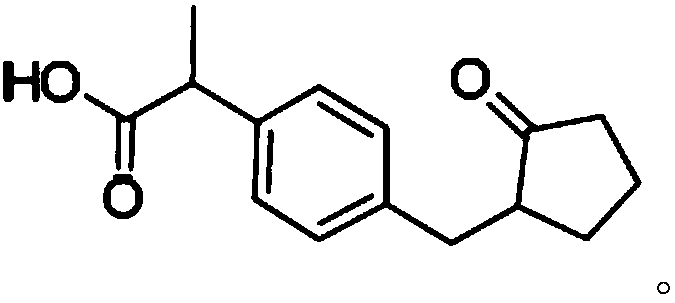
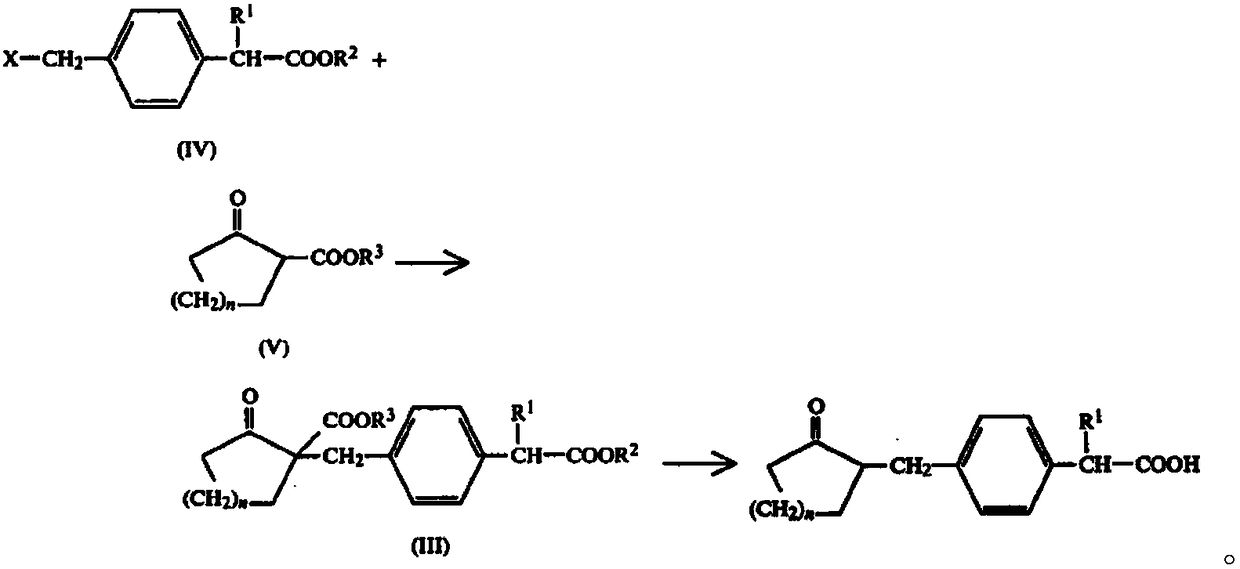
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
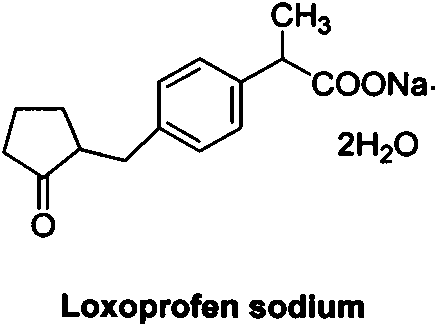
66 results about "Sodium loxoprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
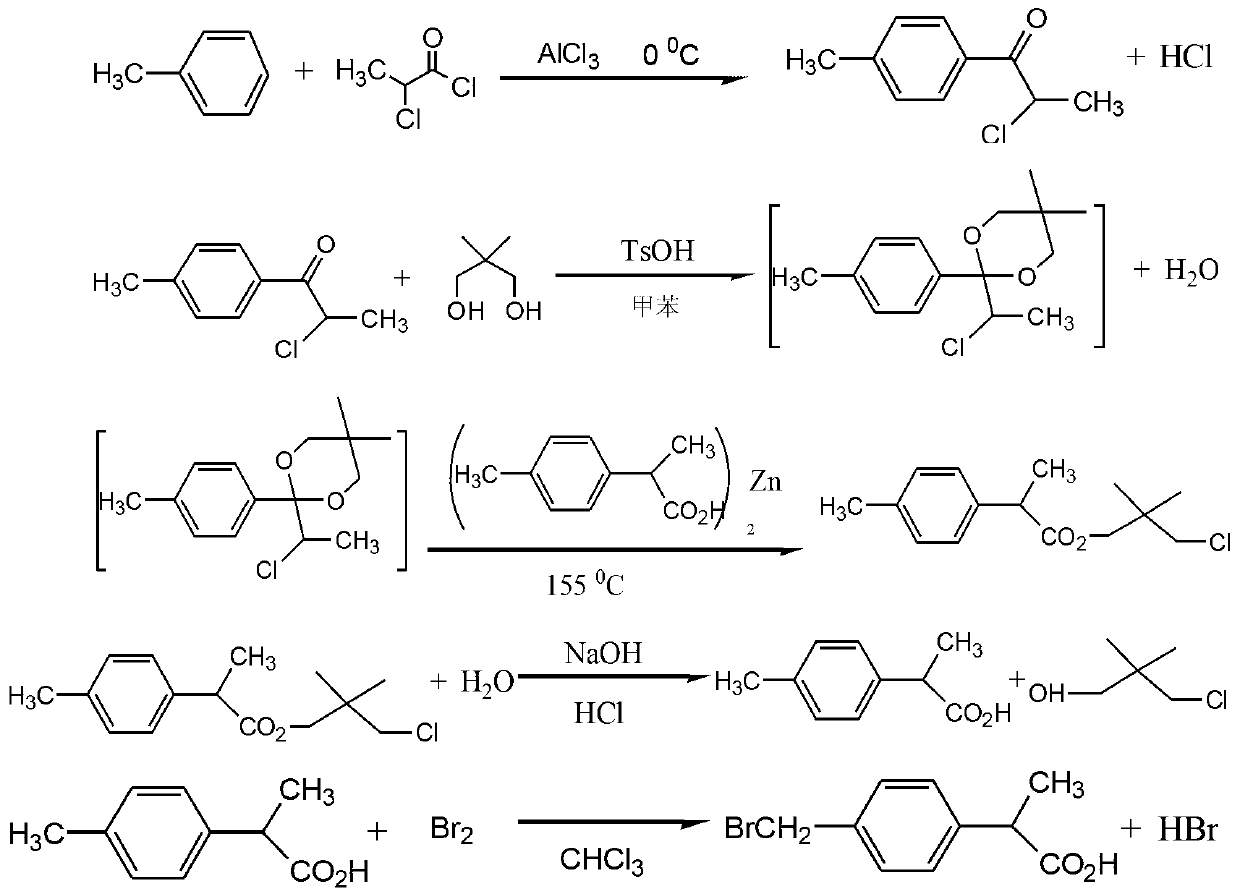
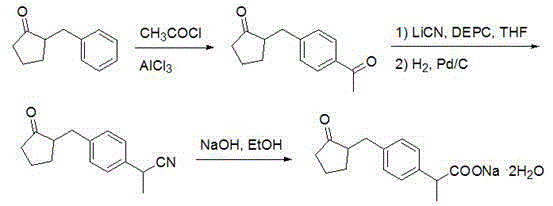
Method for synthesizing loxoprofen sodium
ActiveCN101412670ARaw materials are easy to getUnique craftOrganic compound preparationCarboxylic compound preparationSolventHydrolysis
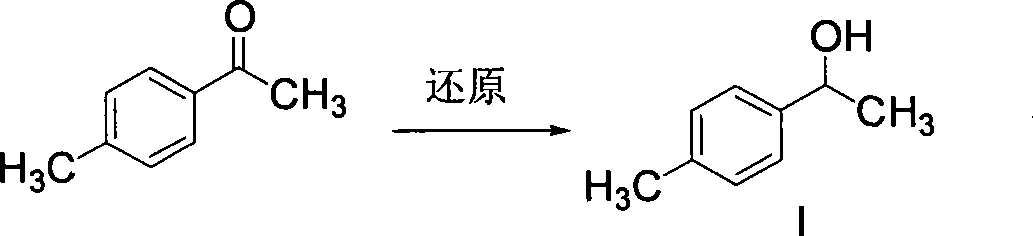
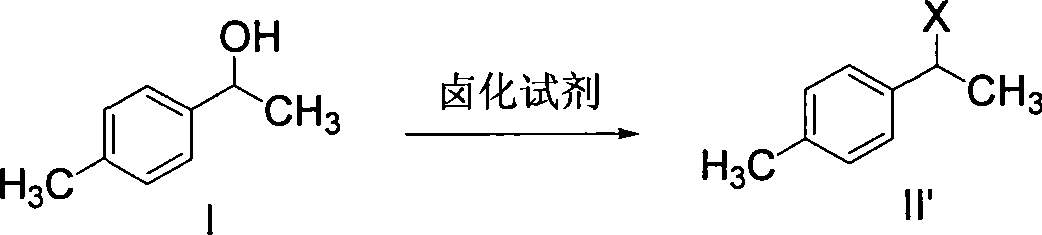
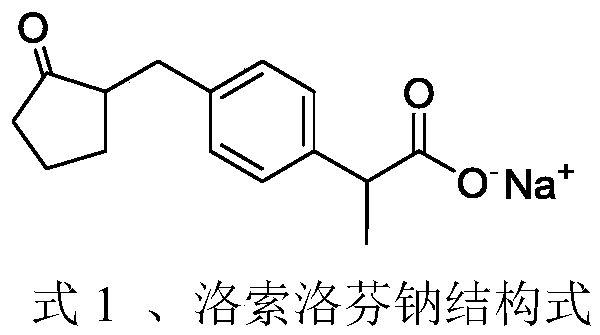
The invention discloses a synthetic method for loxoprofen sodium, which is prepared by taking methyl acetophenone as an initial raw material through reduction, acylation or halogen substituent, cyanation, hydrolysis, bromination, condensation, decarboxylation and salifying. The method has the advantages of easily obtained raw material, unique technology, simple and stable operation, and high productive rate in each step of reaction; and all solvents used in the synthesis process can be recycled, so the production cost is reduced greatly. Tests show that the obtained product has reliable quality and stable performance, and can be further used for making preparation of non-steroidal anti-inflammatory drugs such as the loxoprofen sodium.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Improved cataplasm ground-mass and use thereof
InactiveCN101416955AImprove adhesionGood molding effectPharmaceutical non-active ingredientsSheet deliveryCross-linkAnalysis method
The invention discloses an improved substrate of a cataplasma and the application thereof to loxoprofen sodium cataplasma. The substrate of the cataplasma adopts the combined application of two or more cross-linking agents and achieves the purposes of increasing the viscosity of a paste and improving the ductibility and moldability of the paste. The application of the substrate can reduce the variety and usage of thickening accessories or can increase the viscosity of the cataplasma to the extent required by a viscous force even without adding special thickening agents. The substrate has advantages in ensuring the good ductibility and moldability of the cataplasma and quickly establishing analytical methods, and the like, thus being significant for the further promotion and application ofthe cataplasma.
Owner:CHONGQING PHARMA RES INST
Synthetic methods of loxoprofen sodium and intermediate thereof
InactiveCN104710309ASimple and fast operationEasy to operateOrganic compound preparationCarboxylic acid esters preparationPropanoic acidLoxoprofen
The invention discloses synthetic methods of loxoprofen sodium and an intermediate thereof; with benzene as a starting material, the key intermediate of loxoprofen sodium is obtained through acylation, halogenation, ketalation, rearrangement and Blanc chloromethylation, then the key intermediate is subjected to condensation, decarboxylation and salt forming reaction to obtain loxoprofen sodium. The methods have the advantages of easily obtained raw materials, simple operation, stable process and high yield; 2-(4-chloromethyl phenyl)propionic acid or propionate is prepared by adopting a method of direct carrying out chloromethylation on a substituted benzene ring, the operation is simple, the yield is high, the use of a chloromethyl ether reagent or stannic chloride and other Lewis acids is avoided, the environmental protection property is high, and relatively good industrial application value is achieved.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Method for catalytic esterification resolution of 2-(4-methylphenyl) propionic acid enantiomer via stereoselective enzyme
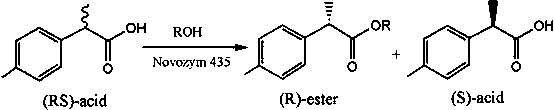
The invention discloses a method for catalytic esterification resolution of a 2-(4-methylphenyl) propionic acid enantiomer via stereoselective enzyme. On the basis of high catalysis efficiency and high stereoselectivity of lipase, catalytic esterification resolution of racemic 2-(4-methylphenyl) propionic acid is conducted in an organic solvent medium, so that the (S)-2-(4-methylphenyl) propionicacid is prepared. The reaction system, with the application of an organic solvent system, can improve thermal stability and catalysis efficiency of the lipase to a great extent, so that a substrate conversion rate and optical activity are greatly improved, and the optical activity of the substrate is greater than or identical to 97.84%. In comparison with other resolution technologies, the methodis gentle in reaction conditions, simple to operate and low in environmental pollution, and the obtained (S)-2-(4-methylphenyl) propionic acid is relatively high in optical purity; and with the (S)-2-(4-methylphenyl) propionic acid serving as a key intermediate product, a feasible method is provided for the preparation of loxoprofen sodium.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium
InactiveCN108440274AHigh HPLC contentThe reaction mechanism is simpleOrganic compound preparationCarboxylic acid esters preparationCompound cMethyl propionate
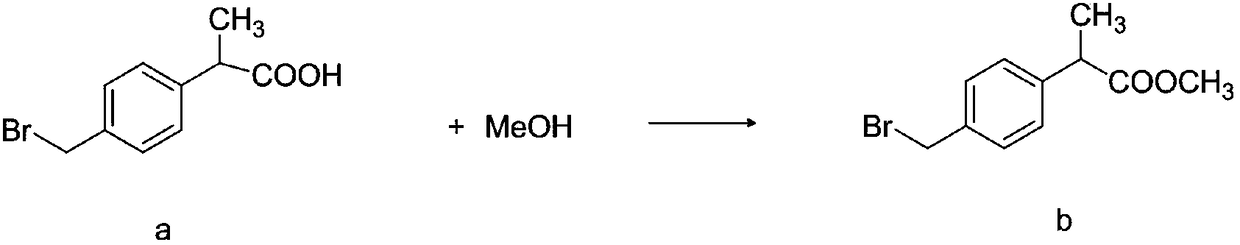
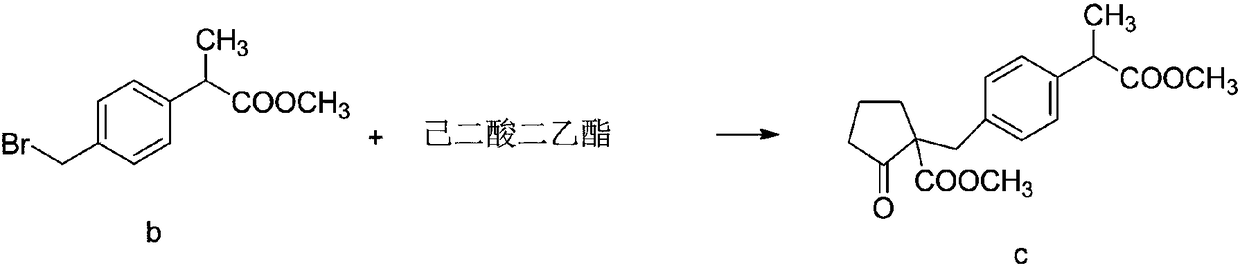
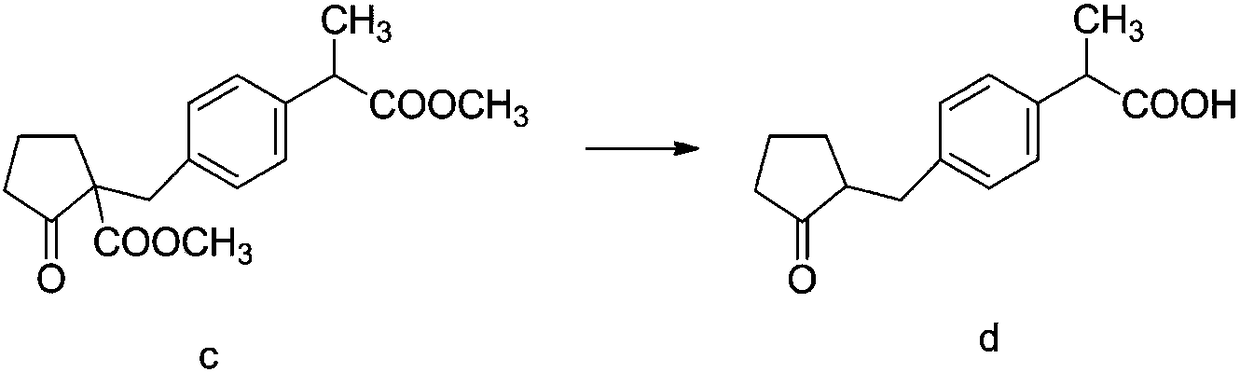
The invention discloses a method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium, 2-(4-bromomethylphenyl) methyl propionate b is synthesized by esterification of raw material a and methanol, intermediate compound c and loxoprofen acid are synthesize sequentially, and the loxoprofen sodium is finally synthesized. The reaction mechanism is simple, by-products arefew, synthesis steps are easy to control, the raw material is easily available, and the impurity content of each step is strictly controlled. The purifying method is easy to operate and suitable for industrial production. The white flake crystal loxoprofen sodium is prepared. Finally, HPLC analysis shows that the loxoprofen sodium content detected by HPLC is as high as 99.95%.
Owner:大桐制药(中国)有限责任公司
Industrial production method of high purity loxoprofen sodium dehydrate
InactiveCN104326903AHigh yieldImprove qualityOrganic compound preparationCarboxylic acid esters preparationDistillationAdipic acid
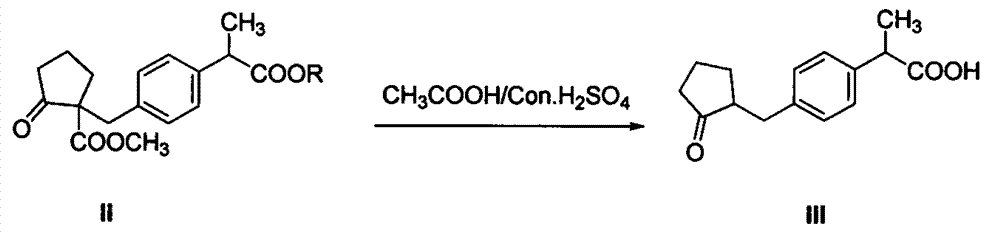
The invention discloses an industrial production method of high purity loxoprofen sodium dehydrate; according to the method, adipic acid diester is taken as a starting material for Dieckmann condensation reaction with 2-(4-bromomethyl phenyl) propionate under strong alkaline conditions, the yield and quality of compound II are effectively improved, because the adipic acid diester on the market is lower in cost than 2-ethoxy carbonyl cyclopentanone, the production cost can be greatly reduced; the compound obtained by reaction is processed sequentially by hydrolysis and decarboxylation under acidic conditions, in the hydrolysis and decarboxylation process, generated low boiling point alcohol solvents and carbon dioxide gas can be removed by atmospheric distillation, the reaction can be effectively promoted, the reaction time is shortened, the conversion rate is improved; and finally the final product loxoprofen sodium dehydrate is generated under the condition of aqueous sodium hydroxide solution. The preparation method can greatly reduce the manufacturing cost of loxoprofen sodium, and is more suitable for the industrialized production than the existing technology.
Owner:合肥远志医药科技开发有限公司
Preparation method for 2-(4-brooethylphenyl) propionic acid
InactiveCN103342636AHigh purityGood anti-rheumatic effectOrganic compound preparationCarboxylic compound preparationPropanoic acidRheumatism
The invention relates to the technical field of 2-(4-brooethylphenyl) propionic acid, and in particular relates to a preparation method for 2-(4-brooethylphenyl) propionic acid. The preparation method comprises the following steps of: taking p-methylphenyl-2-chloro-1-ketone and 2-p-methylphenyl propionic acid as main raw materials, and finally preparing a finished product via a dry acylation reaction, a condensation reaction and a bromination reaction, wherein the finished product is high in purity and high in yield which is up to more than 98%, is one of key intermediates for synthesising loxoprofen sodium, and is clinically indicated to be the greatest in the effects of easing pain, resisting inflammation and resisting rheumatism within the presently known aryl propionic acid medicines, thus being greatly preferred by national and international experts and scholars, and promoted by the medical circle and patients. Additionally, the preparation method disclosed by the invention is simple in production process, low in production cost, and less in pollution.
Owner:NANTONG TAITONG CHEM TECH
Loxoprofen sodium framework tablet
InactiveCN101342147AReduce releaseImprove securityOrganic active ingredientsAntipyreticSide effectActive component
The invention belongs to the technical field of medicines, particularly relates to a composition containing loxoprofen sodium, and more particularly, the invention relates to a loxoprofen sodium matrix tablet. The loxoprofen sodium matrix tablet is composed of loxoprofen sodium of an active component, hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC) and acrylics of polymer materials and filling agents. The loxoprofen sodium matrix tablet of the invention has longer action time than a common tablet and reduces the side effects. The invention has simple operation process, low cost and easy control and is suitable for industry large scale production.
Owner:FUDAN UNIV
Method for preparing loxoprofen sodium
ActiveCN106699559AHigh purityStrong industrial operationOrganic compound preparationCarboxylic acid esters preparationOrganic synthesisCombinatorial chemistry
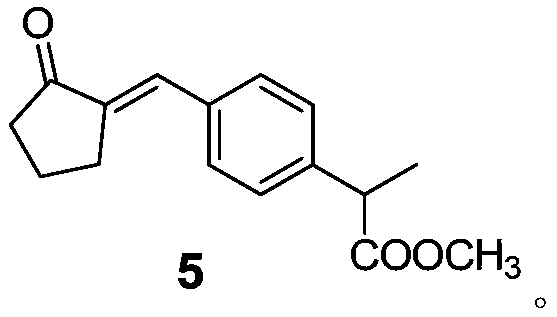
The invention relates to the technical field of organic synthesis, in particular to a method for preparing loxoprofen sodium. The invention provides a compound with the structure shown in formula 5 and a preparation method and application of the compound, (the formula is defined in the description). The loxoprofen sodium obtained according to the scheme is high in purity, high in industrialized operation, and good in application prospect.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Drug composition containing loxoprofen sodium and preparation method of drug composition
InactiveCN105380929AEvenly dispersedImprove solubilityOrganic active ingredientsAntipyreticAdhesive cementCurative effect
The invention relates to a drug composition containing loxoprofen sodium and a preparation method of the drug composition. A loxoprofen sodium patch is successively formed by an antisticking film, a drug layer smeared on the antisticking film and a substrate layer covering the drug layer; and the drug layer is prepared from the following components: loxoprofen sodium, a skeleton material, an adhesive, a transdermal enhancers a skeleton material solvent, and the weight ratio of the loxoprofen sodium to the skeleton material is 1 to (1 to 2). When the drug layer is prepared, adhesive cement is homogenized by adopting a homogenizing machine, so that various components are more uniformly dispersed, the uniformity of the drug release speed is guaranteed, and the curative effect is relatively good.
Owner:蚌埠丰原涂山制药有限公司
Loxoprofen sodium coating agent and preparation method thereof
InactiveCN105106180AGood biocompatibilityGood film formingOrganic active ingredientsAntipyreticDiseaseIrritation
The invention discloses a loxoprofen sodium coating agent smeared on the surface of skin, relieve and cure rheumatoid arthritis, osteoarthritis, lumbago, scapulohumeral periarthritis, and diseases of shoulders, necks and wrists, and a preparation method of the loxoprofen sodium coating agent. The loxoprofen sodium coating agent comprises chitosan and glycerol, wherein chitosan is a deacetylation product of crustaceans, has favorable biocompatibility, film-forming property, bacterium resistance, inflammation resistance and hemostasis, and serves as a favorable auxiliary material for preparation of gelata; glycerol is taken as a plasticizer and humectant, and used for enabling a film to be high in elasticity and low in fracture possibility, and enhancing the percutaneous absorption of medicine; the coating agent made of glycerol and loxoprofen sodium can be used for overcoming the first pass effect of a liver and gastrointestinal tract effect, improving bioavailability and enhancing curative effect through local skin application. The coating agent has the advantages that the effect taking period is short; the effect is durable; the spreadability and the adhesive force are high; skin irritation is avoided; the drug delivery is convenient. The preparation method is relatively reasonable; the specificity of a content determination method is high; the result is accurate; the standards can be used for controlling the quality of the coating agent.
Owner:CHENGDU AIBIKE BIOTECH
Medicine composition soft capsule and its preparing process
InactiveCN1857233AEvenly dispersedDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineActive component
The present invention relates to medicine composition soft capsule with loxoprofen sodium as active component and its preparation process. The soft capsule is prepared through preparing the inclusion with the mixture of loxoprofen sodium and several of diluent, co-solvent, solubilizer, suspending agent and surfactant; and the subsequent preparing soft capsule. The soft capsule has high stability of loxoprofen sodium, raised medicine leaching quantity, high bioavailability, high safety and high effectiveness.
Owner:宛六一 +1
Loxoprofen sodium sustained-release pellet
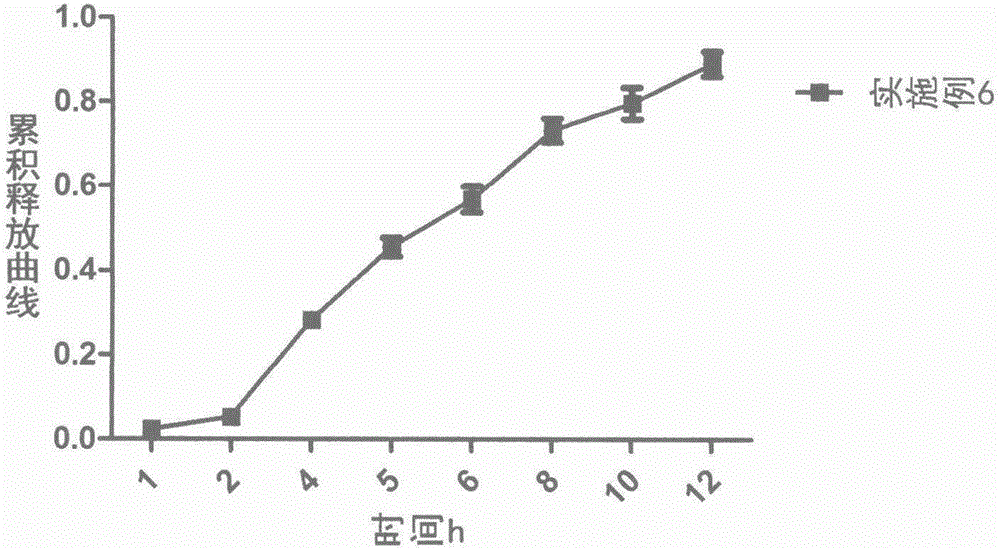
ActiveCN105769773AInhibition of burst releaseGood sustained release propertiesOrganic active ingredientsAntipyreticSustained release pelletsBlood concentration
The invention provides a loxoprofen sodium sustained-release pellet. The pellet comprises a drug-loaded pellet core, an isolating layer coating the drug-loaded pellet core and a sustained-release layer coating the isolating layer, wherein the weight ratio of the drug-loaded pellet core to the isolating layer to the sustained-release layer is 80:(1-15):(2-20). The loxoprofen sodium sustained-release pellet shows an excellent release characteristic in vitro, the frequency of administration can be reduced, the medication compliance of patients can be improved, the blood concentration can be stabilized, and toxic and side effects of drugs on the stomach and intestines can be reduced. The invention further discloses a method for preparing the loxoprofen sodium sustained-release pellet. The method is simple in process, and is easy to industrially produce.
Owner:CHINA PHARM UNIV
Preparation method of loxoprofen sodium ring opened impurity
ActiveCN107353195ASimple processSimple and fast operationOrganic compound preparationCarboxylic preparation by oxidationOrganic solventTetramine
The invention relates to a preparation method of a loxoprofen sodium ring opened impurity, and belongs to the technical field of bulk drug preparation. The preparation method comprises the following steps: step one, carrying out substitution reactions between a compound represented by a formula I and hexamethylene tetramine in an organic solvent A, hydrolyzing the reaction product by inorganic acids to obtain a compound represented by a formula II; carrying out condensation reactions between the compound represented by the formula II and a compound represented by a formula III in an organic solvent B to obtain a compound represented by a formula IV; carrying out oxidation and ring opening reactions of the compound represented by the formula IV under the effect of an oxidant and inorganic alkalis to obtain the loxoprofen sodium ring opened impurity represented by a formula V. The invention provides a preparation method of the loxoprofen sodium ring opened impurity.
Owner:迪嘉药业集团股份有限公司
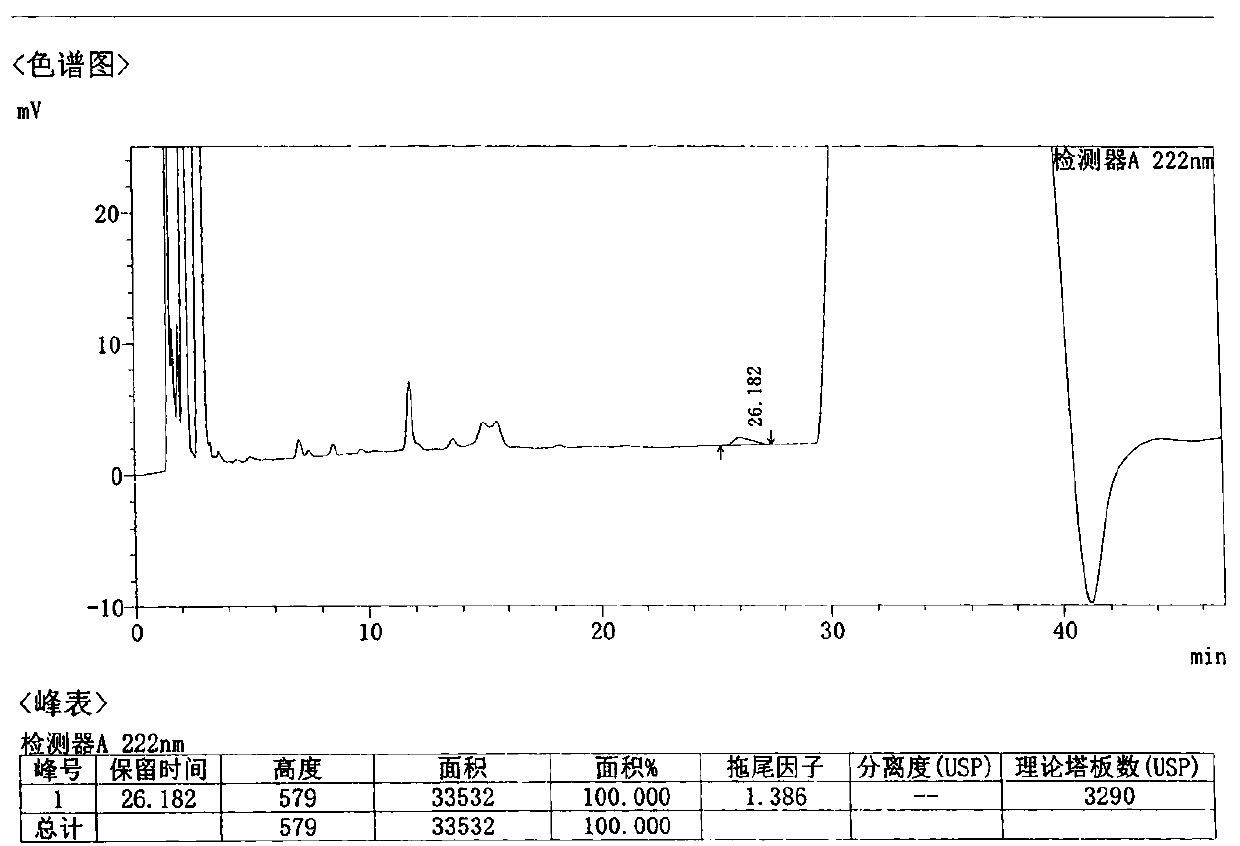
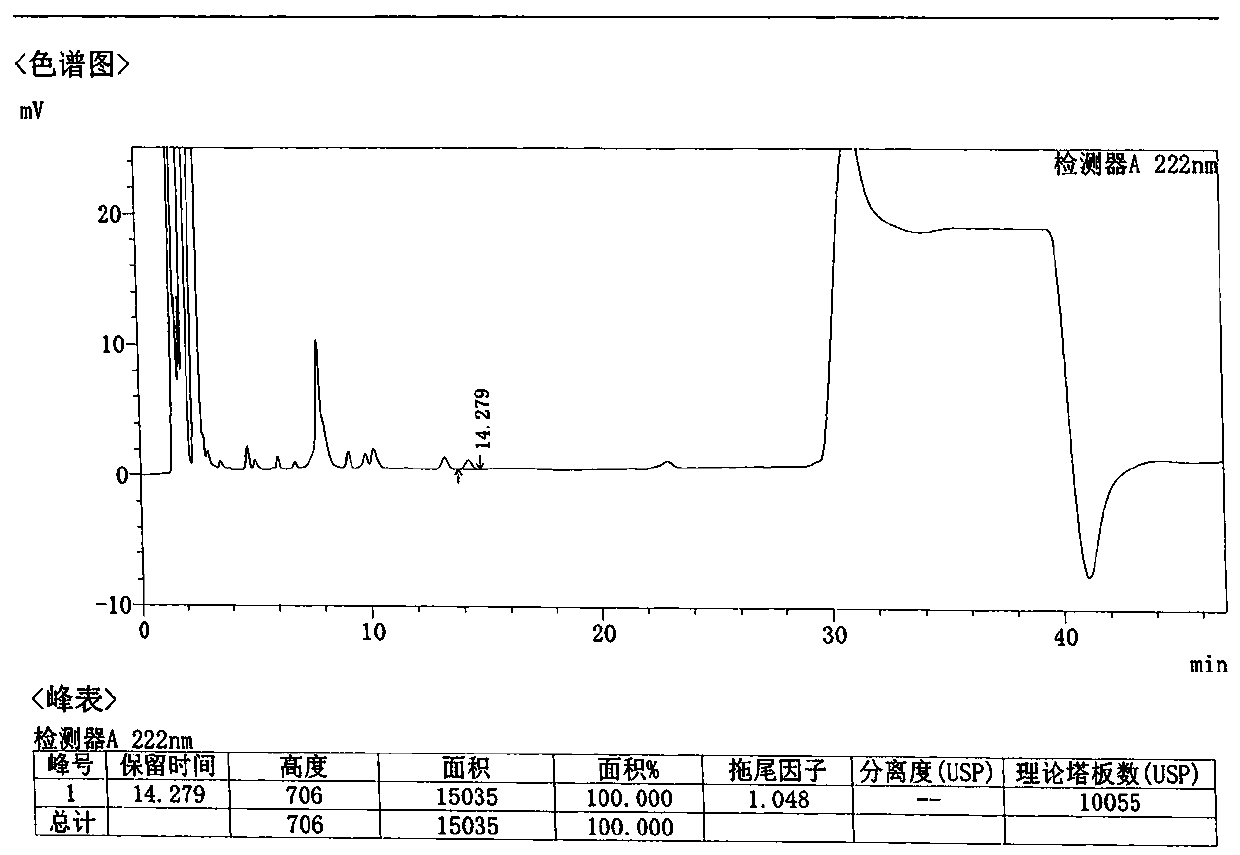
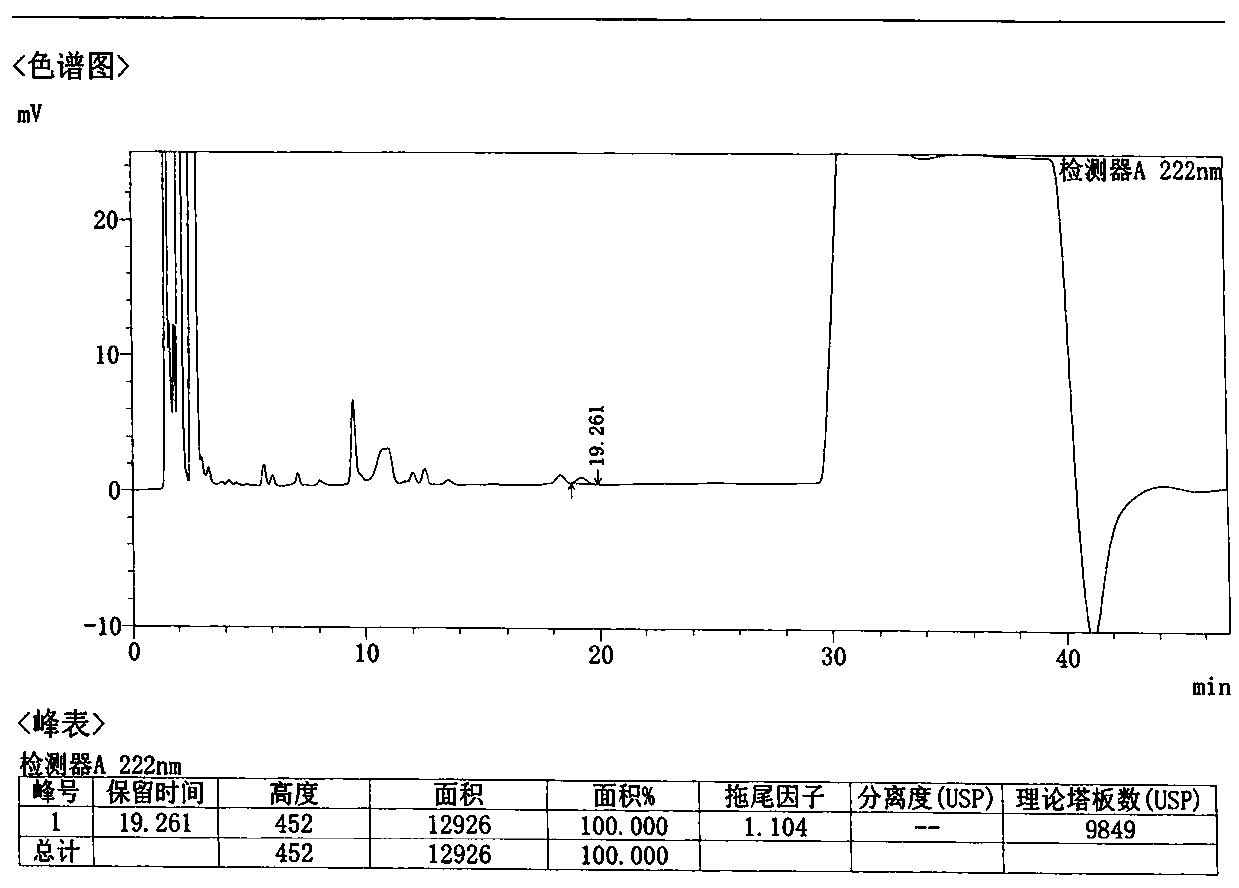
Method for detecting related substances in Loxoprofen or sodium salt thereof
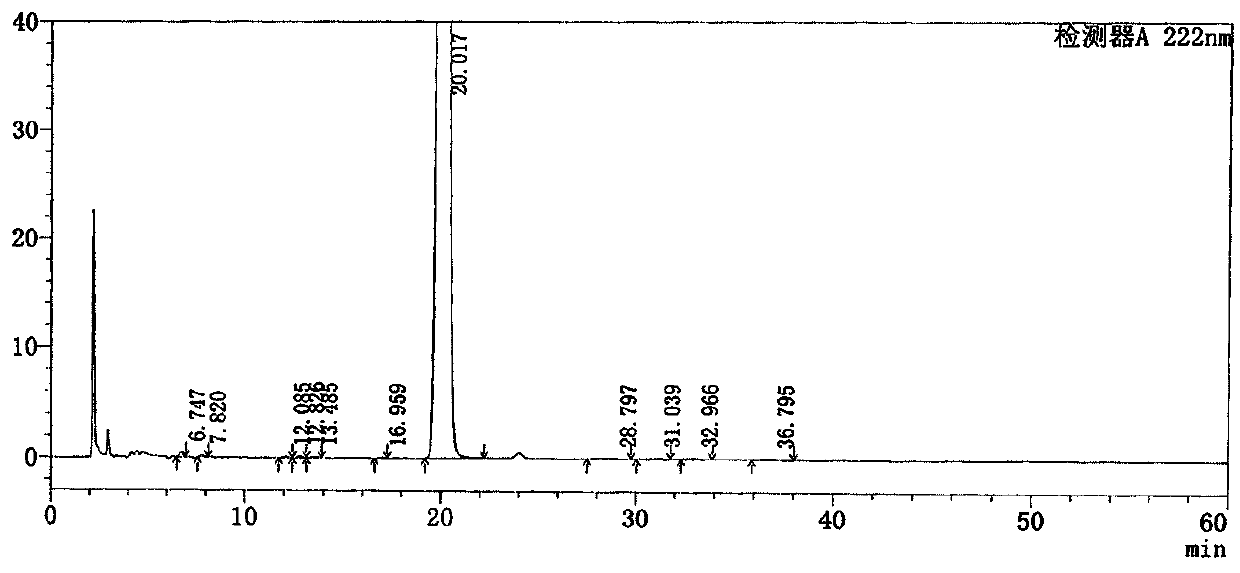
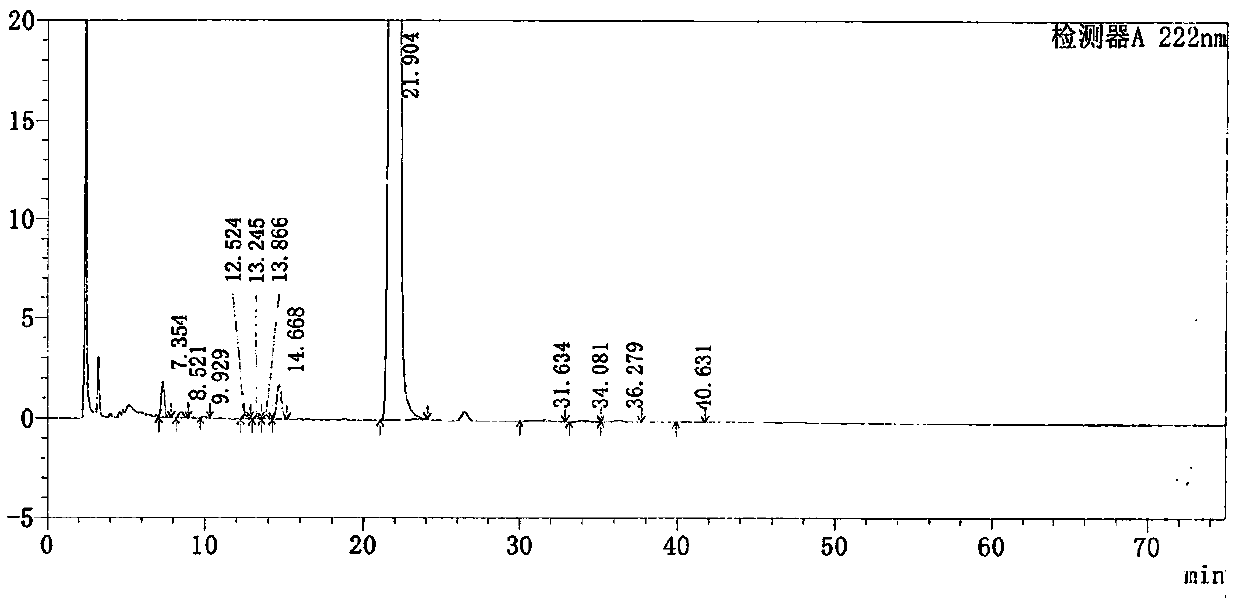
The invention discloses a method for detecting related substances in Loxoprofen or sodium salt thereof. The method uses a high performance liquid chromatography, gives unique chromatographic conditions and successfully detects a process impurity in the Loxoprofen sodium salt. According to the method, the impurities in the process are detected by using a reverse chromatography, the peak shape is better, the separation degree between the peak shape and an adjacent chromatographic peak is high, the research and development cost is saved, the experiment difficulty is reduced, and the reproducibility is good.
Owner:HUNAN JIUDIAN PHARMA +1
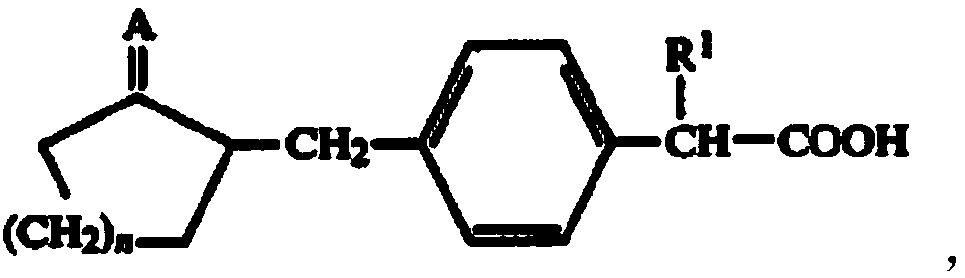
Preparation method of substituted phenylacetic acid derivative
ActiveCN109020808AEasy accessPreparation from carboxylic acid halidesOrganic compound preparationState of artPhenylacetic acid
The invention belongs to the field of drug synthesis and relates to a preparation method of a substituted phenylacetic acid derivative, especially to a preparation method for preparing 2-[4-(2-oxyamylmethyl)phenylpropionic acid]. The preparation method includes a Friedel-Crafts reaction, ring-closure reaction and a coupled reaction which are sequentially exchangeable, and a reduction reaction. Thepreparation method hasn't been enlightened by the prior art and also cannot obtain technical enlightenment from the prior art. The preparation method is suitable for production on a commercial scaleand provides another technical scheme for the industrial production of loxoprofen sodium.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Loxoprofen sodium matrix sustained-release tablet
ActiveCN102525989AIncreased diffusion resistanceReduced Diffusion ResistanceOrganic active ingredientsAntipyreticSustained Release TabletLoxoprofen
The invention relates to a novel matrix sustained-release tablet containing loxoprofen sodium, in particular to a locoprofen sodium matrix sustained-release tablet with a regulation layer, which is capable of stably releasing for 8 hours, good in releasing effect, simple in process and low in cost.
Owner:北京天衡药物研究院有限公司
Loxoprofen sodium composition
ActiveCN102670531AWell mixedReduce viscosityOrganic active ingredientsAntipyreticDissolutionMoisture absorption
The invention relates to a loxoprofen sodium composition. Due to the high viscosity of loxoprofen sodium, on one hand, the dispersible tablet of the loxoprofen sodium is easy to clot after being disintegrated, so that the dispersible tablet cannot completely pass through a 2# sieve easily; and on the other hand, the dispersible tablet is easy to absorb moisture, and after the moisture absorption, the tablet is fluffy, the content of the main medicament is decreased, and particularly, the dissolution rate is decreased obviously. According to the loxoprofen sodium composition, a preparation process is changed, and silicon dioxide in the formula is added by an interior addition, so that the composition with high dispersible uniformity and stability is obtained.
Owner:DISHA PHARMA GRP
Preparation method of micropowder for pharmaceutical preparation of loxoprofen sodium dihydrate
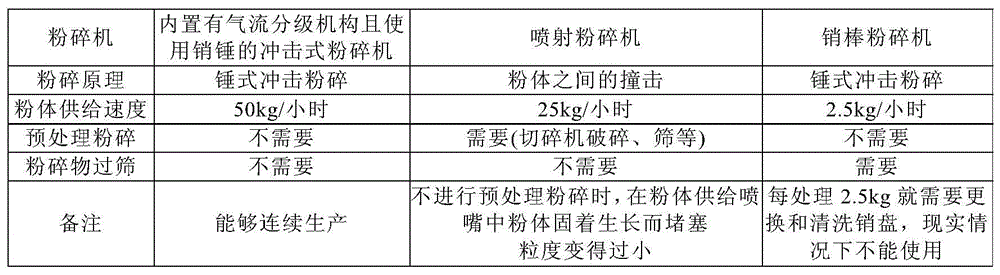
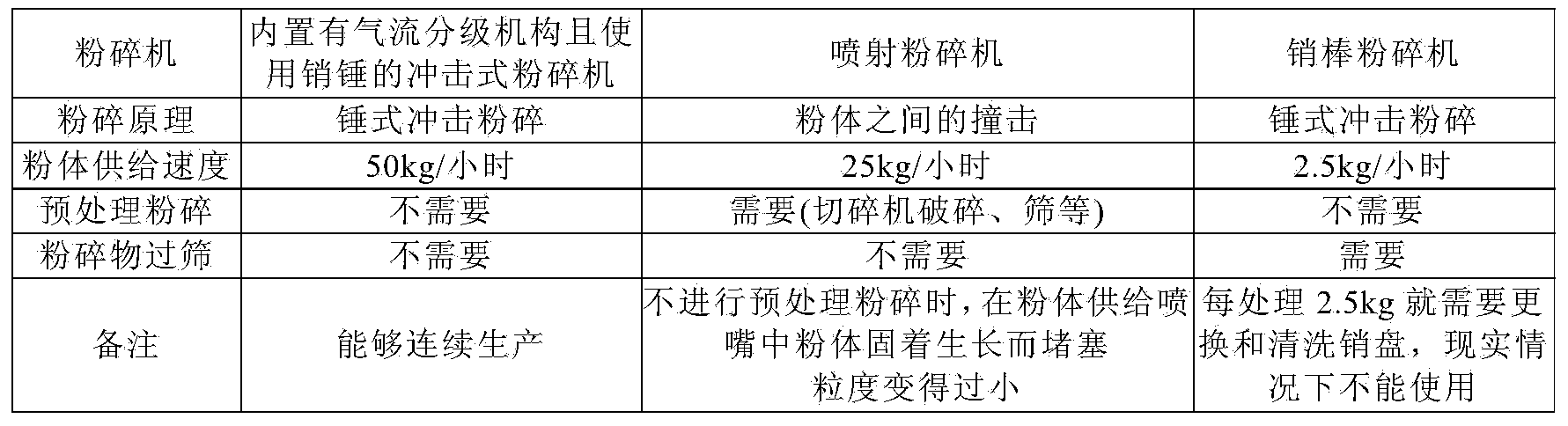
ActiveCN103533931BImprove productivityContinuous preparationOrganic active ingredientsPowder deliveryDrugs preparationsChemistry
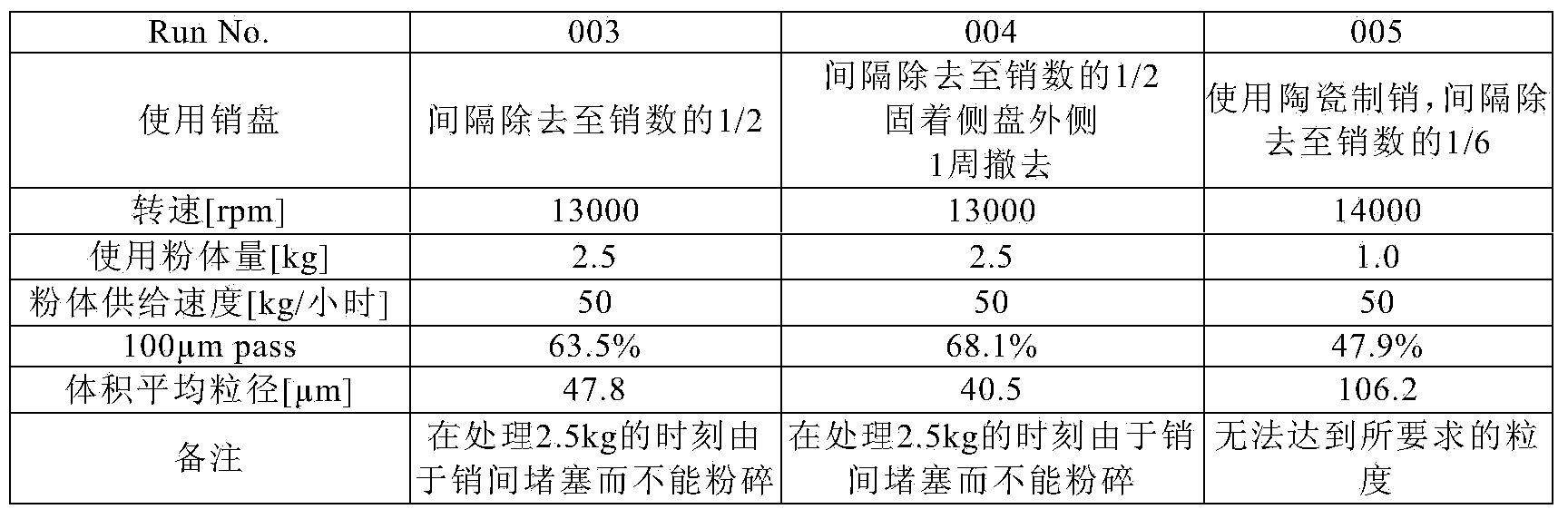
A kind of manufacture method of the pharmaceutical preparation micropowder of loxoprofen sodium dihydrate, wherein, use the impact pulverizer that is built-in air classification mechanism and use pin hammer, preferably atomization pulverizer, to loxoprofen sodium Dihydrate crystals or crystalline powders are pulverized to continuously produce fine powders of loxoprofen sodium dihydrate with a volume average particle diameter measured by a laser diffraction particle size distribution meter in the range of 20 to 120 μm.
Owner:HAMARI CHEM LTD
Synthesis method of loxoprofen sodium
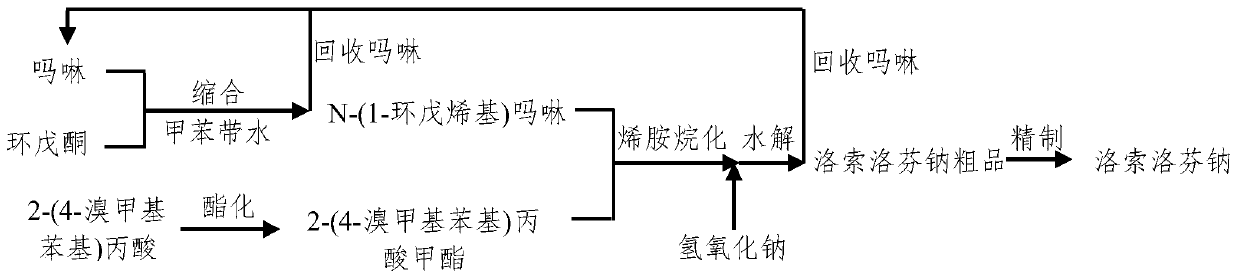
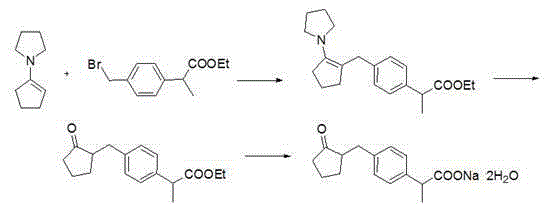
InactiveCN109776300AReduce consumptionReduced responsePreparation from carboxylic acid saltsOrganic compound preparationAlkyl transferReflux
The invention discloses a synthesis method of loxoprofen sodium, which comprises the following steps of: step 1), preparing N-(1-cyclopentenyl) morpholine; step 2), preparing 2-(4-bromomethyl phenyl)methyl propionate, the method also comprises the following step 3), preparing loxoprofen sodium by an enamine alkylation method: first dissolving 2-(4-bromomethyl phenyl) methyl propionate in a solvent, adding N-(1-cyclopentenyl) morpholine and the solvent in a container, dropwise adding 2-(4-bromomethyl phenyl) methyl propionate solution under reflux conditions; continuing reacting under reflux conditions after the completion of the dropwise addition; adding alkali solution after obtained reaction liquid is cooled, hydrolysising and separating to obtain an organic phase and an aqueous phase respectively, extracting the aqueous phase with an extractant to obtain extract liquid and the aqueous phase after extraction respectively, and post-treating the aqueous phase after extraction to obtain loxoprofen sodium. The preparation of loxoprofen sodium by the above method has the advantages of simple reaction steps, high yield of loxoprofen sodium and low production cost.
Owner:ZHEJIANG UNIV
Method for producing fine powder of loxoprofen sodium dihydrate for pharmaceutical preparations
ActiveCN103533931ASharp particle size distributionEasy formulationPowder deliveryOrganic active ingredientsPharmaceutical formulationParticle Size Analyzer
A method for producing a fine powder of loxoprofen sodium dihydrate for pharmaceutical preparations, said method comprising milling loxoprofen sodium dihydrate crystals or a crystalline powder of loxoprofen sodium dihydrate with an impact mill, preferably a pulverizer-type mill, which is provided with a built-in air-classifying system and comprises a pin hammer, to thereby continuously produce a fine powder of loxoprofen sodium dihydrate the volume-average particle size of which is in the range of 20-120 [mu]m, when measured with a laser diffraction particle size analyzer.
Owner:HAMARI CHEM LTD
Improved cataplasm ground-mass and use thereof
InactiveCN101416955BEasy to graspReduce typesPharmaceutical non-active ingredientsSheet deliveryBiotechnologyEngineering
The invention discloses an improved substrate of a cataplasma and the application thereof to loxoprofen sodium cataplasma. The substrate of the cataplasma adopts the combined application of two or more cross-linking agents and achieves the purposes of increasing the viscosity of a paste and improving the ductibility and moldability of the paste. The application of the substrate can reduce the variety and usage of thickening accessories or can increase the viscosity of the cataplasma to the extent required by a viscous force even without adding special thickening agents. The substrate has advantages in ensuring the good ductibility and moldability of the cataplasma and quickly establishing analytical methods, and the like, thus being significant for the further promotion and application of the cataplasma.
Owner:CHONGQING PHARMA RES INST
Method for separating related substances in loxoprofen or sodium salt thereof
ActiveCN110907583ASolve the swelling problemReduce experiment costComponent separationReversed-Phase Liquid ChromatographyBiochemistry
The invention discloses a method for separating related substances in loxoprofen or sodium salt thereof. According to the method, unique chromatographic conditions are used, and the related substancesin the loxoprofen sodium salt are successfully separated. According to the method, reversed-phase chromatography is used for detection, the peak shape is good, the degree of separation from adjacentchromatographic peaks is high, the research and development cost is saved, the experimental difficulty is reduced, and the reproducibility is good.
Owner:HUNAN JIUDIAN PHARMA
New method for synthesizing loxoprofen sodium
InactiveCN109809987ALow costEasy to operateOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsOrganic matter
The invention belongs to the field of synthesis of organic matters and specifically relates to a new method for synthesizing loxoprofen sodium. The synthesis method is characterized by taking 2-(4-bromomethyl) phenylpropionic acid as a raw material and performing a 4-step reaction to prepare the loxoprofen sodium. The new method for synthesizing the loxoprofen sodium adopted by the invention has the effects that the yield is increased and the industrial prospect is good.
Owner:PANJIN GELIN KAIMO TECH CO LTD
Loxoprofen sodium preparation method
InactiveCN109694326AReasonable price and easy to buyFew synthetic stepsOrganic compound preparationCarboxylic acid esters preparationAlkyl transferSingle measure
The invention discloses a loxoprofen sodium preparation method, which comprises: directly carrying out alkylation on 2-(p-bromomethyl)isophenylpropionic acid as a starting raw material under a NaOH and DMF system, carrying out hydrolysis decarboxylating to obtain a crude product, separating the by-product produced in the reaction through high-vacuum distillation to obtain loxoprofen acid, and carrying out salt forming to obtain the loxoprofen sodium. According to the present invention, the method has characteristics of less synthesis step, mild reaction condition, reasonable price and easy purchase of the raw material, low pollution and low production cost, and is suitable for industrial production, wherein the product content can reach more than 99.8%, and the single impurity is less than0.05%.
Owner:HUBEI XUNDA PHARMA
Loselofen sodium injection and its prepn
InactiveCN1357324AQuick resultsLow toxicityOrganic active ingredientsAntipyreticOral medicationChronic rheumatic arthritis
The present invention provides loselofen sodium injection and its preparation. Available loselofen sodium dosage forms include oral tablet, capsule and granule only. The Rosorolfine sodium injection of the present invention is used for relieving pain and diminishing inflammation of chronic rheumatic arthritis, rheumatoid arthritis, lumbago, etc. and especially relieving serious pain of postoperation, fracture, strain, etc. It has obvious effects and no addiction. It is prepared by dissolving Rosorolfine sodium in water, adding proper amount of medicinal supplementary material, eliminating pyretogen, disinfection or filtering and may be prepared into water injection, transfusion agent to freezed dried powder for injection.
Owner:FUDAN UNIV
Loxoprofen sodium gel and preparation method thereof
InactiveCN104188899AEasy to useQuick effectOrganic active ingredientsAntipyreticArthritisOrganic chemistry
The invention belongs to the technical field of medicines, and relates to loxoprofen sodium gel and a preparation method thereof. The loxoprofen sodium gel is suitable for inflammation diminishing and pain stopping of arthritis deformans, courbature and wounds. Compared with an oral product in the market at present, the loxoprofen sodium gel disclosed by the invention has the characteristics that the preparation technology is simple and feasible, and the loxoprofen sodium gel directly acts on the wound, takes a quick effect and is convenient to use.
Owner:天津市聚星康华医药科技有限公司
Loxoprofen sodium gel
InactiveCN102525875AHigh permeability in vitroImprove transmittanceOrganic active ingredientsAntipyreticSolventPropylene glycol
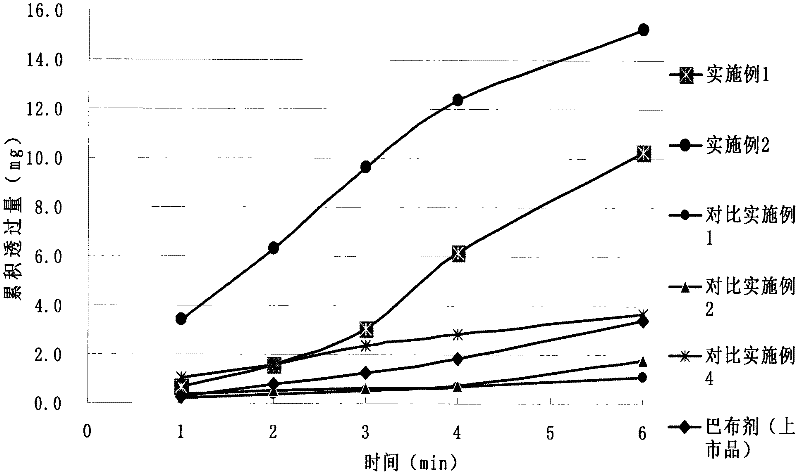
The invention relates to loxoprofen sodium gel, which comprises the following components in percentage by weight: 0.1-5.0 percent of loxoprofen sodium, 0.05-25.0 percent of a penetration enhancer, 0.5-8.0 percent of a gel substrate and 65-95 percent of a solvent, wherein the penetration enhancer is selected from azone, oleic acid or a mixture of azone and propylene glycol or / and oleic acid or a mixture of propylene glycol and oleic acid. Due to the adoption of the gel, the endermic permeation amount of loxoprofen sodium can be increased greatly; and the formed gel is uniform and transparent, and is used for treating swelling and pain caused by arthritis deformans and trauma.
Owner:CHONGQING PHARMA RES INST
Loxoprofen sodium gel paste matrix without transdermal penetration enhancer and preparation method thereof
The invention discloses a loxoprofen sodium gel paste matrix and a preparation method thereof. The gel paste matrix comprises, by weight, 0.5 to 2.0 parts of loxoprofen sodium, 40 to 60 parts of purified water, 5 to 10 parts of framework material, 25 to 45 parts of humectant, 1 to 10 parts of tackifier, 1 to 8 parts of filler, 1 to 5 parts of surfactant, 0.4 to 2.0 parts of pH regulator, 0.1 to 2.0 parts of aluminum hydroxide, 0.2 to 1.0 part of preservative, 1 part of ethanol and 0.05 to 0.5 part of edetate disodium. The gel paste matrix provided by the invention does not contain transdermalpenetration enhancers such as N-methyl pyrrolidone, crotamicin and azone. The purpose of an ideal transdermal effect is achieved by improving the release behavior of the matrix. The risk of potentialharm of an organic solvent to a human body is reduced. Ethyl p-hydroxybenzoate is added into the matrix of the plaster as a preservative, which overcomes the defect that a gel plaster is easy to mildew.
Owner:HUNAN JIUDIAN PHARMA
Method for synthesizing loxoprofen sodium
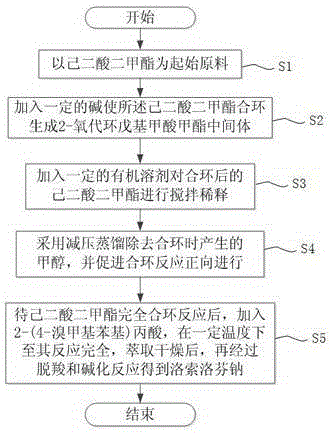
InactiveCN105218351AThe reaction route is simpleReact route securityOrganic compound preparationCarboxylic acid esters preparationDistillationAdipate
The invention discloses a method for synthesizing loxoprofen sodium. The method comprises the following steps: (a) using dimethyl adipate as an initial raw material; (b) adding a certain amount of alkali to enable the dimethyl adipate to perform ring closing and generate a 2-oxocyclopentyl carboxylate intermediate; (c) adding a certain amount of an organic solvent into the ring-closed dimethyl adipate to perform stirring and diluting; (d) removing methanol generated in ring-closing by adopting reduced pressure distillation, and promoting forward performance of a ring-closing reaction; and (e) adding 2-(4-bromomethyl)phenylpropionic acid after complete ring-closing reaction of the dimethyl adipate, performing complete reaction at a certain temperature, performing decarboxylation and quaternization reactions after extracting and drying to obtain the loxoprofen sodium. According to the method, the generated intermediate does not need post-treatment and separation purification, and directly reacts with the 2-(4-bromomethyl)phenylpropionic acid. The method is simple to operate, has simple, safe, environment-friendly reaction route, has low cost, mild reaction condition, good regioselectivity and relatively high production conversion rate.
Owner:上海立科化学科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com