Method for separating related substances in loxoprofen or sodium salt thereof
A separation method and related substance technology, applied in the direction of material separation, analysis of materials, measuring devices, etc., can solve the problems of main peak tailing, impurity cannot be accurately detected, test solution cannot be filtered or centrifuged, etc., and achieve great benefits , reduce the cost of the experiment, and the reagents are simple and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
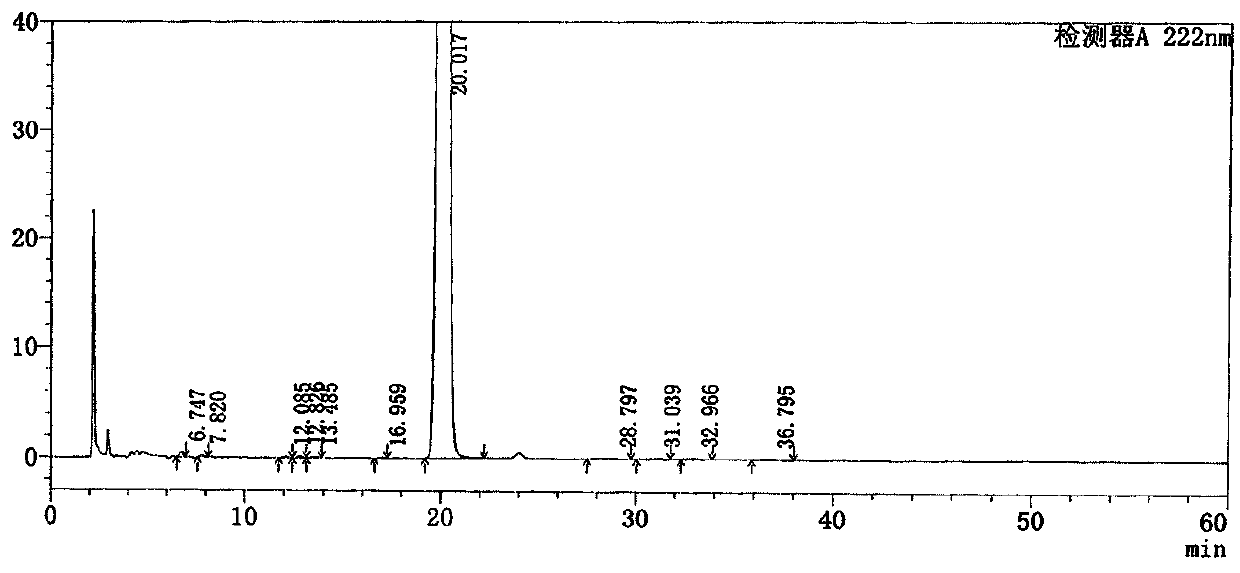
[0040] Take loxoprofen sodium gel patch, cut 35cm 2 (containing loxoprofen sodium 25mg), chopped into 150ml Erlenmeyer flask, added 50ml methanol, weighed and ultrasonicated for 30min, cooled to room temperature and supplemented with methanol to make the concentration 0.5mg / ml, filtered to obtain Test solution:
[0041] Get need testing solution 1.0ml, dilute to 50ml with methanol as reference substance solution;
[0042] The 0.01mol / L sodium dihydrogen phosphate solution with methanol and phosphoric acid to adjust the pH value to 1.7 is the mobile phase, the volume ratio of sodium dihydrogen phosphate and methanol is 50:50, measure the reference substance solution and the test sample prepared above 10 μl of each solution was injected into the chromatograph, and the chromatogram was recorded.
[0043] Among them, the chromatographic conditions for the determination are that the specification of the chromatographic column is 4.6*250mm, 5um, preferably a C18 chromatographic co...
Embodiment 2
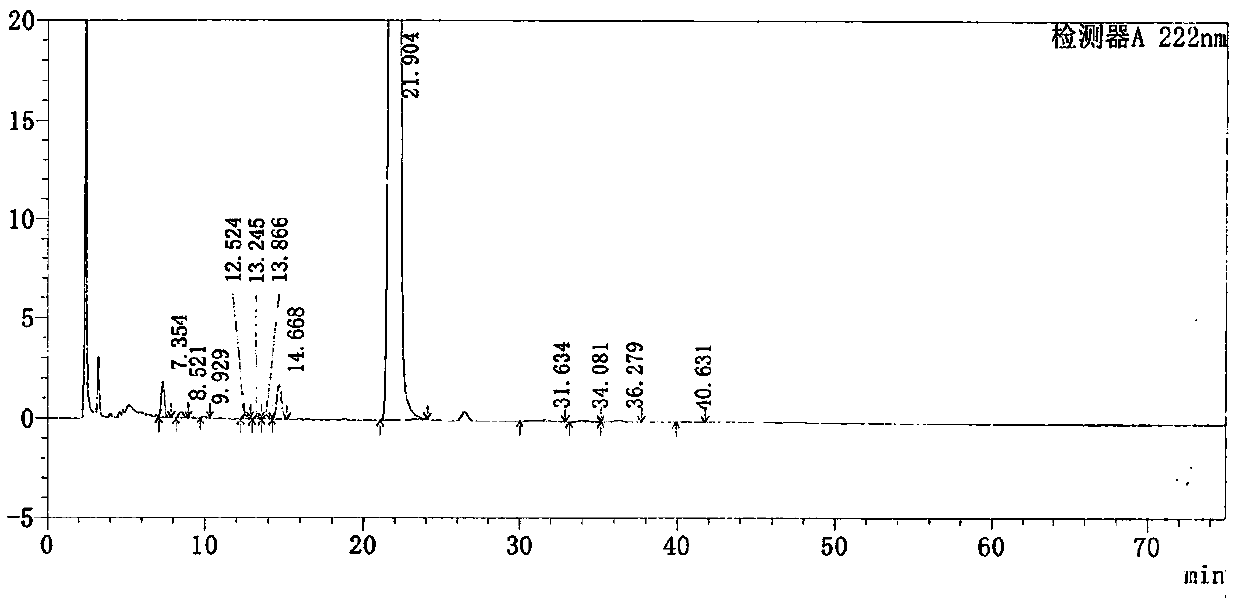
[0050] Take loxoprofen sodium gel patch, cut 35cm 2 (containing loxoprofen sodium 25mg), chopped into 150ml Erlenmeyer flask, added 50ml methanol, weighed and ultrasonicated for 30min, cooled to room temperature and supplemented with methanol to make the concentration 0.5mg / ml, filtered to obtain Test solution:
[0051] Get need testing solution 1.0ml, dilute to 50ml with methanol as reference substance solution;
[0052] The 0.01mol / L sodium dihydrogen phosphate solution with methanol and phosphoric acid to adjust the pH value to 2.5 is the mobile phase, the volume ratio of sodium dihydrogen phosphate and methanol is 50:50, measure the reference substance solution and the test sample prepared above 10 μl of each solution was injected into the chromatograph, and the chromatogram was recorded.
[0053]Among them, the chromatographic conditions for the determination are that the specification of the chromatographic column is 4.6*250mm, 5um, preferably a C18 chromatographic col...
Embodiment 3
[0060] Take loxoprofen sodium gel patch, cut 35cm 2 (containing loxoprofen sodium 25mg), chopped into 150ml Erlenmeyer flask, added 50ml methanol, weighed and ultrasonicated for 30min, cooled to room temperature and supplemented with methanol to make the concentration 0.5mg / ml, filtered to obtain Test solution:
[0061] Get need testing solution 1.0ml, dilute to 50ml with methanol as reference substance solution;
[0062] The 0.01mol / L sodium dihydrogen phosphate solution with methanol and phosphoric acid to adjust the pH value to 2.5 is the mobile phase, the volume ratio of sodium dihydrogen phosphate and methanol is 50:50, measure the reference substance solution and the test sample prepared above 10 μl of each solution was injected into the chromatograph, and the chromatogram was recorded.
[0063] Among them, the chromatographic conditions for the determination are that the specification of the chromatographic column is 4.6*250mm, 5um, preferably a C18 chromatographic co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com