Application of dioscin in preparation of Parkinson's disease protection drug
A technology of diosgenin and Parkinson's disease, which is applied in the application field of diosgenin in the preparation of Parkinson's disease protection medicine, and can solve the problems such as the related reports on the protection application of diosgenin that have not been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
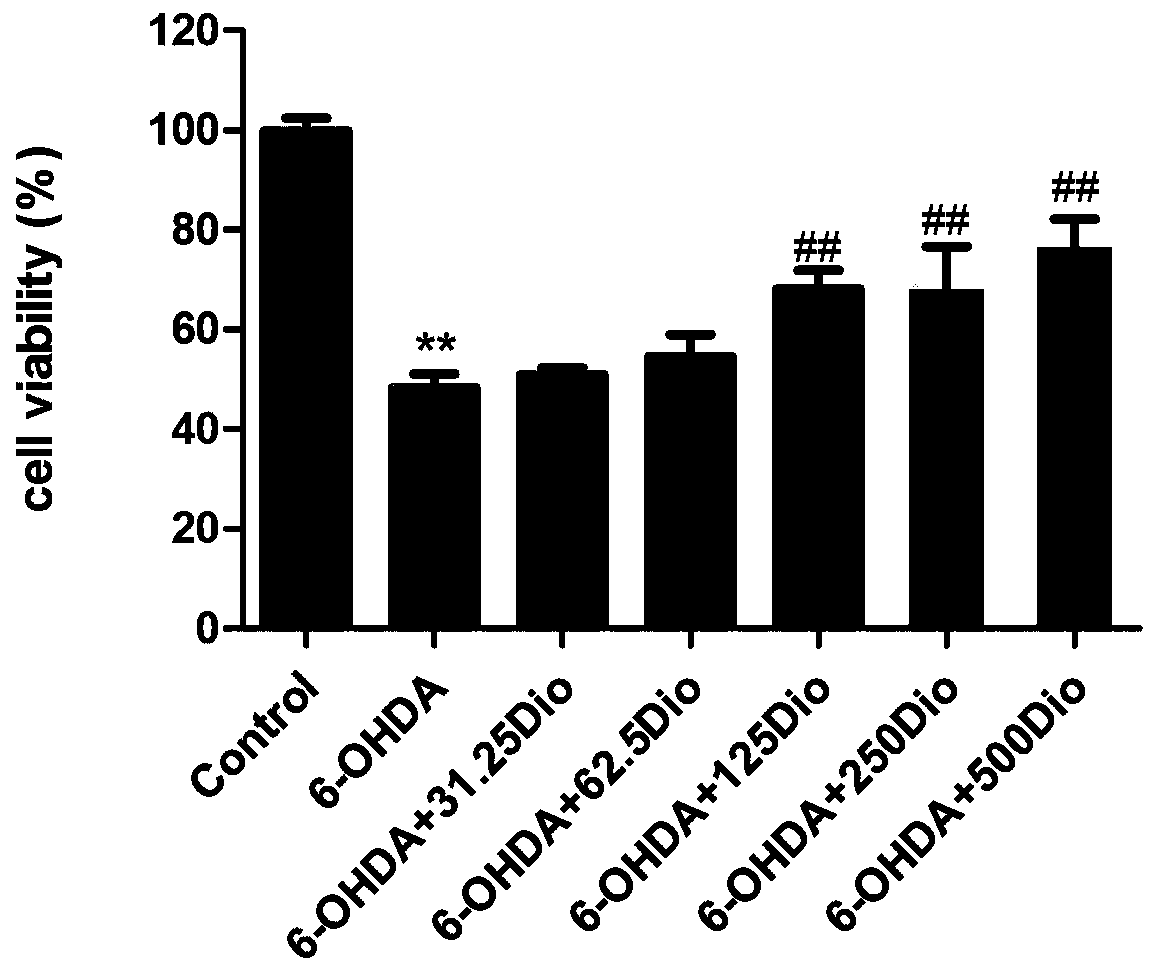
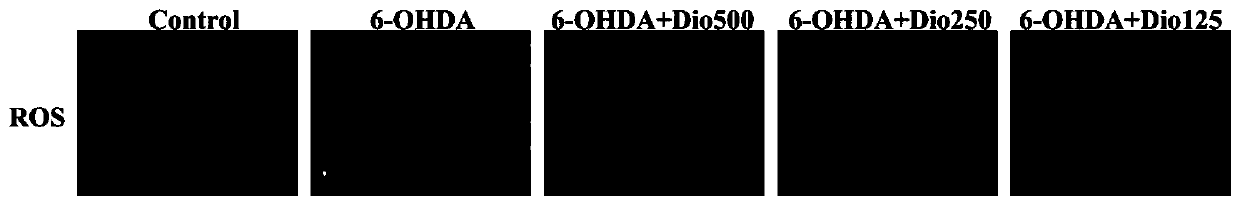
[0017] Example 1 Study on the protective effect of dioscin on 6-OHDA-induced PC12 cell injury
[0018] 1. MTT method to detect cell viability.
[0019] Rat pheochromocytoma cell line PC12 cells in logarithmic growth phase ( CRL-1721TM) was digested with 0.25% trypsin, the collected cell suspension was centrifuged at 1200g / min for 5min and counted, and the cells with a concentration of 1×106 cells / mL (100μL) were inoculated in a 96-well plate, cultured for 24 hours, and divided into the following: Several groups: blank group, 6-OHDA (6-hydroxydopamine) model group, model plus diosgenin administration group with different concentrations. The administration group was given different concentrations of dioscin (31.25, 62.5, 125, 250, and 500ng / ml), and each concentration was provided with 6 replicate wells. After pre-protection for 24h, 6-OHDA 400μM was given together with the model group for 6h. 10 μL of MTT (5.0 mg / ml) was added 4 hours before the end of the experiment. After ...
Embodiment 2
[0027] Example 2 Study on the protective effect of dioscin on MPTP-induced PD mice
[0028] Seventy male C57 mice aged 8-12 weeks were randomly divided into the following groups: blank group, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model group, MPTP modeling plus diosgenin high, medium and low dose administration groups (80, 40, 20 mg / kg) and diosgenin (80 mg / kg) simple administration group, 12 rats in each group. The blank group was intraperitoneally injected with normal saline every day for 7 consecutive days; the MPTP model group was intraperitoneally injected with 20 mg / Kg of MPTP every day, and 2 hours later was given the same amount of normal saline for 7 consecutive days; the MPTP model plus diosgenin administration group Intraperitoneal injection of MPTP 20mg / kg, 2 hours later, different doses (80, 40, 20mg / kg) of dioscin were given by intragastric administration for 7 consecutive days. After MPTP injection, dioscin was administered by intragastric administ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com