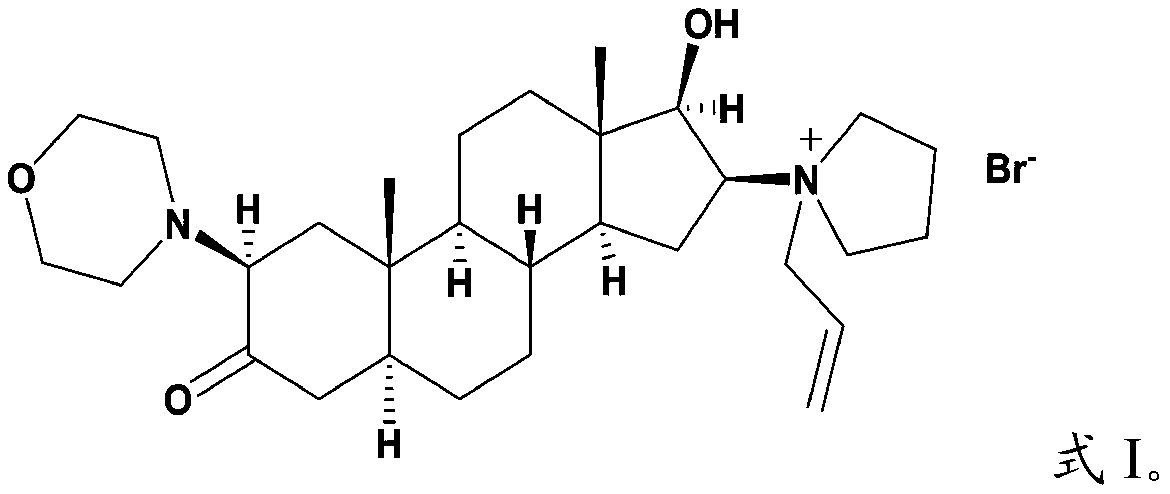
Androstane derivative, preparation method and applications thereof
A derivative, androstane technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides the preparation method of the androstane derivative described in the above scheme, comprising the following steps:
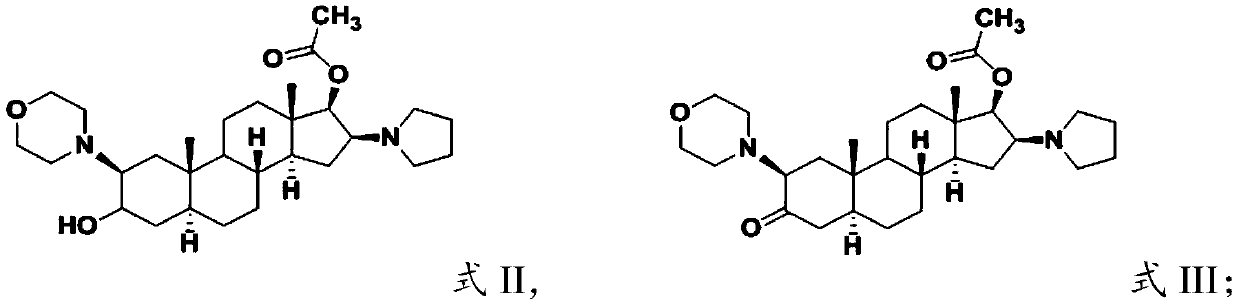
[0029] (1) subjecting the compound having the structure shown in formula II to Sven oxidation reaction to obtain the compound having the structure shown in formula III;
[0030]
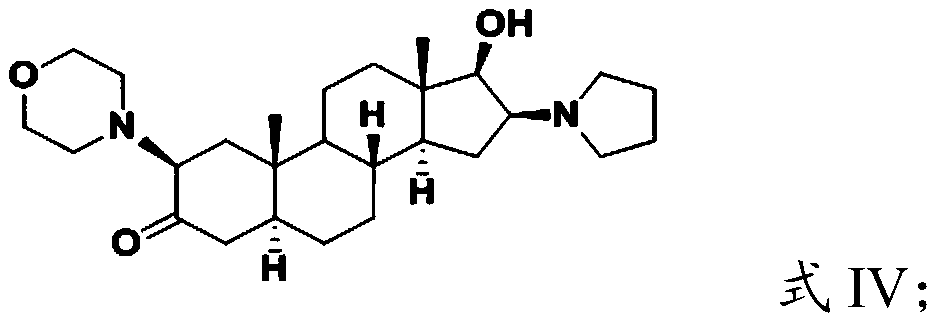
[0031] (2) hydrolyzing the compound having the structure shown in formula III to obtain the compound having the structure shown in formula IV;
[0032]
[0033] (3) performing quaternization reaction on the compound having the structure shown in formula IV and allyl bromide to obtain the androstane derivative having the structure shown in formula I.
[0034] In the present invention, the compound having the structure shown in formula II is subjected to Sven oxidation reaction to obtain the compound having the structure shown in formula III;
[0035]
[0036] The source of the compound having the structure shown in formula II is not partic...
Embodiment 1
[0061] The androstane derivative with the structure shown in formula I is prepared according to the following reaction scheme:
[0062]
[0063] (1) Under stirring conditions, add dichloromethane (500mL) and oxalyl chloride (220mmol) into a 3000mL three-necked reaction flask in sequence, and after cooling down to -60°C, add dichloromethane-DMSO mixed solution (440mmol DMSO dissolved in 100 mL of dichloromethane). After stirring at the same temperature for 10 minutes, a dichloromethane solution (500 mL) of a compound having a structure represented by formula II (15.6 g, 32 mmol) was added dropwise, and after stirring at -60°C for 15 minutes, the reaction solution was cooled to -78°C, and then triethyl ether was added Amine (860 mmol). Stirring was continued at the same temperature for 30 min, and the reaction ended. After the reaction solution was slowly raised to room temperature, it was washed with saturated NaCl solution and extracted with dichloromethane. The organic l...
Embodiment 2
[0079] (1) Under stirring conditions, add dichloromethane (500mL) and oxalyl chloride (160mmol) into a 3000mL three-necked reaction flask in sequence, and after cooling down to -70°C, add dichloromethane-DMSO mixed solution (320mmol DMSO dissolved in 100 mL of dichloromethane). After stirring at the same temperature for 20 minutes, a dichloromethane solution (500 mL) of a compound (15.6 g, 32 mmol) having a structure represented by formula II was added dropwise. After stirring at -70°C for 15 minutes, the reaction solution was cooled to -78°C, and then triethyl ether was added. Amine (800 mmol). Stirring was continued at the same temperature for 60 min, and the reaction ended. After the reaction solution was slowly raised to room temperature, it was washed with saturated NaCl solution and extracted with dichloromethane. The organic layers were combined and dried over anhydrous sodium sulfate, filtered, concentrated, and purified by silica gel column chromatography to obtain 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com