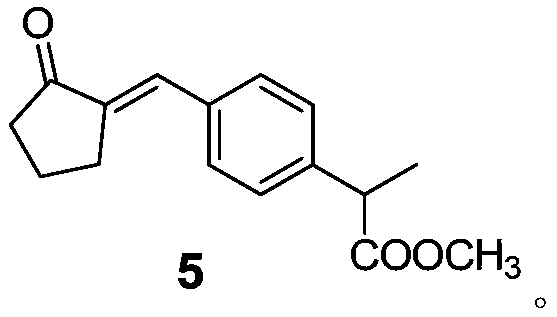
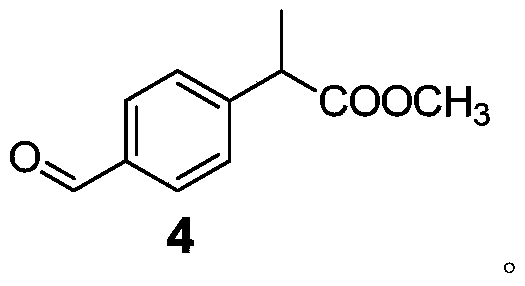
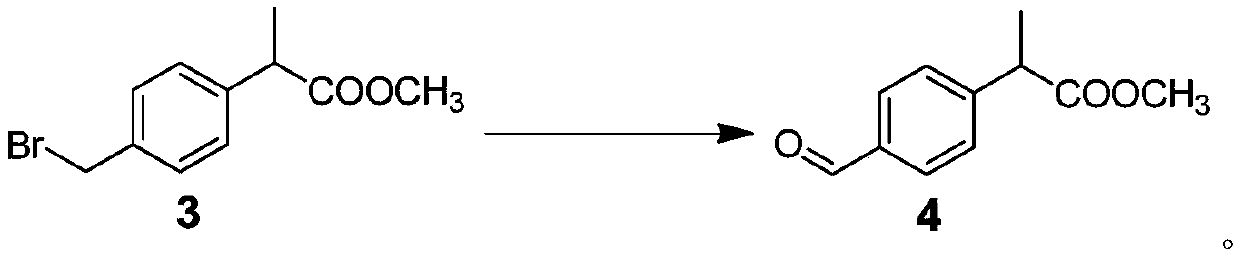
Method for preparing loxoprofen sodium
A compound and reaction technology, applied in the field of loxoprofen sodium preparation technology, can solve the problems of large environmental pollution, long reaction route, unfavorable industrialization, etc., and achieve the effect of strong industrial operation, high purity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of 2-(4-methylphenyl) propionic acid (compound 1)
[0063]
[0064]Take a 250ml three-neck flask, add a constant pressure dropping funnel, put magnesium chips (2.70g 112.2mmol) into the reaction flask, and replace it with nitrogen three times for protection. 1-(1-Chloromethyl)-4-methylbenzene (15.76g 102.0mmol) was added into THF (100ml) and mixed evenly, then placed in a constant pressure dropping funnel. Add 1-(1-chloromethyl)-4-methylbenzene / THF (10ml) solution dropwise to the reaction flask at room temperature (20-25°C), turn on magnetic stirring, and the temperature in the reaction flask gradually rises to 50°C. Add the remaining 1-(1-chloromethyl)-4-methylbenzene / THF solution dropwise at 45-50°C. After the dropwise addition is completed, keep the reaction at 45-50°C for 2.0 hours. After the heat preservation is over, lower the temperature to 0°C, and pass carbon dioxide gas into the reaction liquid at 0-5°C until the reaction is ...
Embodiment 2
[0065] Embodiment 2: the preparation of 2-(4-methylphenyl) propionic acid (compound 1)
[0066]
[0067] Take a 250ml three-neck flask, add a constant pressure dropping funnel, put magnesium chips (3.30g 135.8mmol) into the reaction flask, and replace it with nitrogen three times for protection. 1-(1-Chloromethyl)-4-methylbenzene (20.0g 129.3mmol) was added into THF (150ml) and mixed evenly, then placed in a constant pressure dropping funnel. Add 1-(1-chloromethyl)-4-methylbenzene / THF (10ml) solution dropwise to the reaction flask at room temperature (20-25°C), turn on the magnetic stirring, and gradually increase the temperature in the reaction flask to 35°C Quickly transfer the reaction vial to a low temperature bath to cool down to 10°C. Add the remaining 1-(1-chloromethyl)-4-methylbenzene / THF solution dropwise at 10-15°C. After the dropwise addition is completed, keep the reaction at 10-15°C for 1.5h. After the heat preservation is over, lower the temperature to 0°C,...
Embodiment 3
[0068] Embodiment 3: Preparation of 2-(4-methylphenyl) propionic acid (compound 1)
[0069]
[0070] Take a 3000ml three-neck flask, add a constant pressure dropping funnel, put magnesium chips (56.6g 2.33mol) and THF (300ml) into the reaction flask, and replace it with nitrogen three times for protection. 1-(1-Chloromethyl)-4-methylbenzene (300.0g 1.94mmol) was added to THF (1500ml) and mixed evenly, then placed in a constant pressure dropping funnel in batches. Add 1-(1-chloromethyl)-4-methylbenzene / THF (30ml) solution dropwise to the reaction flask at room temperature (20~2-*5°C), turn on the mechanical stirring, and the temperature in the reaction flask gradually rises to After 30°C, quickly transfer the reaction bottle to a low-temperature bath to cool down to 0°C. Add the remaining 1-(1-chloromethyl)-4-methylbenzene / THF solution dropwise at -5~5°C. After the dropwise addition was completed, the mixture was incubated at -5 to 5°C for 1.5 hours. After the heat preser...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com