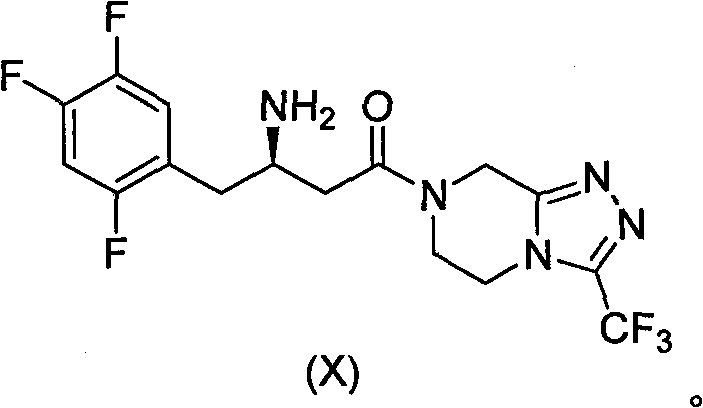
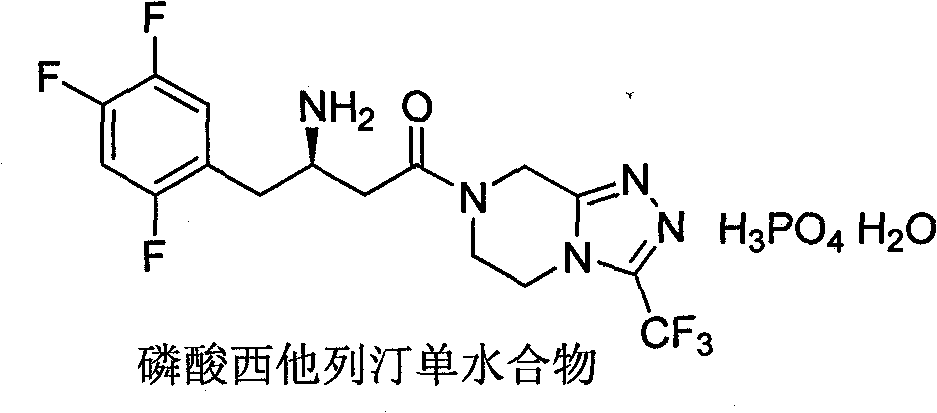
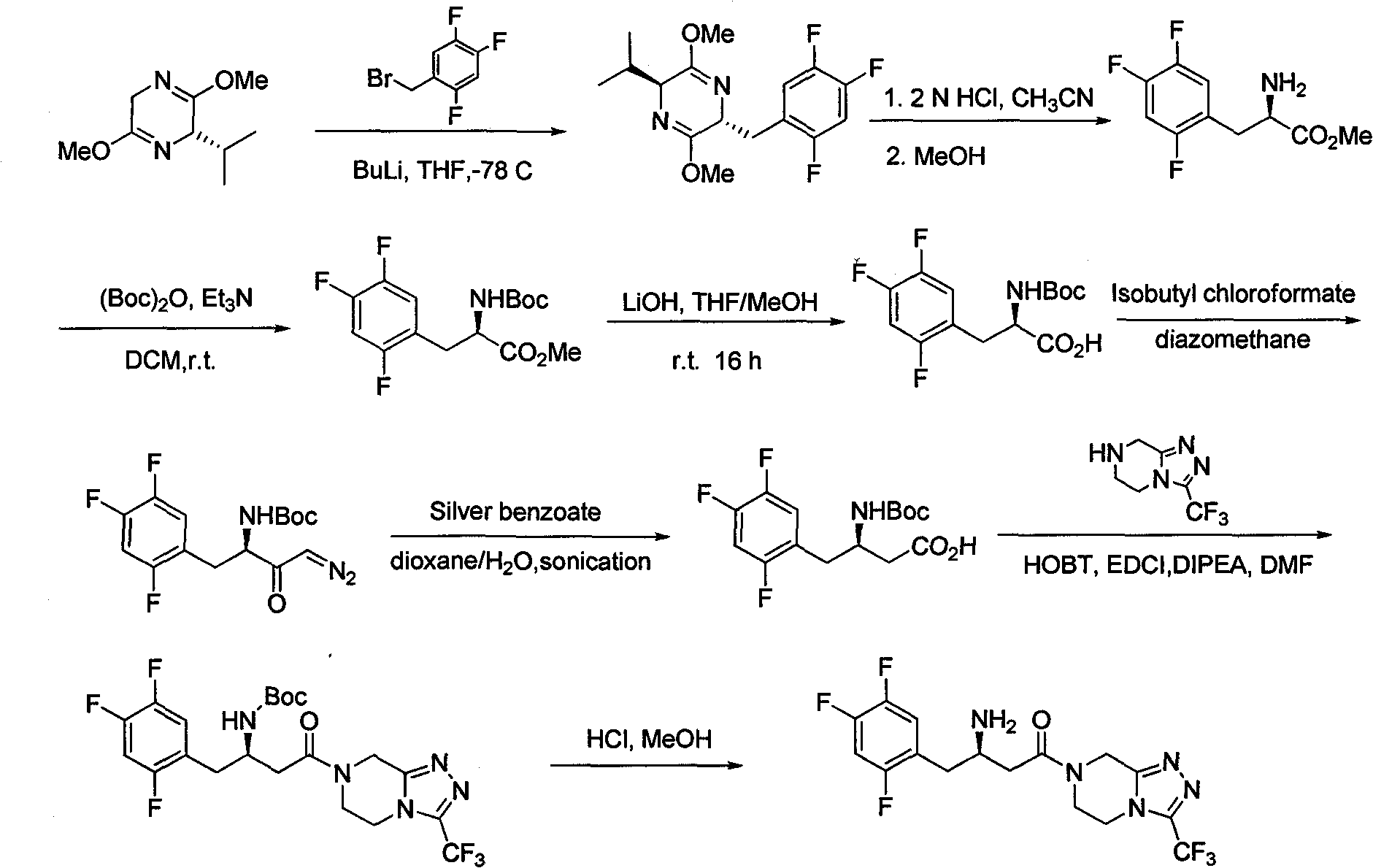
Novel method for synthesizing sitagliptin
A technology of sitagliptin and its compound, which is applied in the field of synthesizing sitagliptin, can solve the problems of high cost, high price, unfavorable industrial production, etc., and achieve the effects of low environmental pollution, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Synthesis of 3-oxo-4-(2,4,5-trifluorophenyl) benzyl butyrate
[0061] At room temperature, add 5-[1-hydroxy-2-(2,4,5-trifluorophenyl)ethylene]-2,2-dimethyl-1,3-di Oxane-4,6-dione (31.6 g), toluene (200 mL) and benzyl alcohol (10.8 g). When the system is uniformly mixed, heat it up and stir and react for 5-6 hours under reflux. After the reaction was completed, the system was cooled to room temperature, 100 mL of water was added, and liquid separation was performed. The organic phase was washed with 100 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 29 g of off-white solid with a yield of 90%. 1 H NMR(400MHz, CDCl 3 ) δ 3.61 (s, 2H), 3.84 (s, 2H), 5.21 (s, 2H), 6.94-6.98 (m, 2H), 7.37-7.41 (m, 5H).
Embodiment 2
[0062] Example 2: Synthesis of 3-amino-4-(2,4,5-trifluorophenyl)-2-butenoic acid benzyl ester
[0063] At room temperature, 3-oxo-4-(2,4,5-trifluorophenyl) benzyl butyrate (29 g), acetonitrile (200 mL), and ammonium acetate (35 g) were sequentially added to the eggplant-shaped flask. When the system is uniformly mixed, heat it up and stir and react for 5-6 hours under reflux. After the reaction was completed, the system was cooled to room temperature, concentrated under reduced pressure to remove the organic solvent, then 200 mL of ethyl acetate and 100 mL of water were added for separation, the organic phase was washed with 100 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain Off-white solid 26g, yield 90%. 1 H NMR (400MHz, DMSO-d6) δ 3.45 (s, 2H), 4.24 (s, 1H), 4.99 (s, 2H), 7.22 (b, 1H), 7.29-7.36 (m, 5H), 7.50- 7.56 (m, 2H), 7.80 (b, 1H).
Embodiment 3
[0064] Example 3: Synthesis of 3-amino-4-(2,4,5-trifluorophenyl) benzyl butyrate
[0065] At room temperature, add 3-amino-4-(2,4,5-trifluorophenyl)-2-butenoic acid benzyl ester (32g), THF (300mL) and tert-butylamine borane (8.7 g). When the system is uniformly mixed, cool down to 0-5°C. At 0-5°C, slowly drop a pre-prepared solution of concentrated sulfuric acid (20g) in isopropanol (60mL) into the system. After the addition is completed, it is naturally warmed to room temperature, and the reaction is stirred for about 8 hours. After the reaction is complete, add 200 mL of water, adjust the pH to 8-9 with 2N NaOH aqueous solution, concentrate under reduced pressure to remove the organic solvent, then add 300 mL of ethyl acetate and 200 mL of water, separate the layers, wash the organic phase with 200 mL of saturated brine, and anhydrous sodium sulfate Dry, filter, and concentrate under reduced pressure to obtain 28 g of pale yellow oil with a yield of 87%. 1 H NMR(400MHz, CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com