Novel method for preparing dextromethorphan
A compound and reaction technology, which is applied in the field of synthetic route design and preparation of raw materials and intermediates, can solve problems such as difficult to obtain, a large amount of waste liquid and residue, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
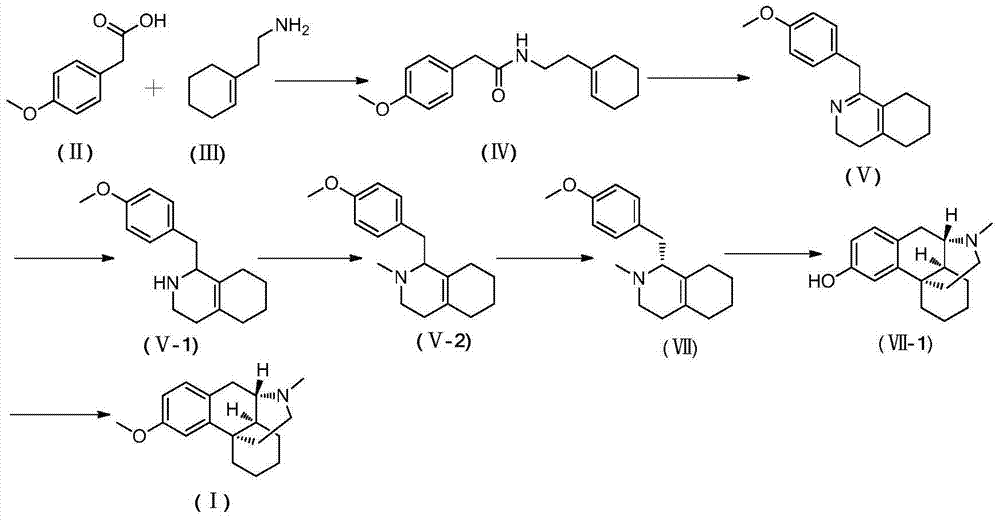
[0098] In the method, the raw material can be obtained by any method, for example, it is prepared by the prior art, or it is purchased through commercial channels. In the present invention, the preferred preparation method comprises steps:
[0099] (1) In an inert solvent, react with a compound of formula (II) and a compound of formula (III) to obtain a compound of formula (IV):
[0100]
[0101] (2) In an inert solvent, carry out a ring-closing reaction with a compound of formula (IV) to obtain a compound of formula (V):
[0102]
[0103] Wherein, in the step (1), the reaction is preferably carried out in the presence of a condensing agent; and the condensing agent is selected from the group consisting of: O-(7-azabenzotriazole-1 -yl)-bis(dimethylamino)carbenium hexafluorophosphate (HATU), O-(benzotriazol-1-yl)-bis(dimethylamino)carbenium hexafluorophosphate (HBTU ), dicyclohexylcarbodiimide (DCC), diisopropylcarbodiimide (DIC), 1-(3-dimethylaminopropyl)-3-ethylcarbod...
Embodiment 1
[0178] (1) Preparation of N-(2-(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide (Ⅳ):
[0179] Add 4-methoxyphenylacetic acid (50.0g, 300.9mmol), cyclohexeneethylamine (37.7g, 300.9mmol) and dichloromethane (300ml) in a 500ml three-neck round bottom flask, stir for 15min, then place on ice The temperature was lowered to 0° C. in a water bath, and DIC (41.8 g, 331.0 mmol) and 4-N,N-lutidine (40.4 g, 331.0 mmol) were added. After stirring at room temperature for 10 h, the reaction was completed. Washed successively with 5% aqueous hydrochloric acid solution (30ml), 5% aqueous sodium carbonate solution (30ml), water (3x50ml) and brine (30ml), dried over anhydrous sodium sulfate, filtered, and concentrated to give a light gray solid as N-(2 -(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide 78.2g, yield 95.0%
[0180] (2) Preparation of 1-(4-methoxy)benzyl-3,4,5,6,7,8-hexahydroisoquinoline (Ⅴ):
[0181] Add N-(2-(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide (IV)...
Embodiment 2
[0189] (1) Preparation of N-(2-(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide (Ⅳ):
[0190] Add 4-methoxyphenylacetic acid (30.0g, 180.5mmol), cyclohexeneethylamine (22.6g, 180.5mmol) and dichloromethane (200ml) in a 500ml three-neck round bottom flask, stir for 15min, then place on ice The temperature was lowered to 0° C. in a water bath, and DCC (40.9 g, 198.6 mmol) and 4-N,N-lutidine (24.3 g, 198.6 mmol) were added. After stirring at room temperature for 8 h, the reaction was completed. Washed successively with 5% aqueous hydrochloric acid (20ml), 5% aqueous sodium carbonate (20ml), water (3x30ml) and brine (20ml), dried over anhydrous sodium sulfate, filtered and concentrated to give off-white solid as N-(2 -(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide 44.4 g, yield 90.0%.
[0191] (2) Preparation of 1-(4-methoxy)benzyl-3,4,5,6,7,8-hexahydroisoquinoline (Ⅴ):
[0192]Add N-(2-(1-ene-1-cyclohexyl)ethyl)-2-p-methoxyphenylacetamide (Ⅳ) (30.0g, 109.7mmol) in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com