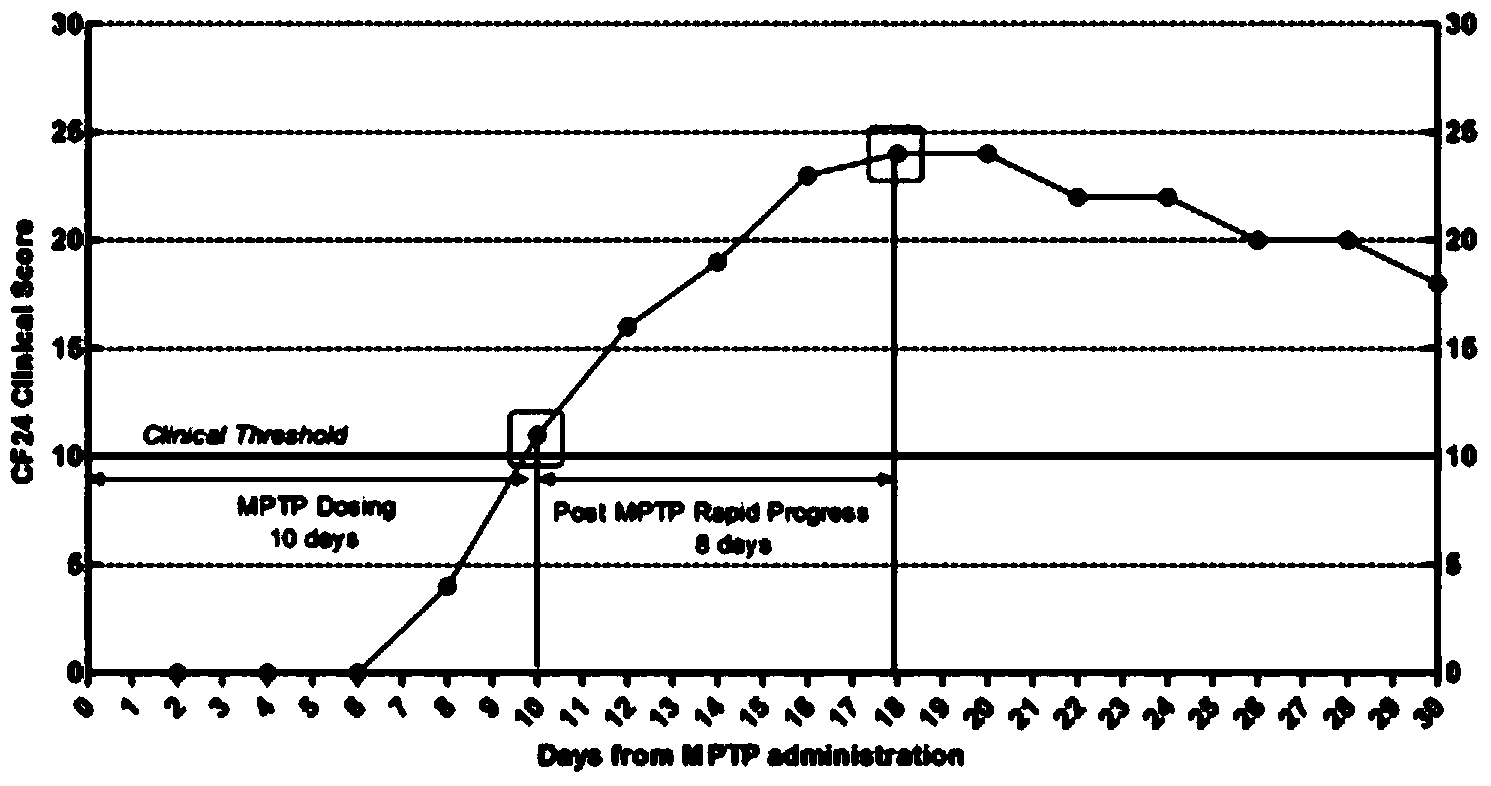
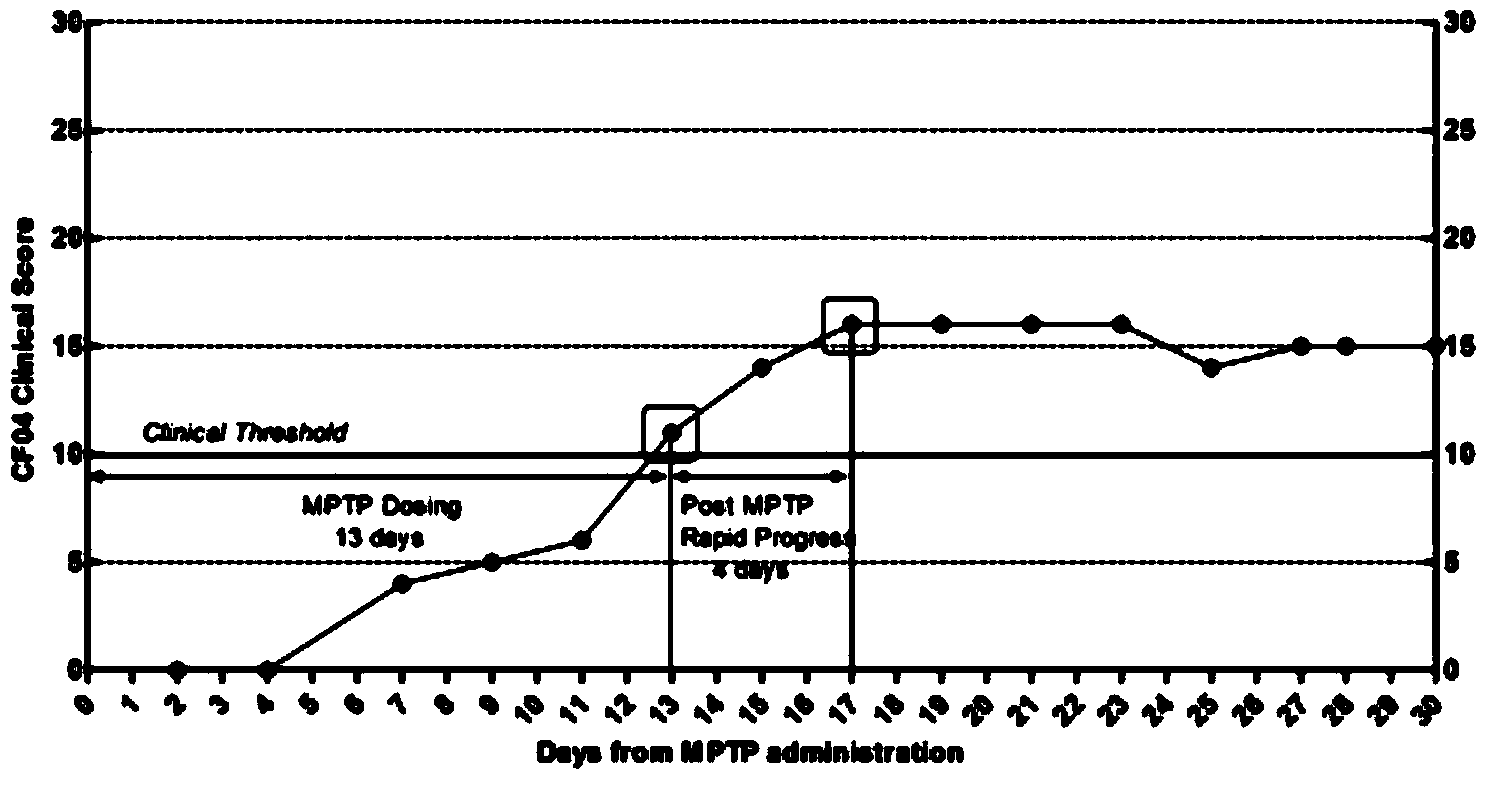
InactiveCN105878240AAccurately reflect individual clinical differencesOrganic active ingredientsPrimateEfficacy
The invention belongs to the technical field of bioscience, and particularly relates to a primates Parkinson's disease (PD) model behavior assessment optimal method. The method comprises the steps of establishing a rhesus monkey PD animal model; injecting MPTP into muscle of a rhesus monkey once a day till typical and stable PD clinical symptoms appear, wherein dosage is 0.2-0.5 mg / kg according to individual sensitivity difference; conducting behavior assessment on the rhesus monkey at two fixed time periods each day while MPTP injection is conducted, and taking the average score of one week as the final score of the week. A set of complete primates PD model behavior assessment method is provided and can be used for new drug efficacy assessment and horizontal and vertical comparative study of different new drugs. The assessment method absorbs the advantages and discards the disadvantages of different rating scales, and can reflect individual clinical difference comprehensively and accurately based on practical experience and clinical performance characteristics of rhesus monkeys, and individual clinical difference includes individual sensitivity difference, disease severity difference, disease sequence difference and individual PD feature difference.
Owner:SICHUAN AGRI UNIV
Application of isocorynoxeine to preparation of medicines with neuroprotective effect
InactiveCN108245512ARescue Toxic EffectsDilated blood vesselsOrganic active ingredientsNervous disorderDiseaseMPTP
The invention provides application of isocorynoxeine to preparation of medicines with a neuroprotective effect. The medicines with the neuroprotective effect, provided by the invention, can effectively rescue the toxic effect of neurotoxin MPTP on dopaminergic neurons and have a protective effect on the dopaminergic neurons; compared with the common effects of lowering the blood pressure, expanding blood vessels and slowing the heart rate of the isocorynoxeine, the invention develops novel application of the isocorynoxeine, and a theoretical basis and an experimental basis are provided for further studying the neuropharmacology of a Chinese medicinal herb uncaria; furthermore, the isocorynoxeine provides a new idea and possibility for development of a Parkinson's disease treating medicine,an antidepressant medicine and an Alzheimer's disease treating medicine; especially, as the Parkinson's disease treating medicine, the isocorynoxeine has a particularly outstanding effect.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Application of dexrazoxane in preparation of drug for treating neurodegenerative diseases
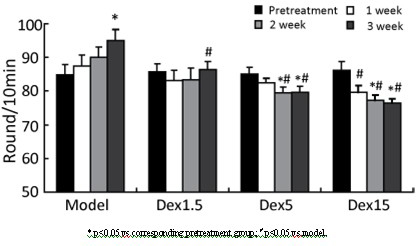
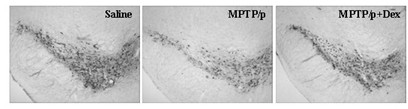
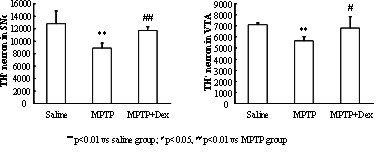
InactiveCN102349908AImprove behavioral disordersAvoid damageOrganic active ingredientsNervous disorderMPTPNeuro-degenerative disease
The invention provides application of dexrazoxane in preparation of drug for treating neurodegenerative diseases. Experiments prove that dexrazoxane can improve the behavior disorder of model rats of Parkinson's disease belonging to neurodegenerative diseases, inhibit the decrease of TH (helper T cell) nerve cells in mouse mesencephalic SNc (substantia nigra compact part) and ventral tegmental area (VTA), which is caused by MPTP / p (1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine / probenecid) modeling, improve the proliferation and activation of astrocyte and microglia, obviously inhibit the over-expression of alpha-synuclein in substantia nigra compact part and reticular part, and inhibit the reduction of survival rate of dopaminergic neuron cell strain SH-SY5Y cells, which is caused by H2O2 and MPP<+> (1-methyl-4-phenylpyridinium ion). The results show that dexrazoxane has the action of treating neurodegenerative diseases. Through the invention, dexrazoxane can be used as an active ingredient to prepare the drug for treating neurodegenerative diseases.
Owner:NANJING MEDICAL UNIV
Application of plumbagin in preparation of medicine for preventing or treating Parkinson's disease
PendingCN113209064ASignificant neuroprotective effectFree from damageOrganic active ingredientsNervous disorderAntagonismMPTP
The invention belongs to the technical field of new application of plumbagin, and provides application of plumbagin in preparation of a medicine for preventing or treating Parkinson's disease. The toxic effect of neurotoxin MPP + / MPTP on Parkinson's disease and the toxic effect of plumbagin on antagonism of MPP <+> / MPTP on Parkinson's disease are observed through cell and animal experiments. Researches show that plumbagin can inhibit SH-SY5Y and PC12 nerve cells and dopaminergic neuron death and dyskinesia caused by neurotoxin MPP <+> / MPTP. Therefore, the plumbagin has good application prospects and huge new medicine development value in the aspects of Parkinson's disease prevention and treatment and neuroprotection.
Owner:GUILIN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com