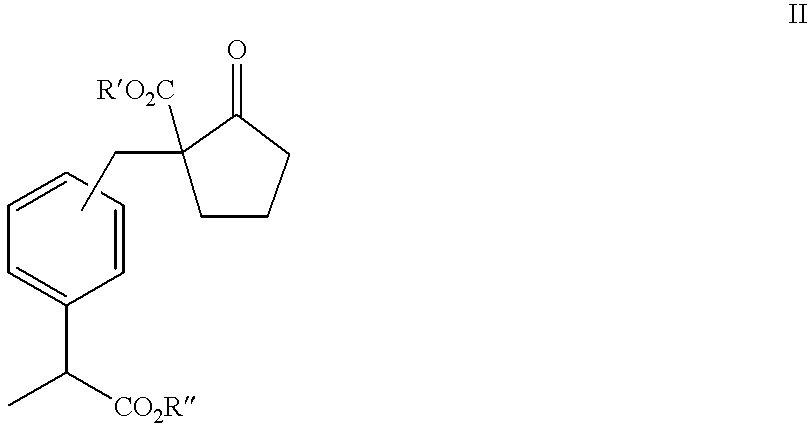
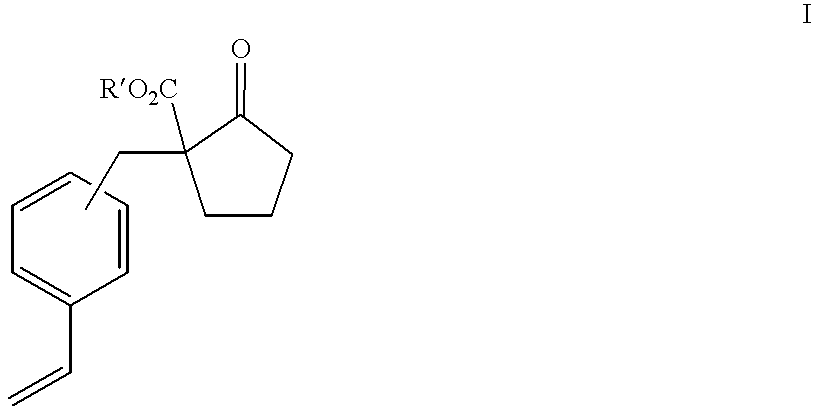
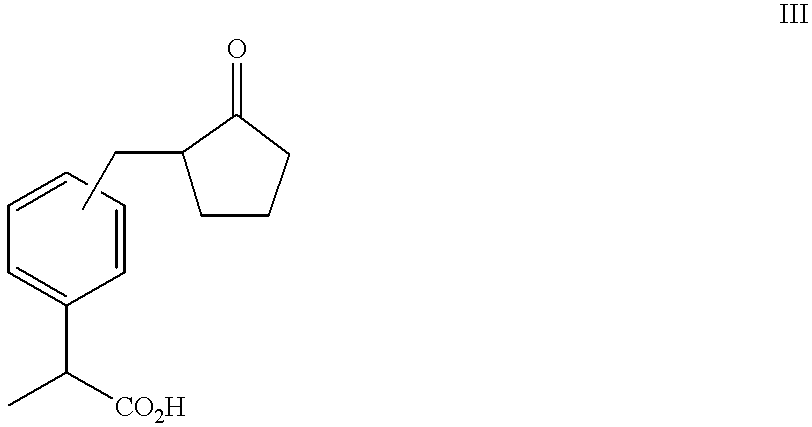
Process for producing 2-substituted propionic acid
a technology of propionic acid and substituted propionic acid, which is applied in the direction of carbon monoxide carboxylic preparation, organic chemistry, biocide, etc., can solve the problems of inconvenient industrial practice, and inability to achieve the effect of reducing the number of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0145] [Step (1-1)]
[0146] Into a reaction vessel of 15 liters were put 7.2 liters of toluene, 243 g (4.27 mol) of sodium methoxide (purity 95%) and 900 g (5.11 mol) of dimethyl adipate (purity 99%), and the mixture was heated with stirring. After the temperature in the vessel reached 60.degree. C., removal of the solvent of toluene was started under reduced pressure (9.3 kPa (70 mmHg) or less). At the same time, a by-product of methanol was also distilled off. The reaction was carried out for 40 minutes. As the result of measurement after the reaction, the total volume of mixed solvents of toluene and methanol was 6 liters. After that, 5.5 liters of acetone was added to the mixture. Further, 612 g (3.81 mol) of chloromethylstyrene (purity 95%; containing 500 ppm of nitrocresol, nitrophenol and 4-tert-butylcatechol in total; para-isomer:meta-isomer ratio=96:4) was added at room temperature, then the mixture was heated with refluxing for 8 hours.
[0147] At the temperature of 60.degree....
example 2
[0161] [Step (2-1)]
[0162] Into a reaction vessel of 300 ml were put 65 ml of toluene, 2.2 g (0.039 mol) of sodium methoxide (purity 95%) and 8.0 g (0.046 mol) of dimethyl adipate (purity 95%), and the mixture was heated with stirring, then the solvent was removed under reduced pressure. After that, 50 ml of acetone was added, then 30 ml of a toluene solution of methyl 2-(bromomethylphenyl)propionate (purity 92.7%; prepared from 9.09 g of 2-(bromomethylphenyl)propionic acid (purity 99.25%) was added at room temperature, and the mixture was heated with refluxing for 4 hours.
[0163] Acetone was distilled off under reduced pressure, then 50 ml of toluene, 30 ml of water and 1 ml of concentrated hydrochloric acid were added, and extraction was conducted by separation of liquids. After the organic phase was washed twice with 30 ml of water, filtration was carried out. With the removal of toluene under reduced pressure, 12.9 g of compound II (R'=R"=Me) in pale yellow was obtained as a raw p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Substance count | aaaaa | aaaaa |
| Equivalent mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com