Method for synchronizing m-benzenyl trifluoride di-cyan acetonphenone
A technology of trifluoromethylbenzene and cyanoacetophenone, applied in the field of organic synthesis, can solve the problems of reduced production cost, high production cost, and high product yield, and achieve low production cost, easy recycling, and high product yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
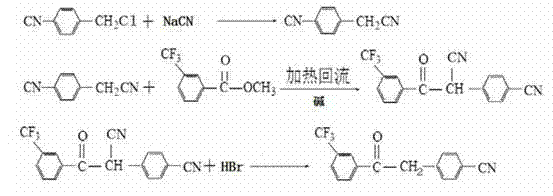
[0022] A kind of synthetic method of m-trifluoromethylbenzene-p-cyanoacetophenone is starting raw material with 4-cyanobenzyl chloride, and concrete steps comprise:
[0023] (1) Add 50 mL of water, 34.3 g of sodium cyanide aqueous solution (dissolving 10.29 g of pure sodium cyanide) and 3 g of catalyst benzyltriethylammonium chloride into a 500 mL four-necked flask equipped with a stirrer, a thermometer and a condenser (TEBAC). After sealing, start stirring while raising the temperature. When the temperature reaches 90-95°C, slowly add 29.73g of 4-cyanobenzyl chloride in toluene solution (121.2g in total) in about 1 hour, and keep reflux during the dropwise addition. Keep warm for 1.5 hours after feeding. After cooling, add 200 mL of water to wash once. The water layer was separated, and the organic layer was dried and then distilled under reduced pressure to obtain 28.82 g of a white solid, namely 4-cyanophenylacetonitrile, with a content of 96% and a yield of 97.4%;
[00...
Embodiment 2
[0027] A kind of synthetic method of m-trifluoromethylbenzene-p-cyanoacetophenone is starting raw material with 4-cyanobenzyl chloride, and concrete steps comprise:
[0028] (1) Add 50mL of water, 51.45g of sodium cyanide aqueous solution (dissolved with 10.29g of pure sodium cyanide) and 3g of catalyst benzyl triethyl chloride into a 500mL four-necked flask equipped with a stirrer, thermometer and condenser ammonium (TEBAC). After sealing, start stirring while raising the temperature. When the temperature reaches 90-95°C, slowly add 28.94g of 4-cyanobenzyl chloride in toluene solution (144.7g in total) in about 1 hour, and keep reflux during the dropwise addition. Keep warm for 1.5 hours after feeding. After cooling, add 200 mL of water to wash once. The water layer was separated, and the organic layer was dried and distilled under reduced pressure to obtain 28.75 g of a white solid with a content of 96.2% and a yield of 97.4%;
[0029] (2) Add 14.34g of 4-cyanophenylaceto...
Embodiment 3
[0032] A kind of synthetic method of m-trifluoromethylbenzene-p-cyanoacetophenone is starting raw material with 4-cyanobenzyl chloride, and concrete steps comprise:
[0033](1) Add 50 mL of water, 27.1 g of sodium cyanide aqueous solution (dissolving 10.29 g of pure sodium cyanide) and 3 g of catalyst benzyl triethyl chloride into a 500 mL four-necked flask equipped with a stirrer, a thermometer and a condenser. ammonium (TEBAC). After sealing, start stirring while raising the temperature. When the temperature reaches 90-95°C, slowly add 30.03g of 4-cyanobenzyl chloride in toluene solution (100.1g in total) in about 1 hour, and keep reflux during the dropwise addition. Keep warm for 1.5 hours after feeding. After cooling, add 200 mL of water to wash once. The water layer was separated, and the organic layer was dried and distilled under reduced pressure to obtain 28.68 g of a white solid with a content of 95.6% and a yield of 96.5%;
[0034] (2) Add 14.34g of 4-cyanophenyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com