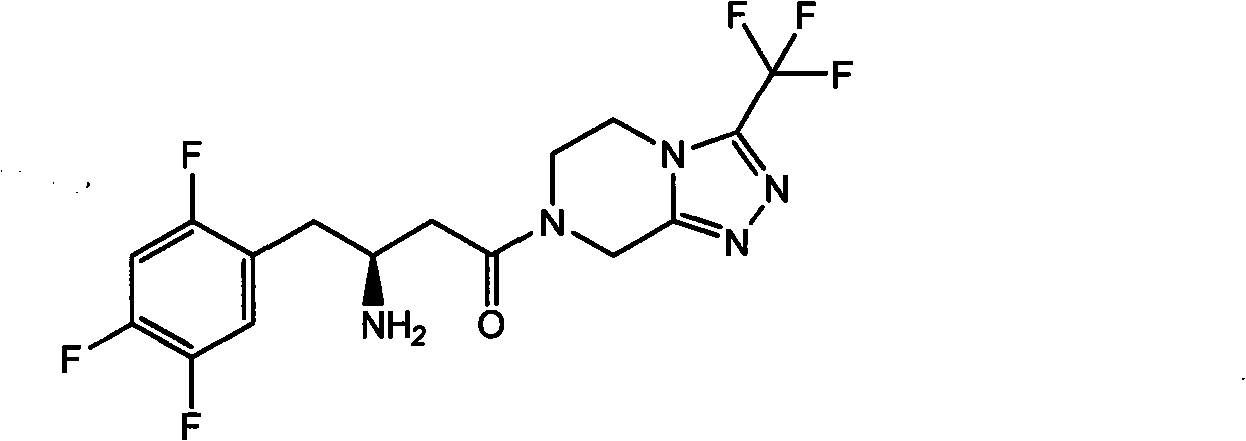
Intermediates of Sitagliptin and preparation method thereof
A technology of sitagliptin and intermediates, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problem of less catalysts, etc., and achieve the effects of improving purity, reducing dosage, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
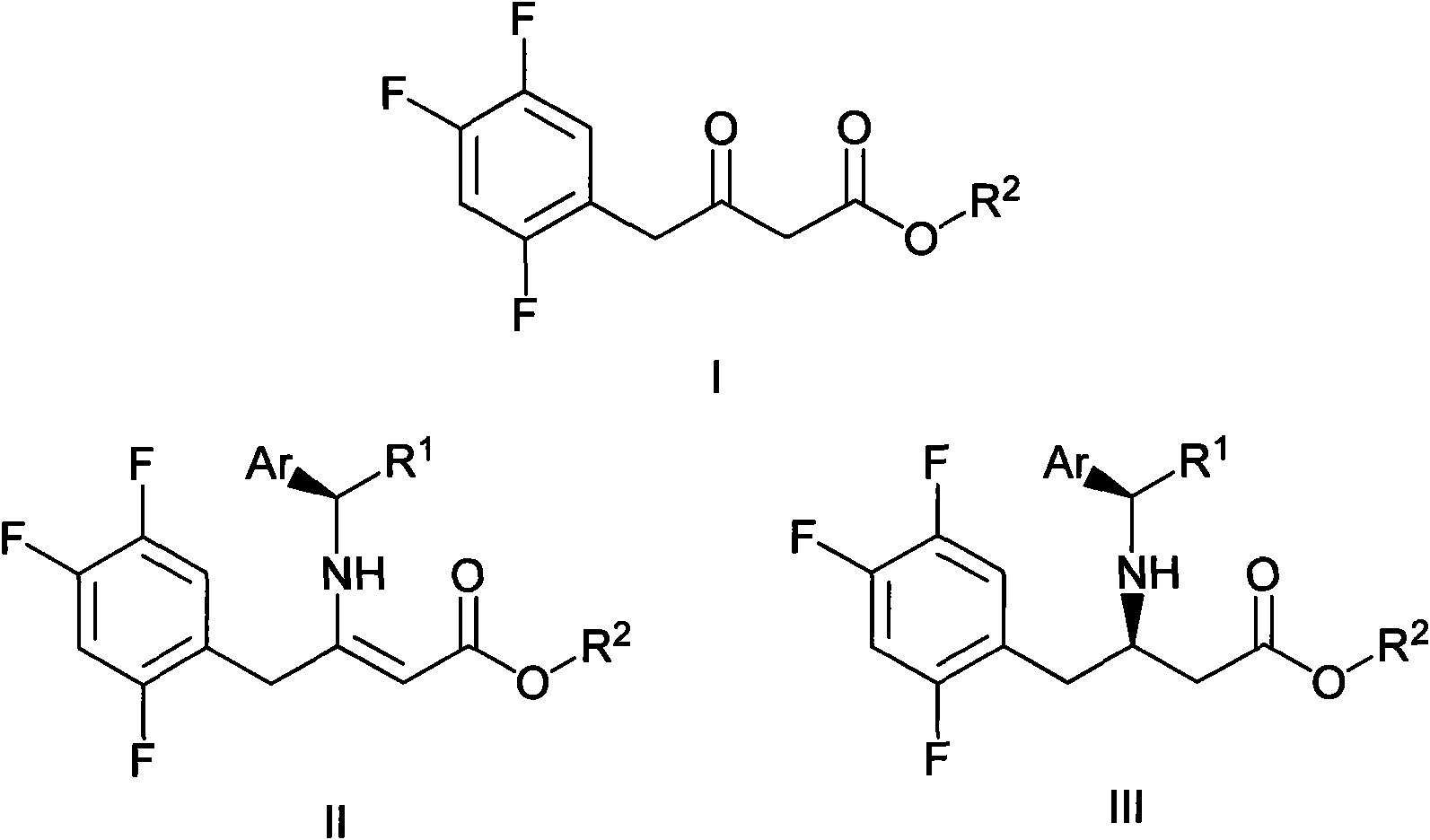
[0033] Example 1 Sitagliptin Intermediate II 1 Synthesis
[0034]
[0035] Using existing methods to prepare I 1 Compound is used as raw material, then the 200g reactant I 1 (0.81mol) and 98g (0.81mol) of (R)-1-phenylethylamine were added to 2.8L of methanol, the temperature was raised to 40°C, 49mL of AcOH was added, the temperature was raised to 48°C, kept for 15min and dropped to within 1h 40°C. After aging for 5 hours, 0.4% seed crystals were added, and the mixture was aged for 1 hour to form a slurry. The mixture was aged for 1 h, 2.8 L of heptane was added over 3 h, the mixture was aged at 40 °C for 3.5 h, and slowly cooled to room temperature over 5.5 h. After a total reaction time of 21 h, the slurry was rapidly filtered and washed with 800 mL of a 1:1 isopropanol / heptane mixture. solid in N 2 258g target product II was produced after drying for 24h 1 , The yield is 90%.
Embodiment 2
[0036] Example 2 Sitagliptin II 2 compound synthesis
[0037]
[0038] Using existing methods to prepare I 2 Compound is used as raw material, then the 200g (0.77mol) reactant I 2 Add 93.1g (0.77mol) of (R)-1-phenylethylamine into 2.8L of isopropanol, raise the temperature to 40°C, add 49mL of AcOH, raise the temperature to 48°C, keep it for 15min and drop it to within 1h 40°C. After aging for 5 hours, 0.4% seed crystals were added, and the mixture was aged for 1 hour to form a slurry. The mixture was aged for 1 h, 2.8 L of heptane was added over 3 h, the mixture was aged at 40 °C for 3.5 h, and slowly cooled to room temperature over 5.5 h. After a total reaction time of 21 h, the slurry was rapidly filtered and washed with 800 mL of 1:1 isopropanol / heptane. The solid was dried under N2 for 24h to produce 243g of the target product II 2 , The yield was 87%.
Embodiment 3
[0039] Example 3 Sitagliptin Intermediate III 1 Synthesis
[0040]
[0041] in N 2 Under protection, add 200g reactant II in 2L autoclave 1 (0.57mol), dissolved in 1.2L of MeOH, added 4g of 5% Pt / Al 2 o 3 (2%) as a catalyst, followed by 16atm of H 2 , heated to 35°C for 15h, TLC detected the reaction and removed the solvent under reduced pressure to obtain 197g of product III 1 , the yield was 98%, ee (enantiomeric excess) > 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com