Substance content determination method and application thereof
A detection method and technology for substances, which are applied in chemical analysis by titration, medical preparations containing active ingredients, and drug combinations, etc., can solve the problems of many operation cost steps, insufficient internal quality of traditional Chinese medicine preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 drug hirudin content detection method of the present invention
[0043] 1. Instruments and reagents
[0044] Analytical balance (Mettler, Switzerland), HW.SY11-KP2 intelligent constant temperature water bath (Beijing Changfeng Instrument Co., Ltd.), 3-18KS centrifuge (Germany Sigma), KQ-500E ultrasonic cleaning machine (Kunshan Ultrasonic Instrument Co., Ltd.), Longshengzhi Capsules (Shaanxi Buchang Co., Ltd., batch number: 181005), leeches (provided by Buchang Co., Ltd.), normal saline (Shijiazhuang No. Erdi Biotechnology Co., Ltd.), tris, hydrochloric acid, thrombin T8020 (batch number: 417G062, Beijing Suolaibao Technology Co., Ltd.)
[0045] 2. Methods and Results
[0046] 2.1 Solution preparation
[0047] 2.1.1 Preparation of the test solution
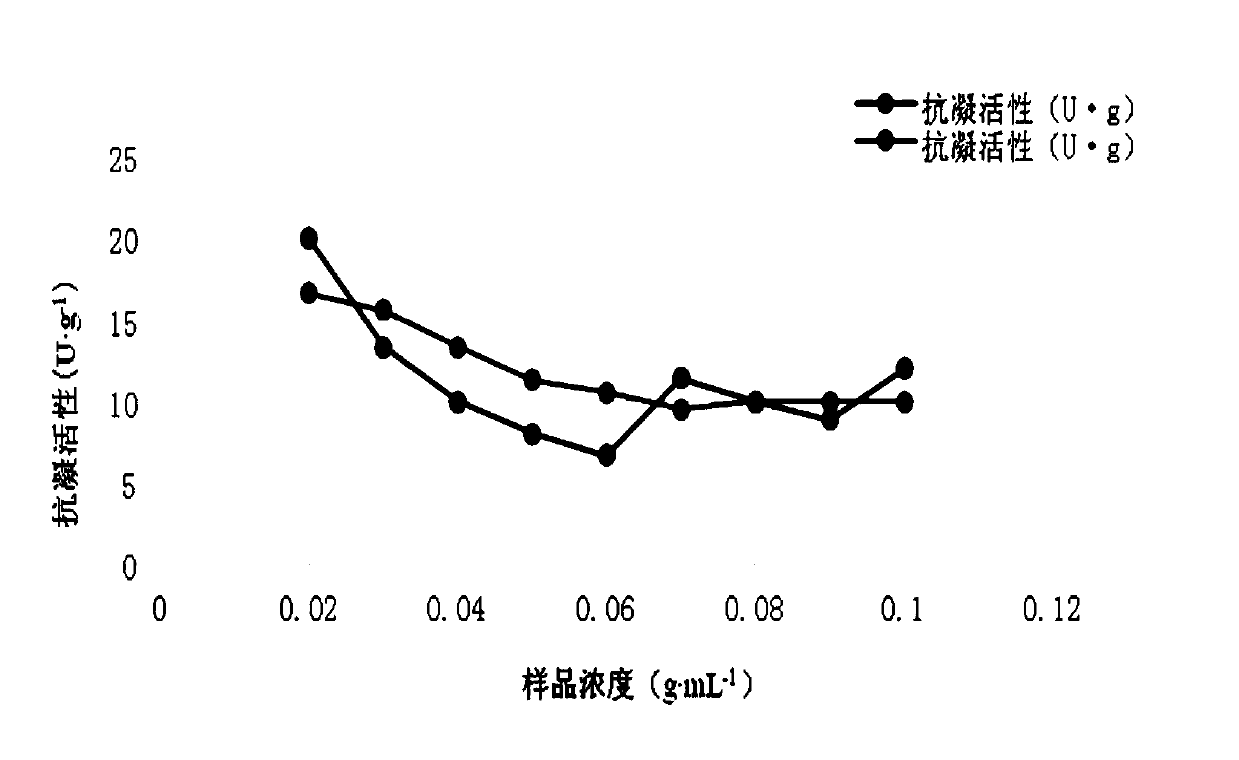
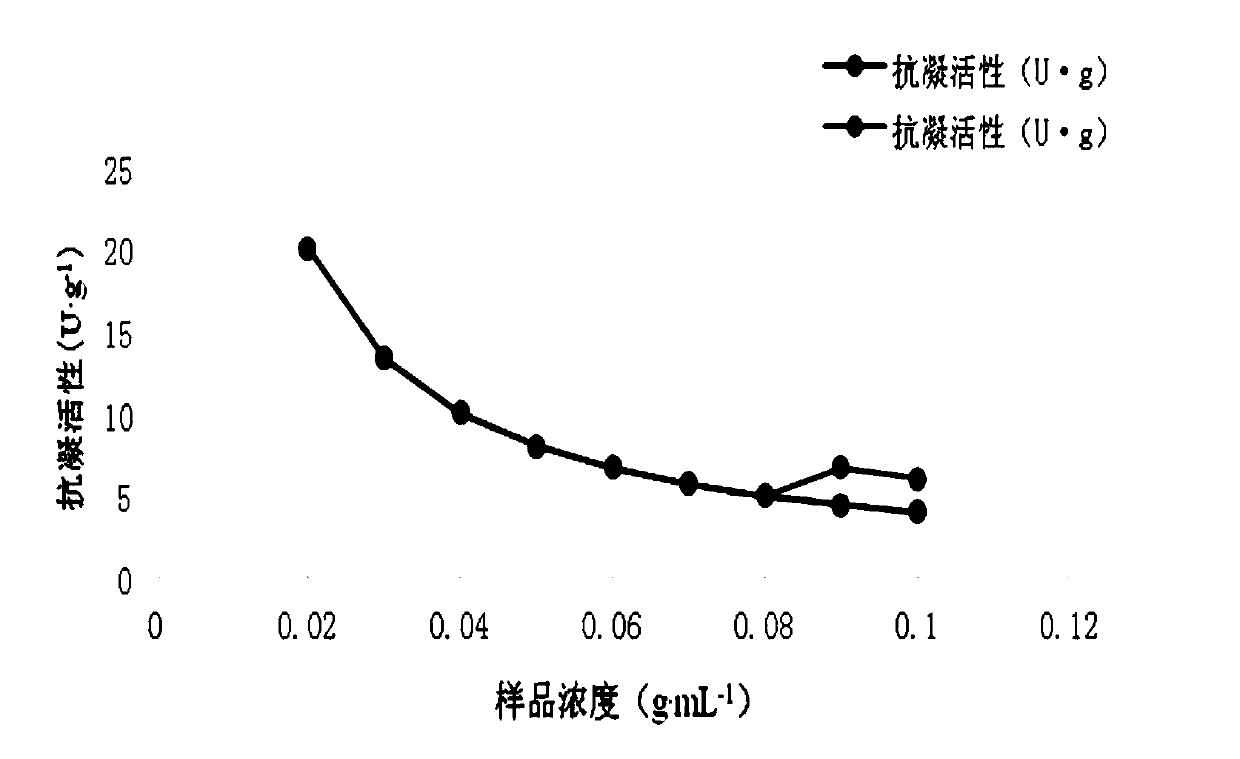
[0048] Take 0.4g, 0.4g, 0.4g, 0.4g, 0.4g, 0.4g, 0.4g, 0.3g, 0.2g, 0.1g respectively, add 2mL, 4mL, 4.44mL, 5mL, 5.7mL, 6.67 mL, 7.5mL, 6.67mL, 5mL of physiological saline, made with a concentration of 0.2g...
Embodiment 2
[0103] Embodiment 2 medicine is prepared as capsule
[0104] Prescription: 225g astragalus, 100g leech, 90g Chuanxiong, 90g angelica, 90g safflower, 113g peach kernel, 90g red peony, 90g woody, 90g calamus, 60g earthworm, 90g mulberry, 35g Acanthopanax extract;
[0105] Preparation method: take leeches, earthworms, and Acanthopanax dry extracts and grind them into fine powder; add water to decoct twice, and boil for the first time The second time is 2 hours, the second time is 1.5 hours, filter, combine the decoction, the relative density of the filtrate is 1.1-1.2 when the filtrate is concentrated to 60 ° C, spray dry into powder, add earthworm, leech, Acanthopanax powder and mix evenly, add medicine With starch, make 1000 grains, that is to say.
[0106] Usage and Dosage: Oral. 3 to 5 capsules at a time, 3 times a day.
Embodiment 3
[0107] Embodiment 3 medicine is prepared as tablet
[0108] Prescription: 225g astragalus, 100g leech, 90g Chuanxiong, 90g angelica, 90g safflower, 113g peach kernel, 90g red peony, 90g woody, 90g calamus, 60g earthworm, 90g mulberry, 35g Acanthopanax extract;
[0109] Preparation method: take leeches, earthworms, and Acanthopanax dry extracts and grind them into fine powder; add water to decoct twice, and boil for the first time The second time is 2 hours, the second time is 1.5 hours, filter, combine the decoction, the relative density of the filtrate is 1.1-1.2 when the filtrate is concentrated to 60°C, spray dry into powder, add earthworm, leech, Acanthopanax powder and mix evenly, add appropriate amount starch slurry, magnesium stearate, blended, granulated, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com