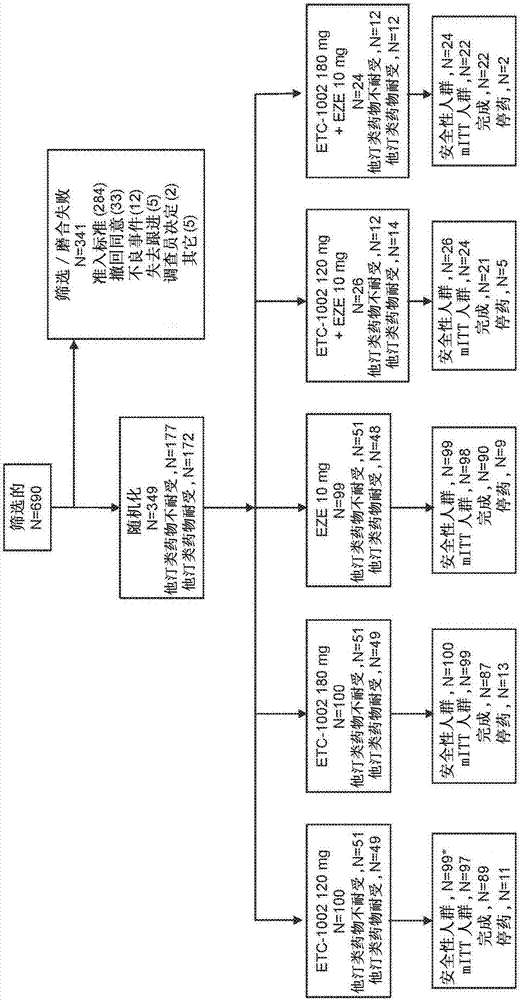
Fixed dose combinations and formulations comprising etc1002 and ezetimibe and methods of treating or reducing the risk of cardiovascular disease
A technology of ETC-1002 and ezetimibe, which can be applied to cardiovascular system diseases, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as negative side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
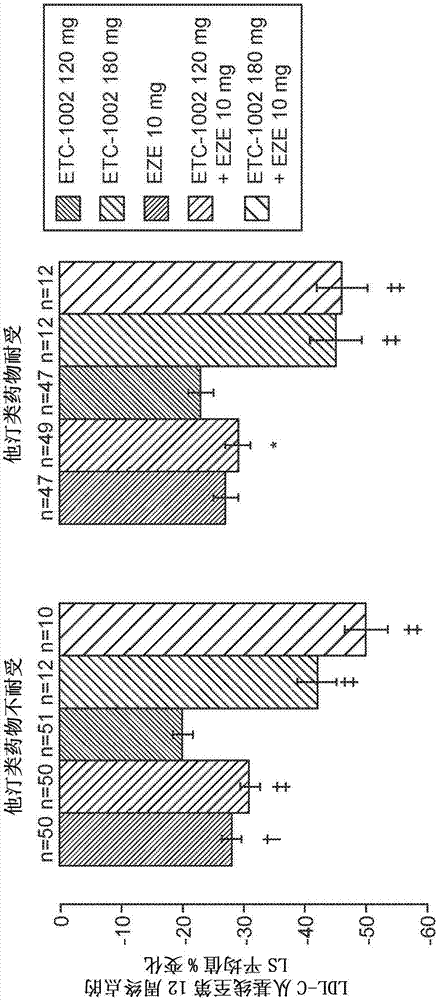
[0127] Example 1: ETC-1002 Alone or in Combination with EZE Reduced from Baseline to Week 12 Compared to EZE Monotherapy more LDL-C
[0128] LDL-C reduction was greatest with the combination of ETC-1002 120 mg (43%) or 180 mg (48%) plus EZE (p figure 2 ). After 2 weeks of treatment, LDL-C decreased significantly and stably ( image 3 ). ETC-1002 alone reduced LDL-C by up to 30%, which was significantly greater than that achieved with EZE monotherapy. The greatest mean reduction in LDL-C occurred with ETC-1002 120 mg or 180 mg in combination with EZE, reaching 43% and 48%, respectively. With ETC-1002, EZE, and the combination, LDL-C reductions occurred within 2 weeks of treatment and were maintained throughout the study. LDL-C lowering in statin-intolerant patients appears to be similar to statin-resistant patients. Considering that statin-intolerant patients had a higher baseline risk of cardiovascular disease than statin-resistant patients, 28% vs. 12%, respectively, ...
Embodiment 2
[0129] Example 2: ETC-1002 alone or in combination with EZE reduces more LDL particles than EZE alone count, apolipoprotein B, total cholesterol, and non-HDL-C
[0130] ETC-1002 alone or in combination with EZE reduced significantly more secondary lipid endpoints than EZE alone, including non-HDL-C, total cholesterol, apolipoprotein B, and LDL particle number. ETC-1002 treatment lowered HDL-C (3% to 6% reduction) and raised HDL-C (5% increase) with EZE alone (p<0.0001 to p<0.0001 to p <0.05) (Table 2).
Embodiment 3
[0131] Example 3: Median CRP was reduced by 30% from baseline to Week 12 endpoint with ETC-1002 120 mg, and 40% reduction with ETC-1002 180mg
[0132] The reduction in CRP in the ETC-1002 monotherapy group was significantly greater (p<0.01, both comparisons) than the 10% reduction observed with EZE alone (Table 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com