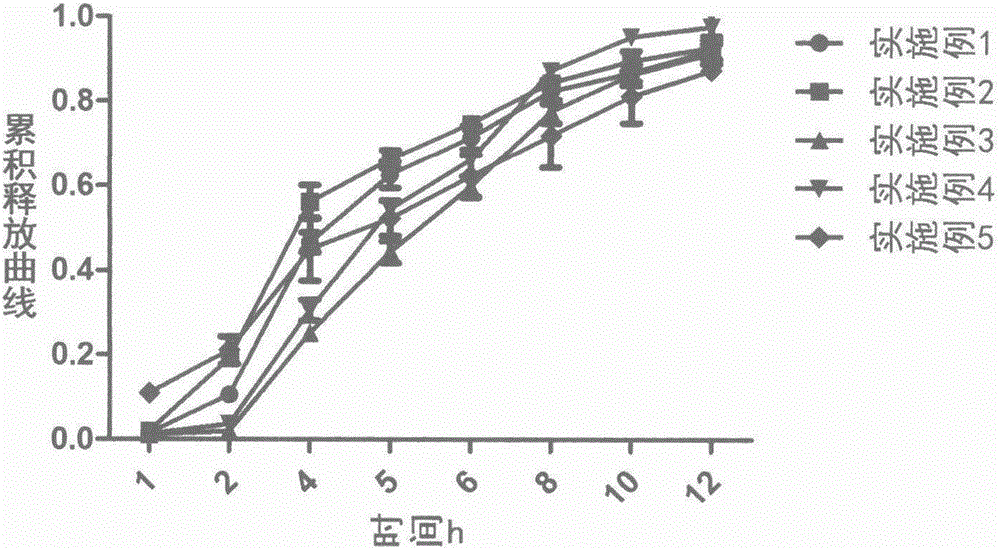
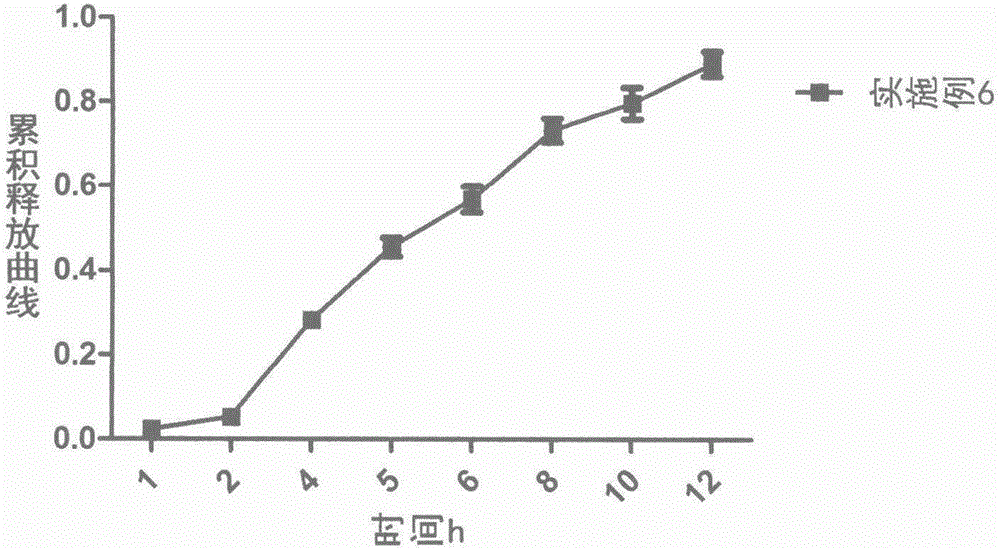
Loxoprofen sodium sustained-release pellet
A technology of loxoprofen sodium and sustained-release pellets, which is applied in the direction of microcapsules, medical preparations of non-active ingredients, and respiratory diseases, etc., which can solve the problems of poor ease of use, reduce irritation, and reduce drug administration The effect of frequency and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 loxoprofen sodium sustained-release pellet capsule (2000)
[0027] prescription:
[0028] 1) Drug-loaded pill core
[0029]
[0030] 2) Isolation layer coating solution
[0031]
[0032] 3) Sustained release layer coating solution
[0033] Surelease water-based dispersion 180g
[0034] Distilled water 120g
[0035] Preparation Process:
[0036] 1) Preparing drug-loaded pill cores: Pass loxoprofen sodium, starch, and microcrystalline cellulose through an 80-mesh sieve respectively, mix evenly according to the prescription ratio, add 50% ethanol, prepare soft materials, and pass through the sieve plate of the extruder Extrude the strips, put the strips into a spheronizer, and spheronize to obtain round particles. After drying at 50°C for 12 hours, sieve 0.6-1.25mm pellets to obtain the core of the pellets containing The appearance of the pill core is smooth, round and hard, suitable for coating.
[0037]2) Prepare the coating solution for the iso...
Embodiment 2
[0041] Embodiment 2 loxoprofen sodium sustained-release pellet capsules (2000)
[0042] prescription:
[0043] 1) drug-loaded pill core is the same as embodiment 1
[0044] 2) Isolation layer coating solution
[0045]
[0046] 3) Sustained release layer coating solution
[0047] Surelease Aqueous Dispersion 240
[0048] Distilled water 160g
[0049] Preparation process: with embodiment 1.
Embodiment 3
[0050] Embodiment 3 loxoprofen sodium sustained-release pellet capsules (2000)
[0051] prescription:
[0052] 1) drug-loaded pill core is the same as embodiment 1
[0053] 2) Isolation layer coating solution
[0054]
[0055] 3) Sustained release layer coating solution
[0056] Surelease water-based dispersion 270g
[0057] Distilled water 180g
[0058] Preparation process: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com