Pharmaceutical compositions of combinations of dipeptidyl peptidase-4 inhibitors with metformin
A technology of dipeptidyl peptidase and metformin hydrochloride, which is applied in drug combinations, active ingredients of heterocyclic compounds, sugar-coated pills, etc., can solve problems such as difficult treatment options, inability to fully control blood sugar, and complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
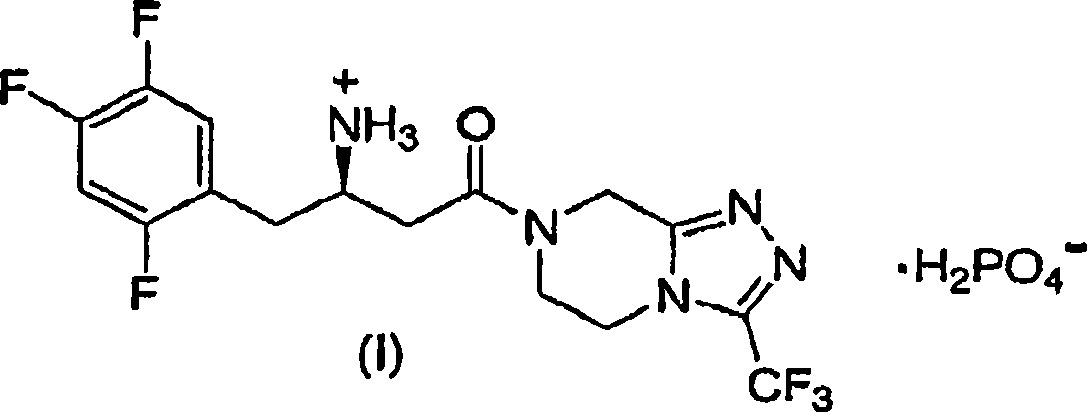
[0020] The preparation of sitagliptin and its pharmaceutically acceptable salts is disclosed in US Patent No. 6,699,871, the entire contents of which are incorporated herein by reference. The preparation of sitagliptin phosphate monohydrate is disclosed in International Patent Publication WO 2005 / 0031335 published on January 13, 2005, the entire content of which is incorporated herein by reference.
[0021] The dose concentration of the DPP-4 inhibitor incorporated in the pharmaceutical composition of the present invention is about 1 mg to about 250 mg of the active part. Preferably, the dose concentration of the DPP-4 inhibitor is about 25 mg to about 200 mg of the active part. Discrete dose concentrations are 25, 50, 75, 100, 150, and 200 mg DPP-4 inhibitor active part equivalents. The "active fraction" refers to the free base form of the DPP-4 inhibitor in the anhydrous form.
[0022] The unit dose concentration of sitagliptin free base anhydrate (active part) incorporated into...
Embodiment 1
[0083] Fixed-dose combination of 50 mg sitagliptin and 500 mg metformin hydrochloride per tablet-wet granulation
[0084]
[0085] * Equivalent to 50mg sitagliptin free base anhydrate.
[0086] ** Removed during processing.
[0087] Production method:
[0088] The sitagliptin phosphate monohydrate and metformin hydrochloride are fed into a high-shear granulator or a fluidized bed granulator. In the case of high-shear granulation, in addition to the polyvinylpyrrolidone binder, purified water containing sodium lauryl sulfate is added to APIs (active pharmaceutical ingredients) within 3-5 minutes. The wet material is either dried in a rack at 40°C or in a fluidized bed dryer at an inlet temperature of 45-60°C for 3-6 minutes. In the case of fluidized bed granulation, the purified water containing polyvinylpyrrolidone and sodium lauryl sulfate is added to the APIs within 30-60 minutes. The wet material is dried in a fluidized bed dryer at an inlet temperature of 45-60°C. Then, the ...
Embodiment 2
[0090] Fixed-dose combination of 50 mg sitagliptin and 850 mg metformin hydrochloride per tablet-wet granulation
[0091]
[0092] * Equivalent to 50mg sitagliptin free base anhydrate.
[0093] ** Removed during processing.
[0094] Production method:
[0095] The tablets were prepared by wet granulation using basically the same method as in Example 1, thereby providing 1117 mg uncoated tablets. Tablets are optionally coated with 27.9mg standard Film-coated formulations to provide 1145mg film-coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com