Transaminase-PLP co-immobilized enzyme and preparation and application thereof
A technology of co-immobilization of enzymes and transaminases, applied in the fields of application, transferase, biochemical equipment and methods, etc., can solve the problems of less recovery batches, poor stability, high temperature, etc., and achieve high product yield and purity, organic solvent Strong tolerance, the effect of reducing the discharge of "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Pretreatment of resin
[0038] a. Epoxy resin pretreatment: take 100g of epoxy resin LX-1000HFA (purchased from Xi'an Lanxiao Science and Technology New Materials Co., Ltd.), soak it in 500mL distilled water for 12h, and then suction filter to obtain the filter cake, which is the pretreated resin, Store at 4°C until use.
[0039] b, amino resin pretreatment:
[0040] ①Solution preparation:
[0041] 0.1M pH 8.0 buffer solution (1L): Add 23.8g of dipotassium hydrogen phosphate and 2.75g of potassium dihydrogen phosphate to 1L of deionized water, dilute to 1000mL, and adjust the pH to 7.8-8.2.
[0042] 2% glutaraldehyde phosphate buffer solution (1L): 40 mL glutaraldehyde (50%), 960 mL water, 4.76 g of dipotassium hydrogen phosphate, after dissolving, adjust the pH to 7.8-8.2 with potassium dihydrogen phosphate.
[0043] ②Vector activation:
[0044] Add 100 mL of 0.1M pH 8.0 buffer to 10 g of the carrier. After constant temperature shaking at 25°C and 300 rp...
Embodiment 2
[0047] Example 2: Cultivation of transaminase genetically engineered bacterial cells
[0048] (1) Construction of transaminase genetically engineered bacteria:
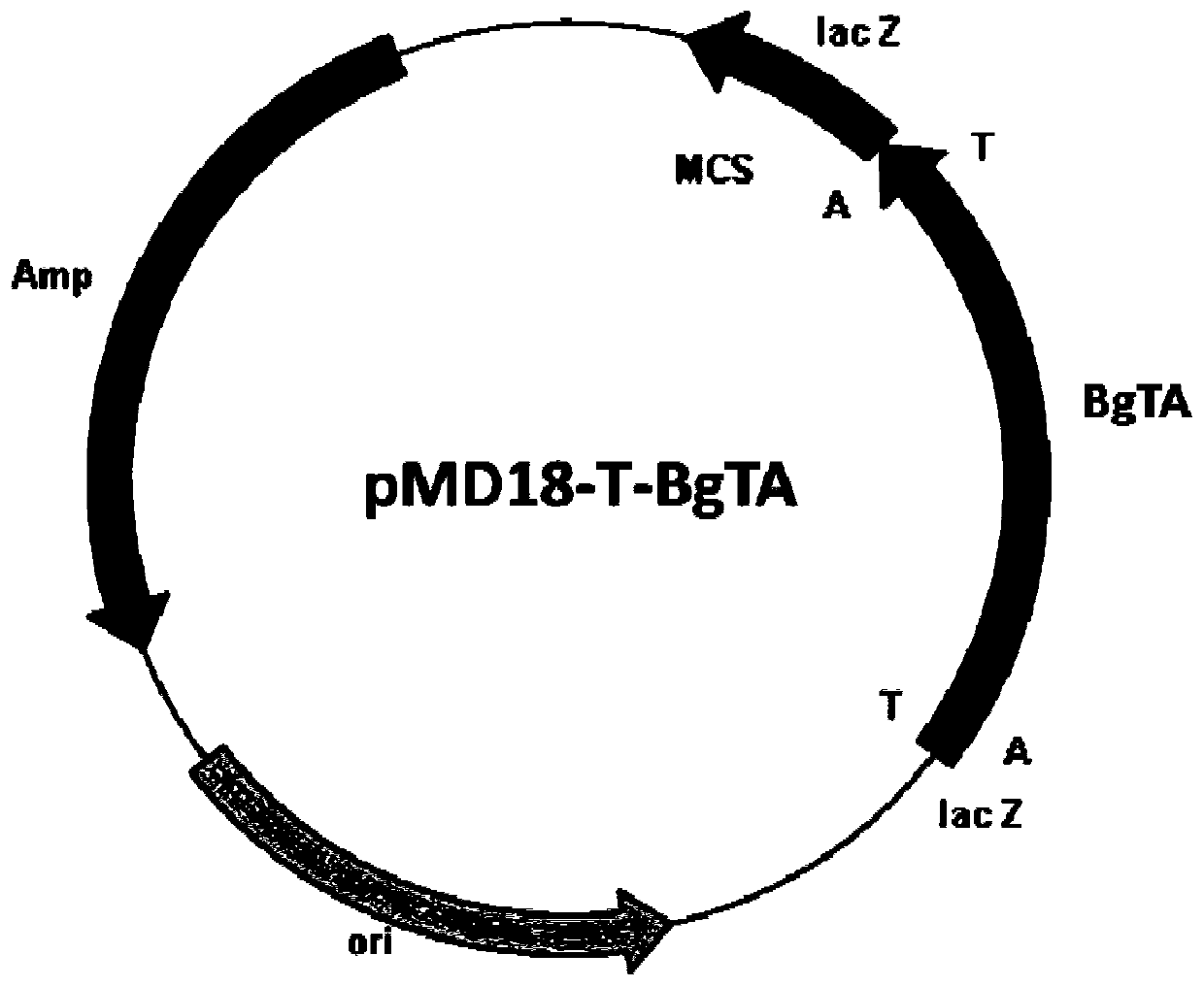
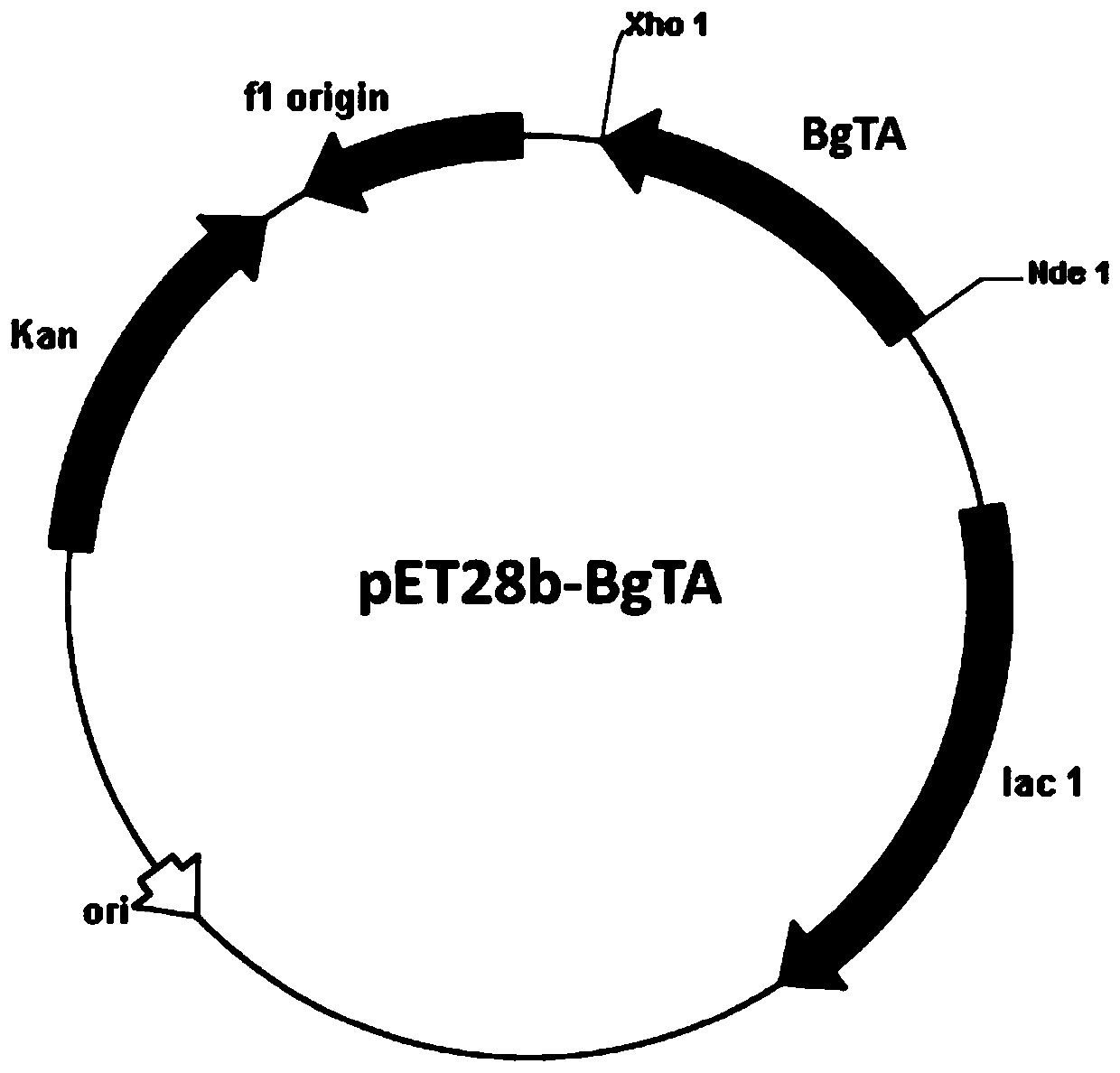
[0049] Primer 1 (CCG 1) was designed according to the gene sequence SEQ ID NO. CATATG GCTATCA TCCAGGTTCAGC), primer 2 (TTG CTCGAG TCAAGCCGGA ACAGAAGAG), and Nde I and Xho I restriction sites (underlined) were introduced in primer 1 and primer 2, respectively. Under the initiation of primer 1 and primer 2, high-fidelity PfuDNA polymerase was used for amplification, and the recombinant plasmid pMD18-T-BgTA ( figure 1 ) as a template, obtain the transaminase BgTA gene sequence, utilize Nde I and Xho I restriction endonucleases (TaKaRa) to process the amplified fragment after sequencing, and utilize T4 DNA ligase (TaKaRa) to use the same restriction for this fragment The endonuclease-treated commercial vector pET28b (Invitrogen) was ligated to construct the expression vector pET28b-BgTA ( figure 2 ). The construct...
Embodiment 3
[0052] Example 3: Preparation of crude transaminase enzyme solution
[0053] The cells were suspended in pH 9.0 Tris buffer according to the concentration (100 g / L), and the cells were disrupted with a high-pressure homogenizer under a pressure of 30 Mpm. After crushing, the crude enzyme liquid is centrifuged at 9000 rpm for 10 min, and the supernatant is taken after centrifugation, which is the crude transaminase enzyme liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com