Method for synthesizing phenylacetonitrile by performing continuous reaction
A phenylacetonitrile, continuous process technology, applied in the preparation of cyanide reaction, organic chemistry and other directions, can solve the problems of large floor space, many equipment, long reaction time, etc., to achieve high production efficiency, reduce side reactions, and shorten the reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
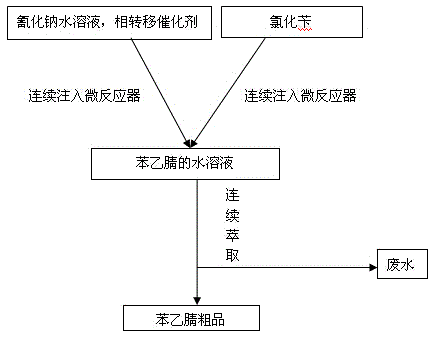
[0022] First adjust the temperature of the microreactor to be controlled at 180°C. Under the action of the plunger pump, the aqueous sodium cyanide solution of 12kmol mass concentration of 13% and the phase transfer catalyst benzyldiethylammonium chloride (the consumption of the phase transfer catalyst is 1‰ of benzyl chloride mass) mixed solution and 10kmol benzyl chloride liquid were continuously injected into the microreactor respectively, and the residence time was controlled at 150s to directly obtain the aqueous solution of benzyl nitrile. The quality of benzyl chloride was monitored by GC, and the purity of the crude product was 98.5%. . Under the action of the continuous extraction and separation equipment (extraction centrifuge), the obtained aqueous solution of benzylnitrile is centrifuged to separate the obtained aqueous solution of benzylnitrile, and the crude product of benzylnitrile is separated from the light phase port, and the yield of the crude product of benz...
Embodiment 2
[0024] First adjust the temperature of the microreactor to be controlled at 250°C. Under the action of the plunger pump, mix 12kmol13% sodium cyanide aqueous solution and the phase transfer catalyst benzyl diethyl ammonium chloride (the amount of the phase transfer catalyst is benzyl chloride 0.5‰ of mass), and 10kmol benzyl chloride liquid were continuously injected into the microreactor, the residence time was controlled at 100s, and the aqueous solution of benzyl nitrile was obtained directly. The quality of benzyl nitrile was monitored by GC, and the purity of the crude product was 98.6%. Under the action of the continuous extraction and separation equipment (extraction centrifuge), the obtained phenylacetonitrile feed liquid is subjected to centrifugal separation, and what is separated from the light phase port is the crude product of phenylacetonitrile, and the yield of the crude product of phenylacetonitrile is 97.7% %, what is separated from the heavy phase is waste sod...
Embodiment 3
[0026] First adjust the temperature of the microreactor to be controlled at 400°C. Under the action of the plunger pump, mix 12kmol13% sodium cyanide aqueous solution and the phase transfer catalyst benzyltriethylammonium chloride (the amount of the phase transfer catalyst is benzyl chloride 0.8‰ of mass), and 10kmol benzyl chloride liquid were continuously injected into the microreactor, the residence time was controlled at 20s, and the aqueous solution of benzyl nitrile was obtained directly. The quality of benzyl nitrile was monitored by GC, and the purity of the crude product was 98.5%. Under the action of the continuous extraction and separation equipment (extraction centrifuge), the obtained phenylacetonitrile feed liquid was subjected to centrifugal separation, and what was separated from the light phase port was the crude product of phenylacetonitrile, and the yield of the crude product of phenylacetonitrile was 97.6% %, what is separated from the heavy phase is waste s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com