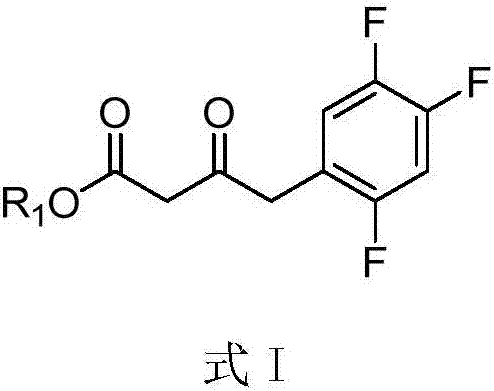
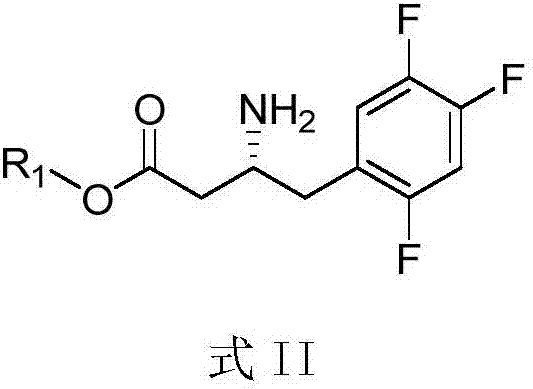
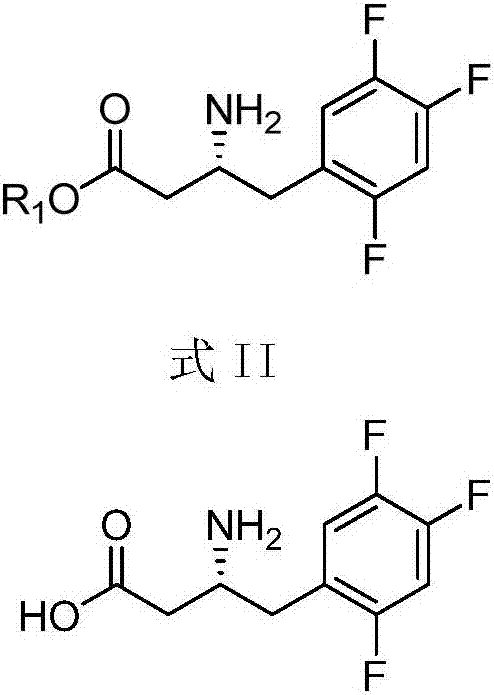
Purpose of compound for preparing Sitagliptin and Sitagliptin preparation method
A technology for sitagliptin and a compound, which is applied in the field of preparing sitagliptin and achieves the effects of large yield, high amino conversion efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 transaminase
[0033] In the following examples, the transaminase is derived from the modified enzyme of the transaminase derived from Arthrobacter by Codexis, and the preparation method of the transaminase is as follows:
[0034] The target gene encoding transaminase (synthesized by Wuhan Tianyi Huiyuan Company) was inserted into the expression plasmid pRSFDuet-1 to obtain a recombinant expression plasmid. The recombinant expression plasmid was transformed into E.coliBL21(DE3) competent cells, and the transformation liquid was spread on the LB plate containing kanamycin, and cultured upside down at 37°C overnight. The resulting recombinant E.coli BL21 (DE3) was inoculated into the LB medium containing kanamycin (the LB medium contains 10g / L protein, 5g / L yeast extract and 10g / LNaCl, pH7.0), Shake culture at 37°C overnight, and insert into 500ml LB medium (the medium is contained in a 2000ml Erlenmeyer flask) according to the inoculum si...
Embodiment 2
[0035] Example 2 Transaminase to the transamination reaction of 3-carbonyl-4-(2,4,5-trifluorophenyl) hydroxyethyl butyrate
[0036] Add 1.1ml 1.83M isopropylamine aqueous solution (pH=8.5), 0.5ml crude transaminase solution, 0.4ml 250mM / L 3-carbonyl-4-(2,4,5-trifluorophenyl) into a 10ml single-necked flask The dimethyl sulfoxide (DMSO) solution of hydroxyethyl butyrate was reacted with magnetic stirring at 45°C for 24 hours, and the conversion rate of the amino group was greater than 99% as determined by high performance liquid chromatography (HPLC). After the reaction, adjust the pH to 11.0, and extract twice with 10 mL of ethyl acetate, combine the extracts, add anhydrous sodium sulfate to dry overnight, remove the solvent by rotary evaporation, and distill under reduced pressure to obtain 25 mg of (R)-3- Amino-4-(2,4,5-trifluorophenyl)-butyric acid hydroxyethyl ester, yield 90.5%, purity>95%, optical purity>98%.
Embodiment 3
[0037] Example 3 The transamination reaction of transaminase to 3-carbonyl-4-(2,4,5-trifluorophenyl) hydroxypropyl butyrate
[0038] Add 1.1ml 1.83M isopropylamine aqueous solution (pH=8.5), 0.5ml crude transaminase solution, 0.4ml 250mM / L 3-carbonyl-4-(2,4,5-trifluorophenyl) into a 10ml single-necked flask The dimethyl sulfoxide (DMSO) solution of hydroxypropyl butyrate was reacted with magnetic stirring at 45° C. for 24 hours, and the conversion rate of amino groups was determined by high performance liquid chromatography (HPLC) to be greater than 99%. After the reaction, adjust the pH to 11.0, and extract twice with 10 mL of ethyl acetate, combine the extracts, add anhydrous sodium sulfate to dry overnight, remove the solvent by rotary evaporation, and distill under reduced pressure to obtain 25 mg of (R)-3- Amino-4-(2,4,5-trifluorophenyl)-butyric acid hydroxypropyl ester, yield 90.5%, purity>95%, optical purity>98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com