Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Interleukin-6 receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 6 receptor (IL6R) also known as CD126 (Cluster of Differentiation 126) is a type I cytokine receptor.
Pharmaceutical composition for treatment of diseases caused by IL-6 production
Pharmaceutical compositions for prevention or treatment of diseases caused by interleukin-6 production, comprising an antibody to interleukin-6 receptor (IL-6R antibody). As the IL-6R antibody, an antibody of animals other than the human such as mice, rats, etc., a chimeric antibody between these and a human antibody, a reshaped human antibody, etc. may be used. The pharmaceutical compositions are useful for prevention or treatment of diseases caused by interleukin-6 production such as plasmacytosis, anti-IgGl-emia, anemia, nephritis, etc.
Owner:KISHIMOTO TADAMITSU +2
Reshaped human antibody to human interleukin-6 receptor
InactiveUS7479543B2Peptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising:(A) an L chain comprising,(1) a human L chain C region, and(2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and(B) an H chain comprising,(1) a human H chain C region, and(2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R.Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer
InactiveUS20060275294A1Halogenated hydrocarbon active ingredientsBiocideAbnormal tissue growthSTAT Transcription Factors
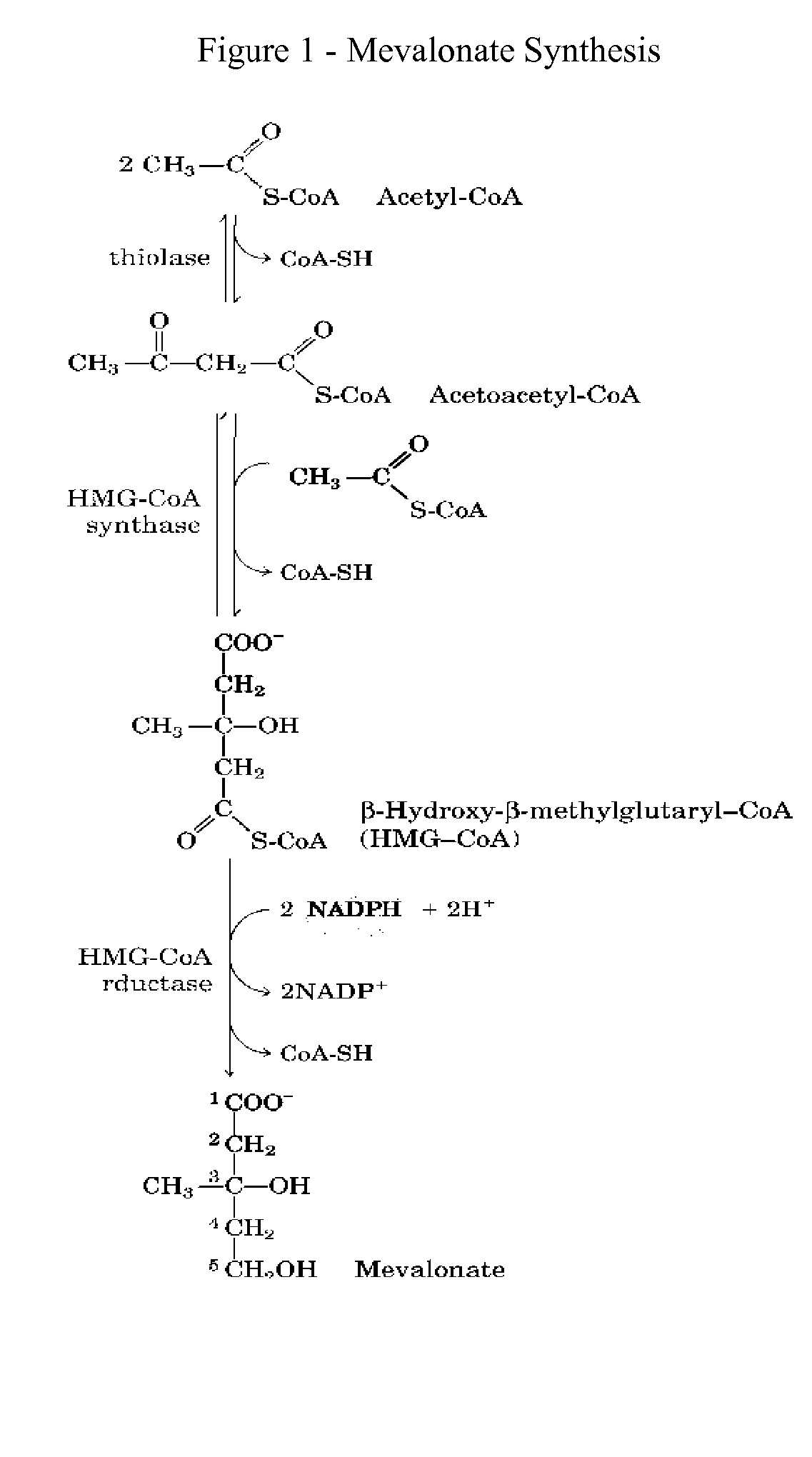
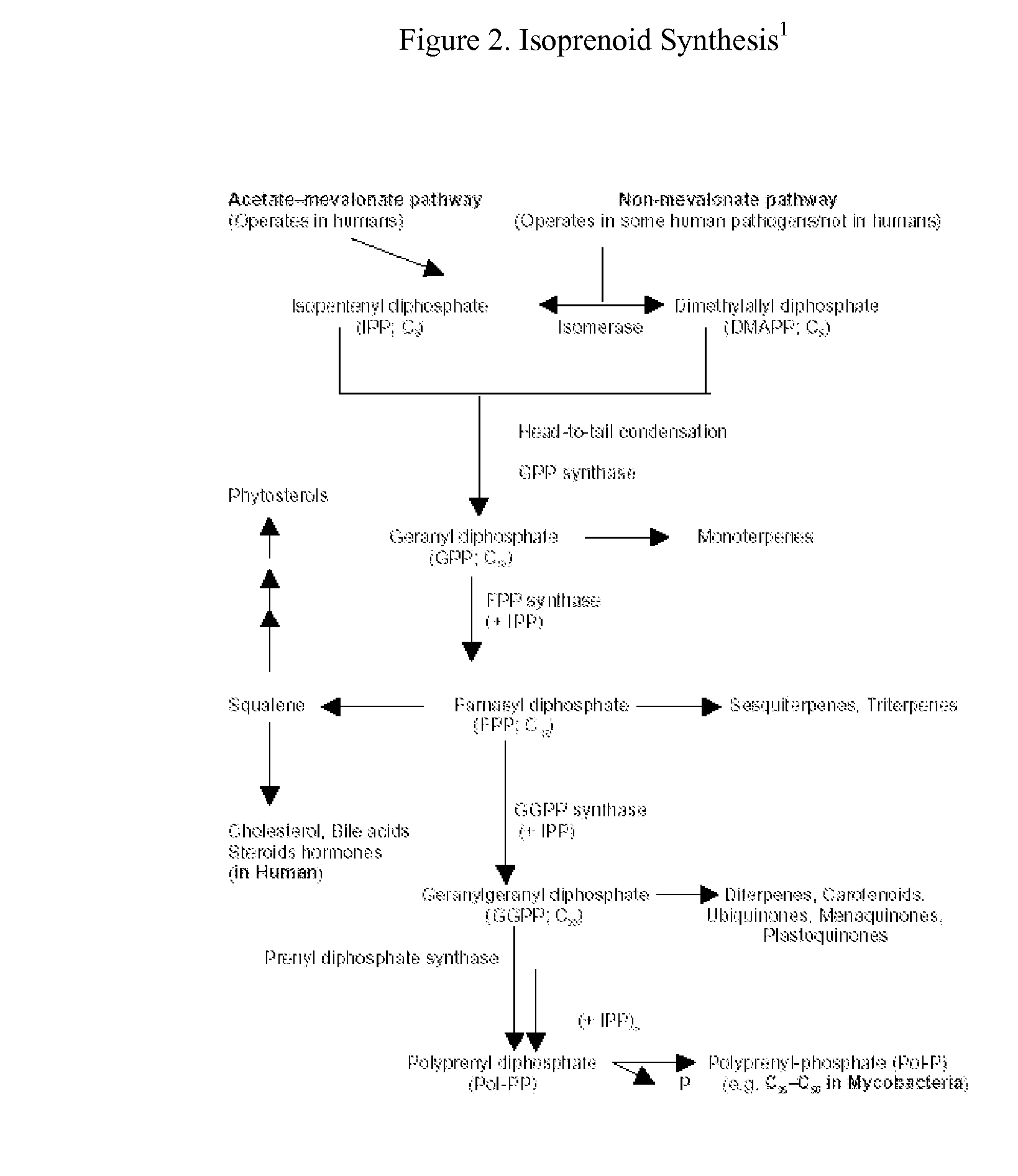
This invention relates to a method for prevention and treatment of aging, age-related disorders and / or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging, age-related disorders and / or age-related manifestations including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway
Owner:OMOIGUI OSEMWOTA SOTA
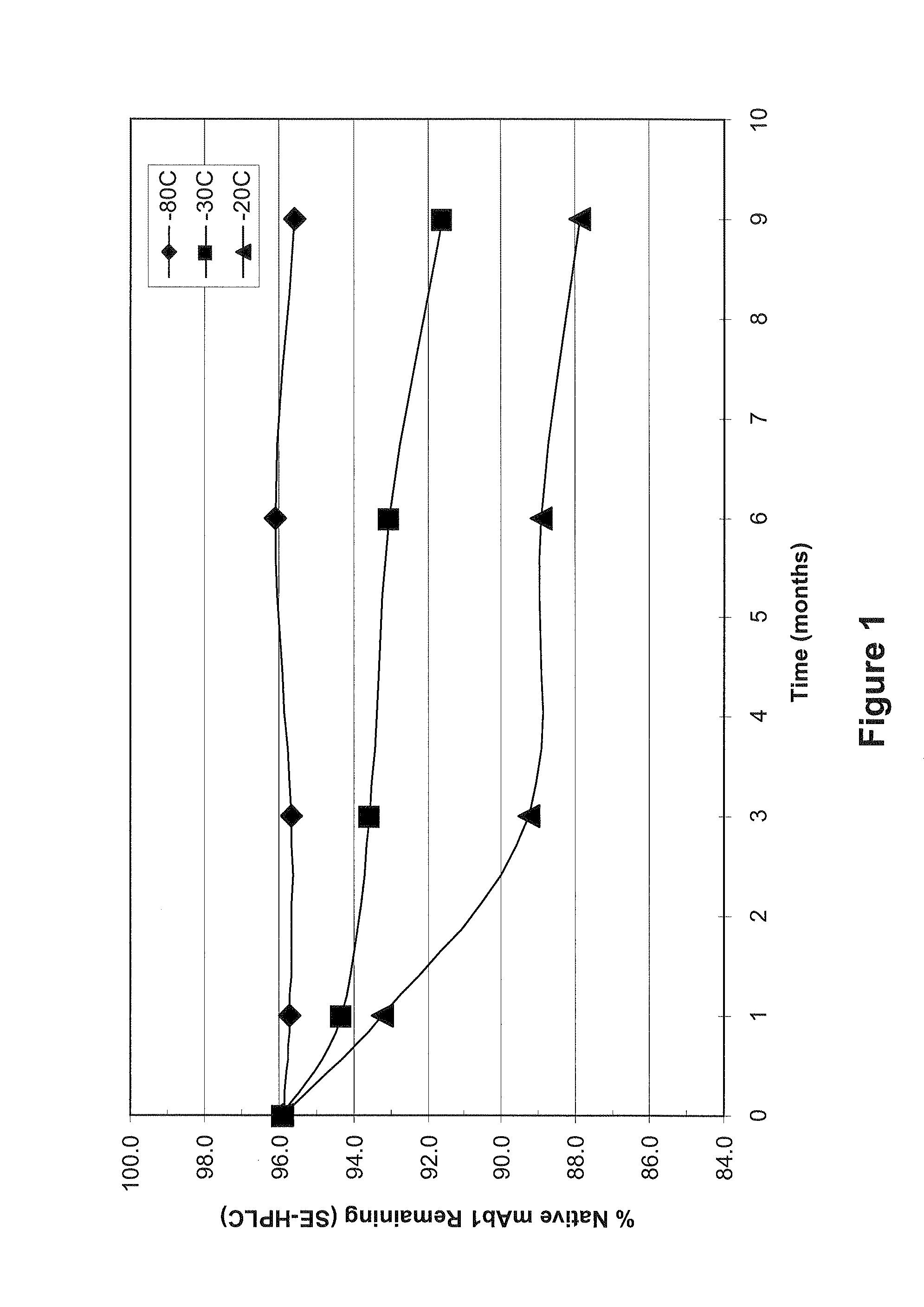
Stabilized formulations containing anti-interleukin-6 receptor (IL-6R) antibodies
ActiveUS9173880B2Organic active ingredientsNervous disorderPharmaceutical formulationSURFACTANT BLEND
The present invention provides pharmaceutical formulations comprising a human antibody that specifically binds to human interleukin-6 receptor (hIL-6R). The formulations may contain, in addition to an anti-hIL-6R antibody, at least one amino acid, at least one sugar, and / or at least one non-ionic surfactant. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability after storage for several months.
Owner:REGENERON PHARM INC
Subcutaneously administered Anti-il-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Nucleic acids encoding interleukin-1 inhibitors and processes for preparing interleukin-1 inhibitors
Compounds are disclosed having the general formula R1—X—R2, wherein R1 and R2 are biologically active groups, at least one of which is polypeptidic. X is a non-peptidic polymeric group. R1 and R2 may be the same or different. Preferred R1 and R2 groups are interleukin-1 receptor antagonist, 30 kDa TNF inhibitor, interleukin-2 receptors and CR1 and muteins thereof. Also included are site selectively modified interleukin-1 receptor antagonist and 30 kDa TNF inhibitor.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Therapeutic agents for ocular inflammatory disease comprising interleukin 6 receptor inhibitor as active ingredient
The present invention relates to therapeutic and / or prophylactic agents for ocular inflammatory disease, which comprise an interleukin 6 (IL-6) receptor inhibitor as an active ingredient.
Owner:OSAKA UNIV +2
Therapeutic agents for graft-versus-host disease comprising interleukin 6 receptor inhibitor as active ingredient
ActiveUS20100255007A1Organic active ingredientsAntibody ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides a novel therapeutic agent for graft-versus-host disease (GVHD).A therapeutic agent for graft-versus-host disease (GVHD), which comprises an interleukin 6 (IL-6) receptor inhibitor as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
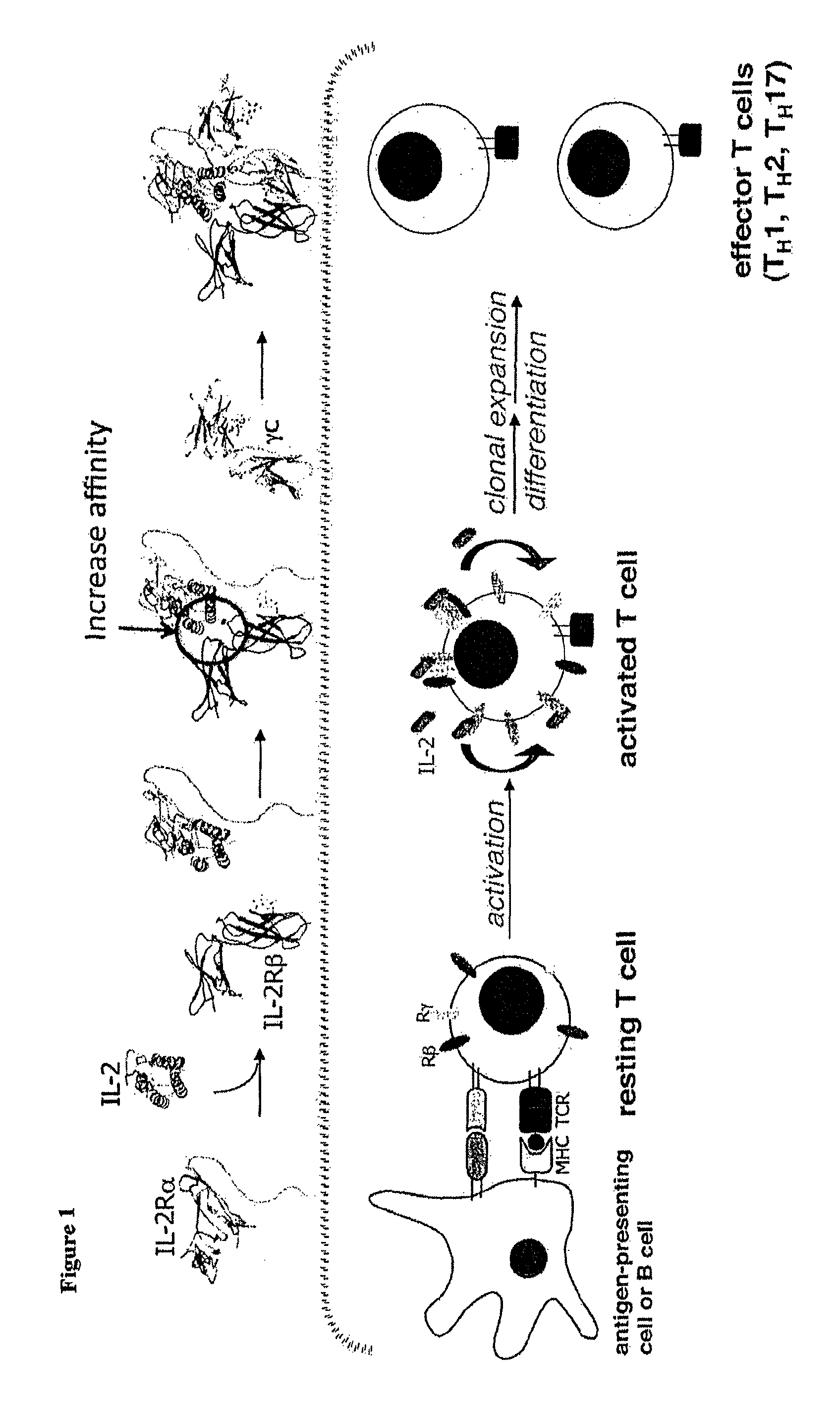
Antagonists of interleukin-2 receptor
ActiveUS9428567B2Reduce interactionPeptide/protein ingredientsDepsipeptidesInterleukin IIMutant protein
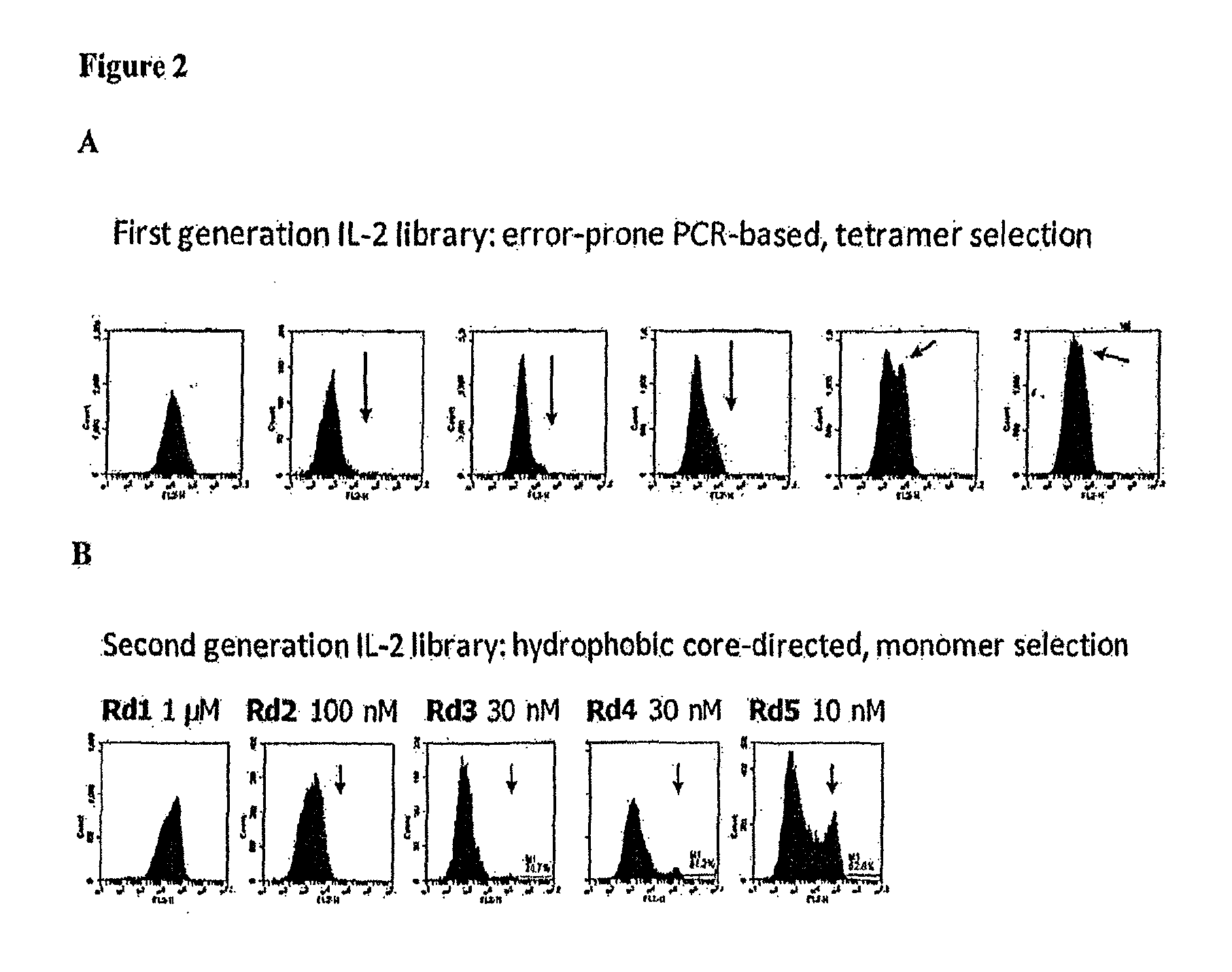
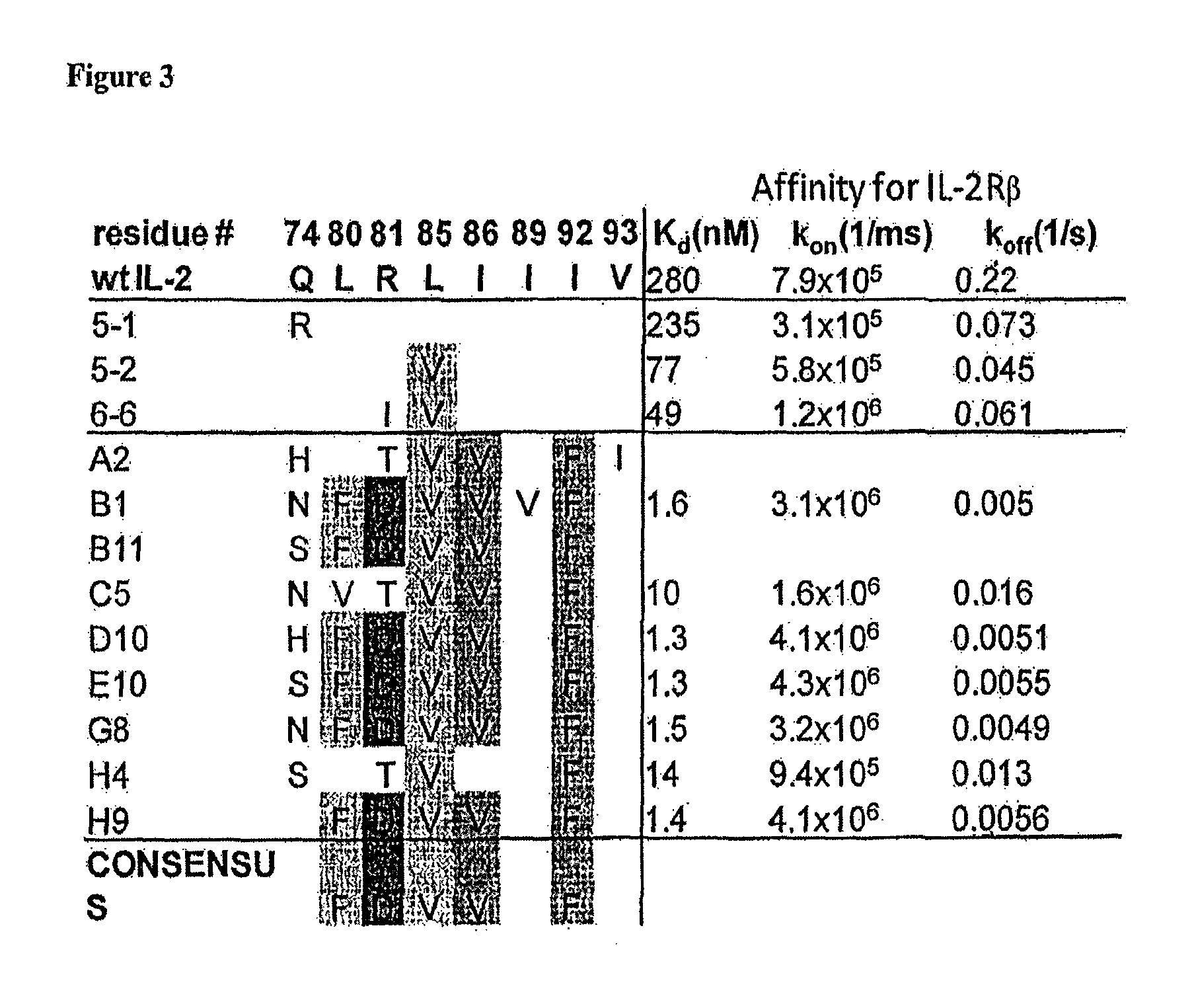
Novel human interleukin-2 (IL-2) muteins or variants thereof, and nucleic acid molecules and variants thereof are provided. Methods for producing these muteins as well as methods for stimulating the immune system of an animal are also disclosed. In addition, the invention provides recombinant expression vectors comprising the nucleic acid molecules of this invention and host cells into which expression vectors have been introduced. Pharmaceutical compositions are included comprising a therapeutically effective amount of a human IL-2 mutein of the invention and a pharmaceutically acceptable carrier. The IL-2 muteins can be used in pharmaceutical compositions for use in treatment of cancer and in stimulating the immune response.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
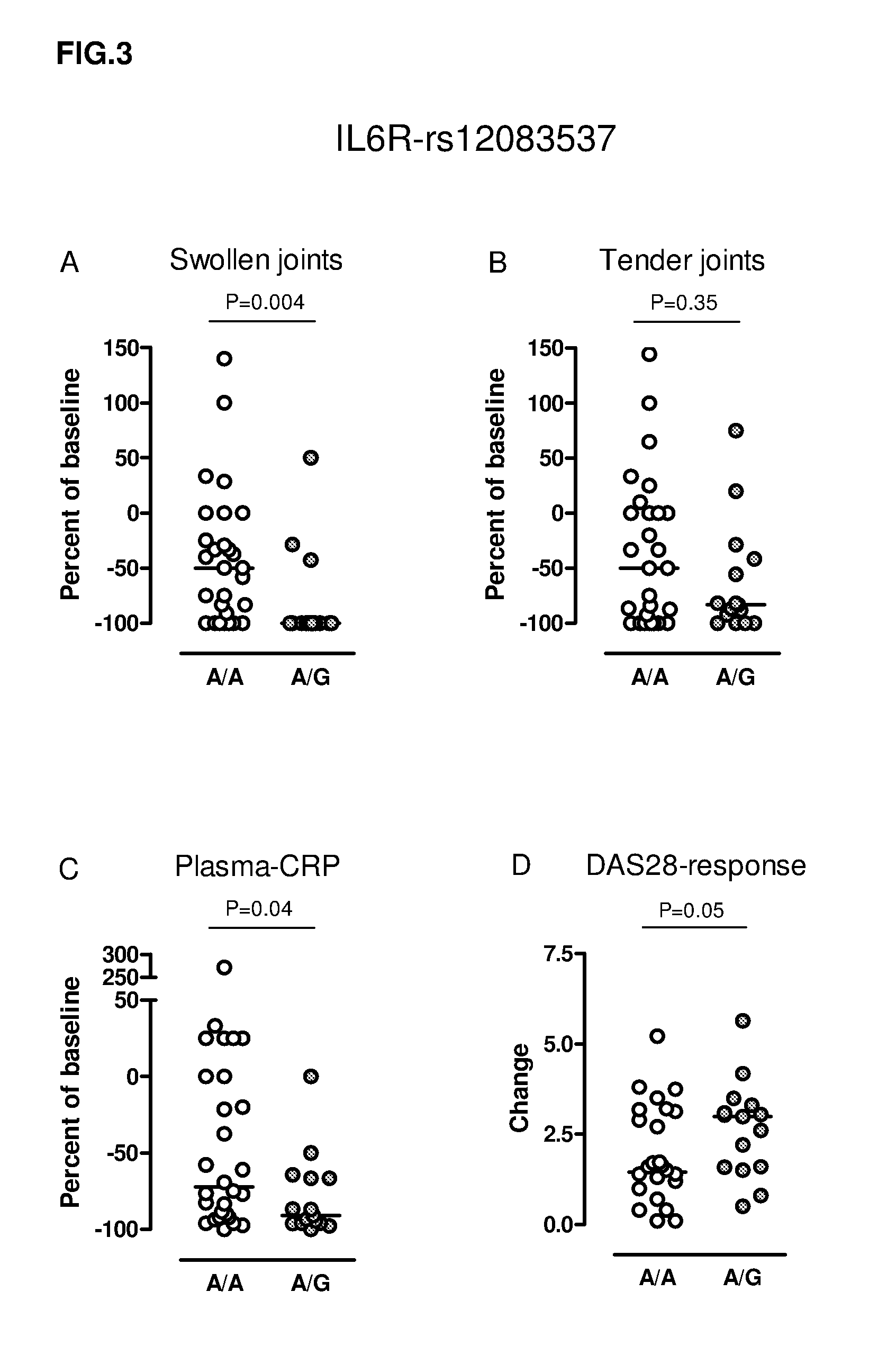
Polymorphism markers for predicting response to interleukin-6 receptor-inhibiting monoclonal antibody drug treatment
InactiveUS20110262462A1Raise the possibilityNucleotide librariesAntipyreticMonoclonal antibodyTocilizumab therapy
The present invention provides single nucleotide polymorphisms (SNPs) associated with clinical responsiveness of rheumatoid arthritis patients to treatment with an interleukin-6 receptor antibody such as tocilizumab, and methods of using such SNPs for predicting clinical response to treatment with the antibody.
Owner:ROCHE MOLECULAR SYST INC
Method for suppressing the development of graft-versus-host-disease by administering interleukin 6 receptor antibodies
ActiveUS8529895B2Organic active ingredientsAntibody ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides a novel therapeutic agent for graft-versus-host disease (GVHD).A therapeutic agent for graft-versus-host disease (GVHD), which comprises an interleukin 6 (IL-6) receptor inhibitor as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
Subcutaneously administered anti-IL-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Activatable Antibodies that Bind Interleukin-6 Receptor and Methods of Use Thereof
ActiveUS20140363430A1Reduce capacitySugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyInterleukin-6 receptor
The invention relates generally to activatable antibodies that include a masking moiety (MM), a cleavable moiety (CM), and an antibody (AB) that specifically binds to interleukin-6 receptor (IL-6R), and to methods of making and using these anti-IL-6R activatable antibodies in a variety of therapeutic, diagnostic and prophylactic indications.
Owner:CYTOMX THERAPEUTICS
Antibodies to interleukin-4 receptors and uses thereof
InactiveUS20050118176A1Preventing allograft rejectionGood immunosuppressive activityPeptide/protein ingredientsMicroorganism based processesIl 4 receptorMammal
Mammalian Interleukin-4 receptor proteins, DNAs and expression vectors encoding mammalian IL-4 receptors, and processes for producing mammalian IL-4 receptors as products of cell culture, are disclosed. A method for suppressing an IL-4-dependent immune or inflammatory response in a mammal, including a human, by administering an effective amount of soluble IL-4 receptor (sIL-4R) and a suitable diluent or carrier.
Owner:IMMUNEX CORP
Subcutaneously administered Anti-il-6 receptor antibody
Owner:CHUGAI PHARMA CO LTD +1
Subcutaneously administered Anti-il-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:F HOFFMANN LA ROCHE INC +1
Activatable antibodies that bind interleukin-6 receptor and methods of use thereof
ActiveUS9487590B2Reduce capacityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyInterleukin-4 receptor
The invention relates generally to activatable antibodies that include a masking moiety (MM), a cleavable moiety (CM), and an antibody (AB) that specifically binds to interleukin-6 receptor (IL-6R), and to methods of making and using these anti-IL-6R activatable antibodies in a variety of therapeutic, diagnostic and prophylactic indications.
Owner:CYTOMX THERAPEUTICS
Therapeutic agents for ocular inflammatory disease comprising interleukin 6 receptor inhibitor as active ingredient
Owner:OSAKA UNIV +2
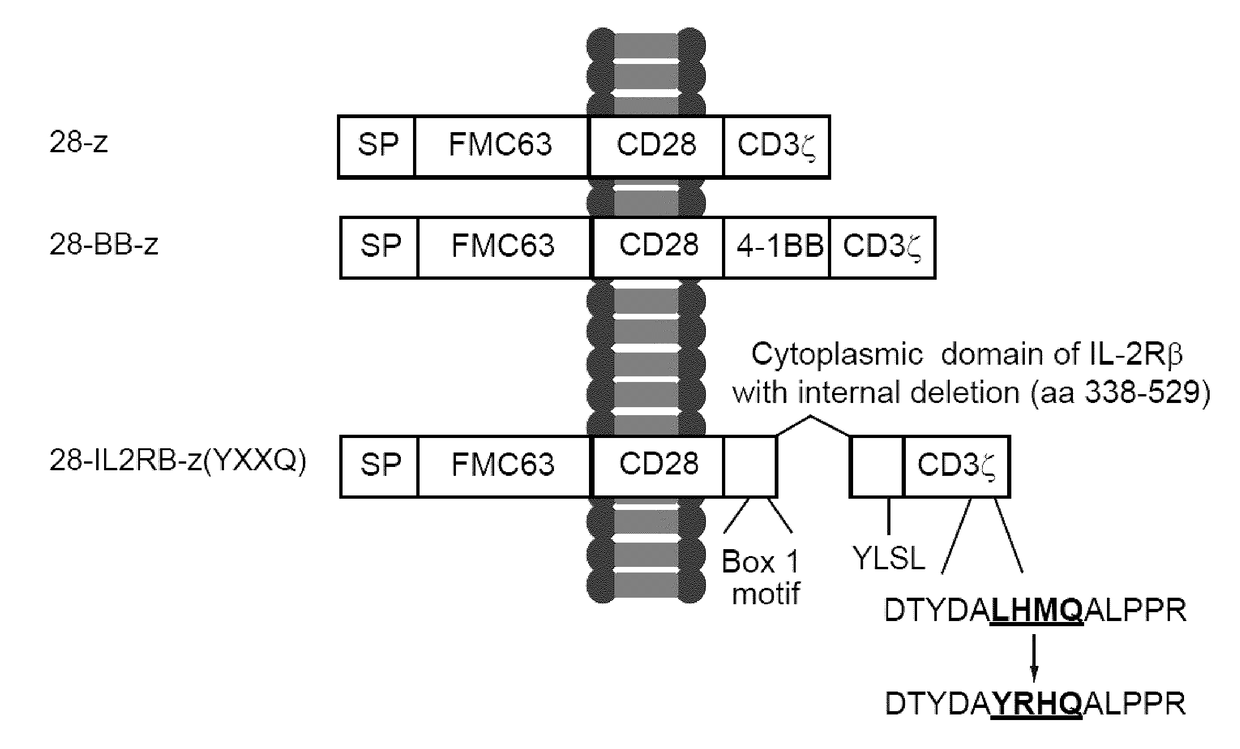
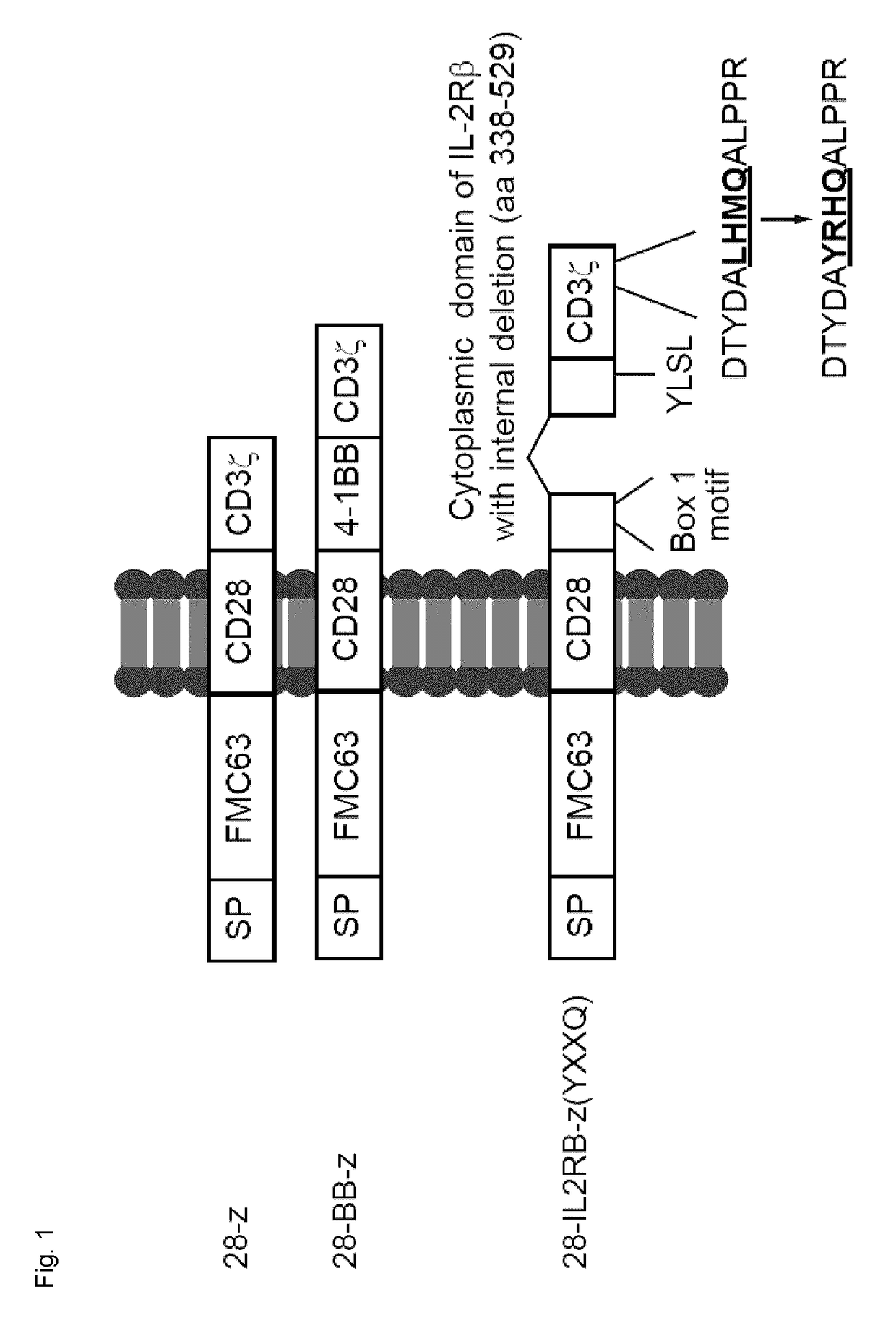
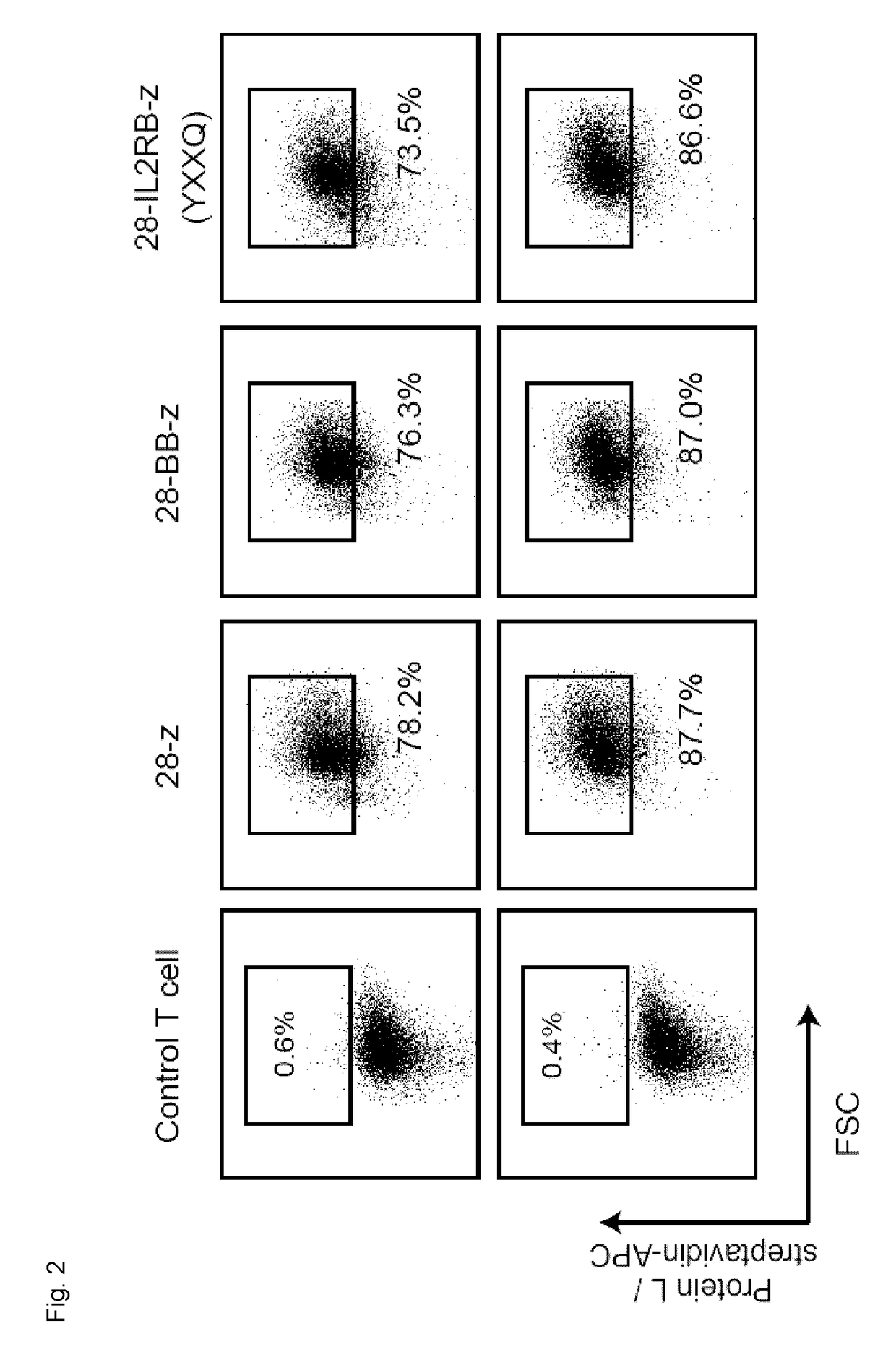
Chimeric Antigen Receptors
ActiveUS20180037630A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenIntracellular signalling
Disclosed herein are chimeric antigen receptors (CARs) comprising an intracellular segment comprising an interleukin receptor chain, a JAK-binding motif, a Signal Transducer and Activator of Transcription (STAT) 5 association motif and / or a CD3ζ intracellular signaling domain comprising an exogenous STAT3 association motif, as well as cells and 5 compositions comprising said CARs and uses thereof.
Owner:UNIV HEALTH NETWORK +1
Genetic variations in the interleukin-6 receptor gene as predictors of the response of patients to treatment with interleukin-6 receptor inhibitors
The present invention relates to a method for predicting the response of patients to treatment with Interleukin-6 Receptor (IL6R) inhibitors, such as antibodies directed against the IL6R. The method comprises the analysis of one or more genetic variations, in particular single nucleotide polymorphisms, in or associated with the Interleukin-6 Receptor gene. The present invention further relates to a kit for use in predicting the response of patients to treatment with IL6R inhibitors, such as Tocilizumab. The patients suffers from rheumatoid arthritis.
Owner:RIGSHOSPITALET
Antibody-containing aqueous formulation and use thereof
ActiveUS20190300615A1Reduce formationOrganic active ingredientsSkeletal disorderMedicineSURFACTANT BLEND
Owner:RICHTER GEDEON NYRT
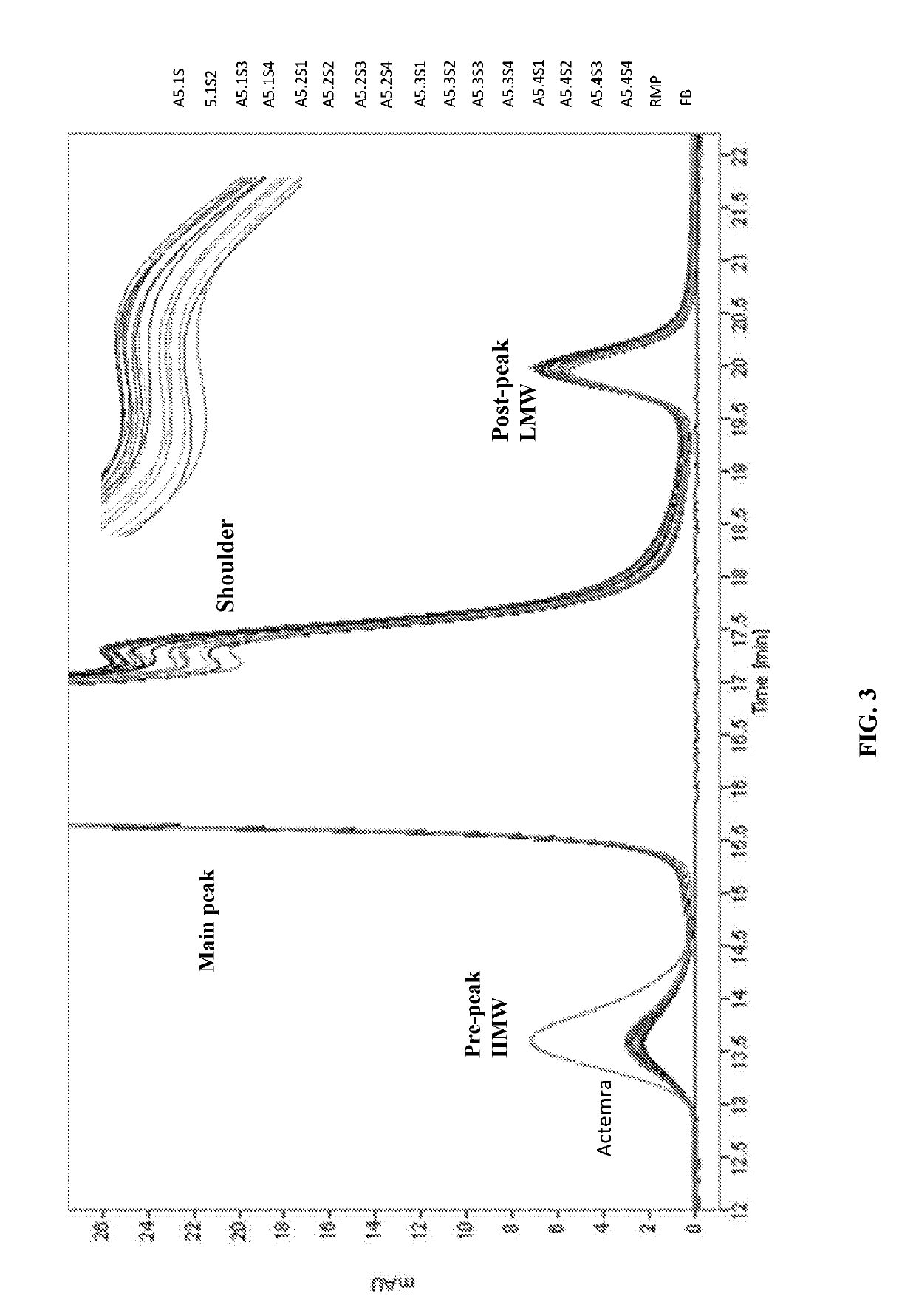
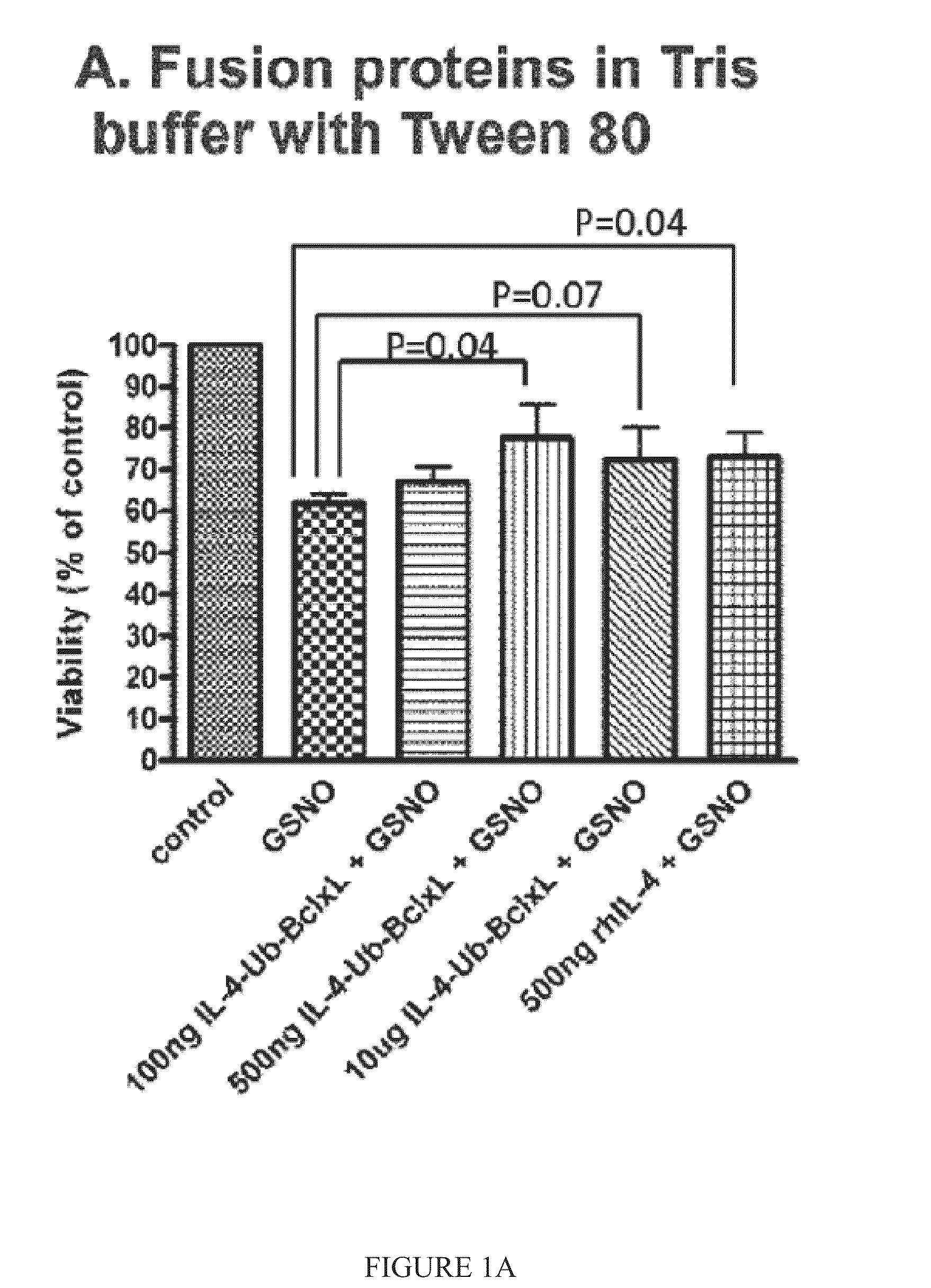
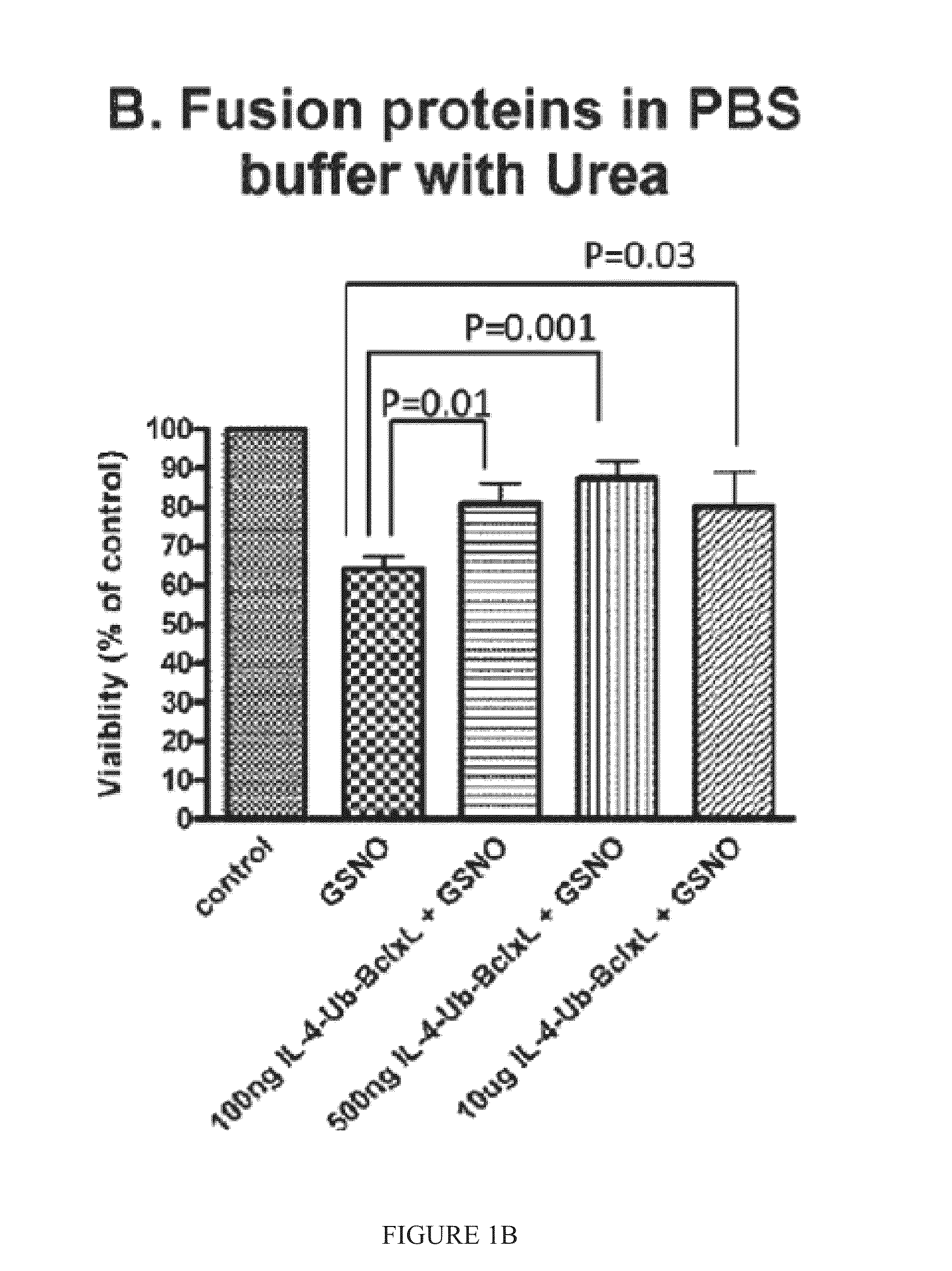
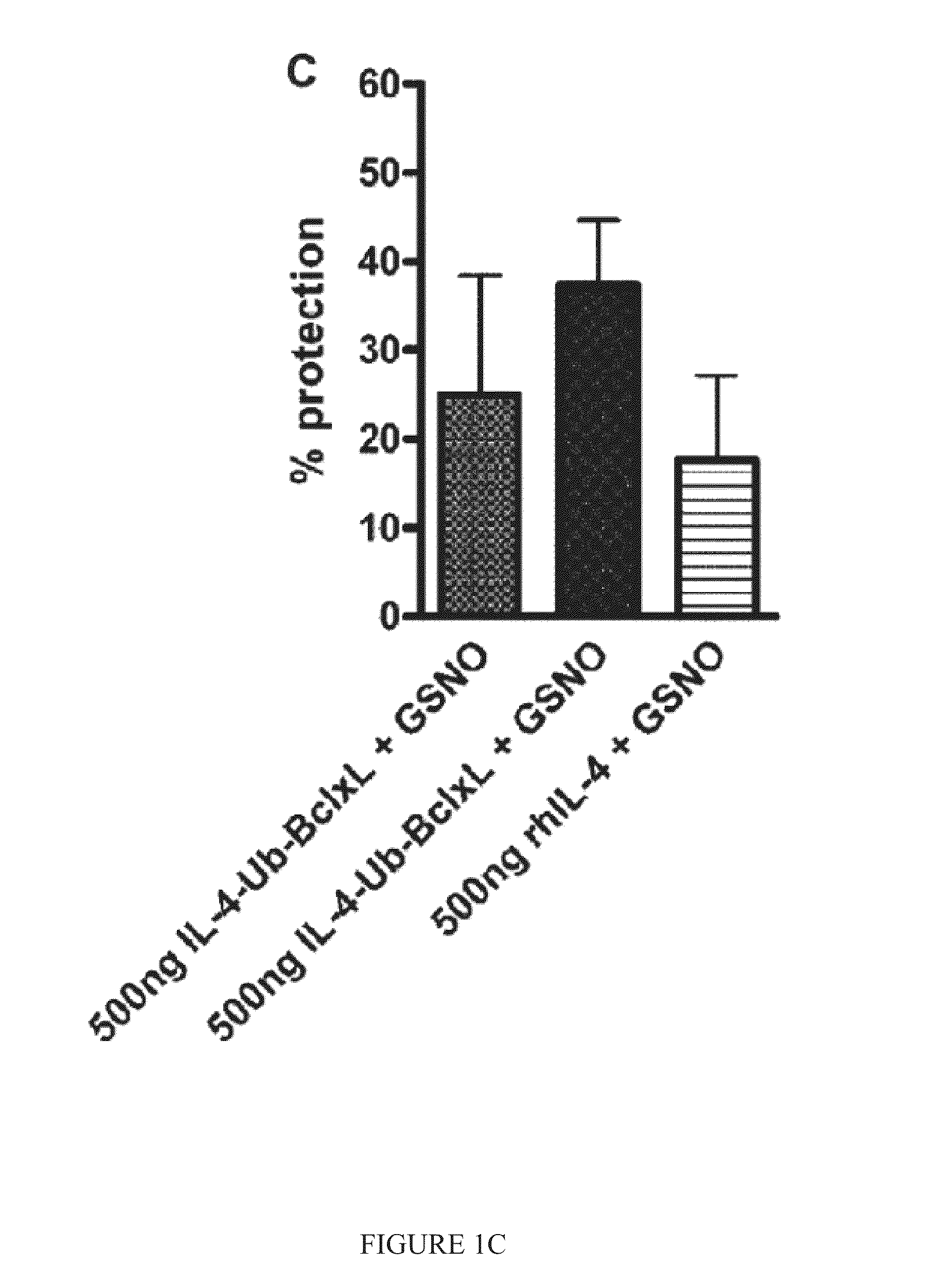
Interleukin-4 receptor-binding fusion proteins and uses thereof
ActiveUS20160237135A1Enhance cell viabilityIncreased activationSenses disorderNervous disorderWhite blood cellInterleukin 5
The present invention relates to interleukin-4 receptor-binding fusion proteins. More specifically, the invention provides, in part, fusion proteins that include an interleukin-4 or interleukin-13 protein moiety joined to an anti-apoptotic Bcl-2 family member protein moiety.
Owner:MEDICENNA THERAPEUTICS +1
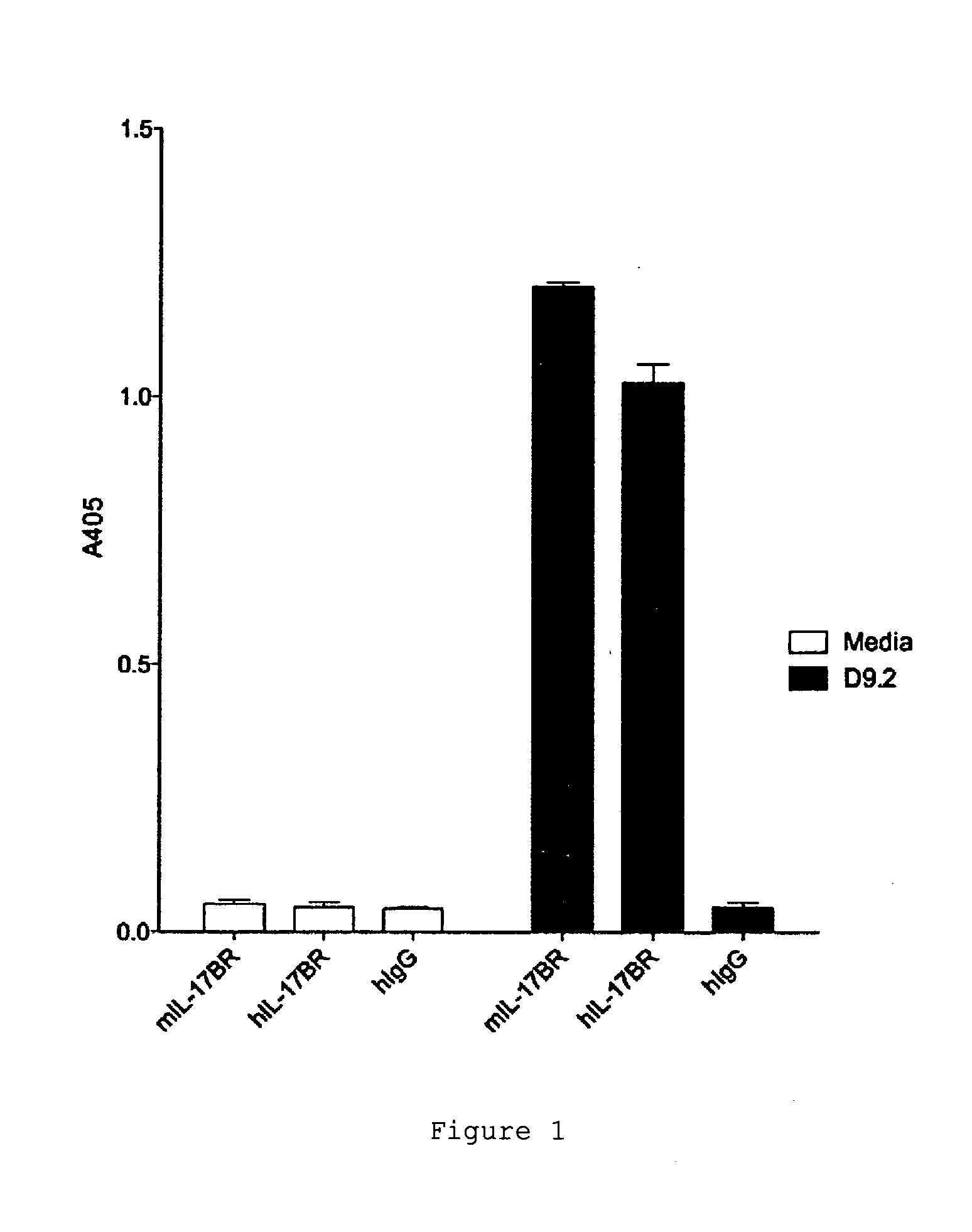
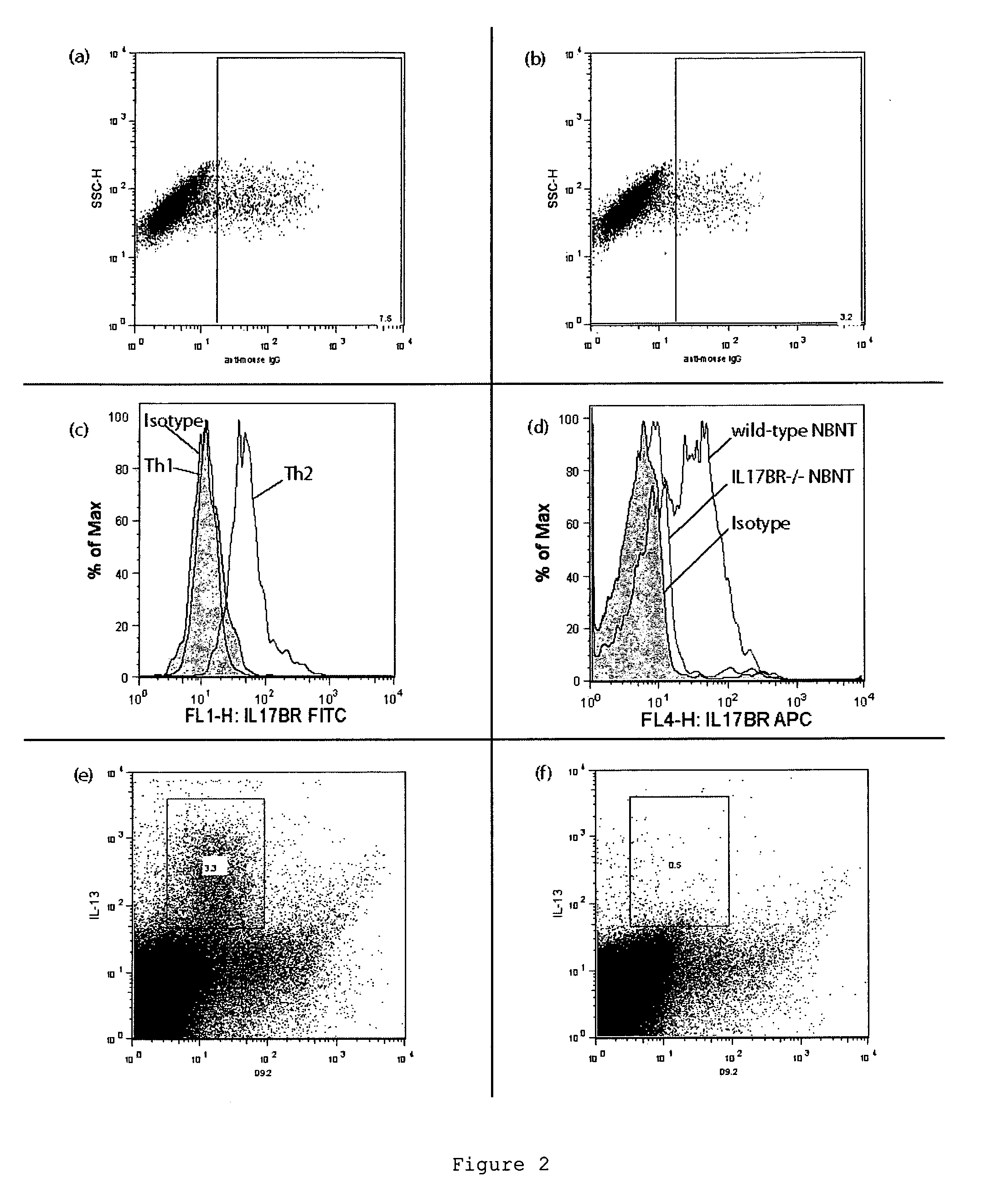
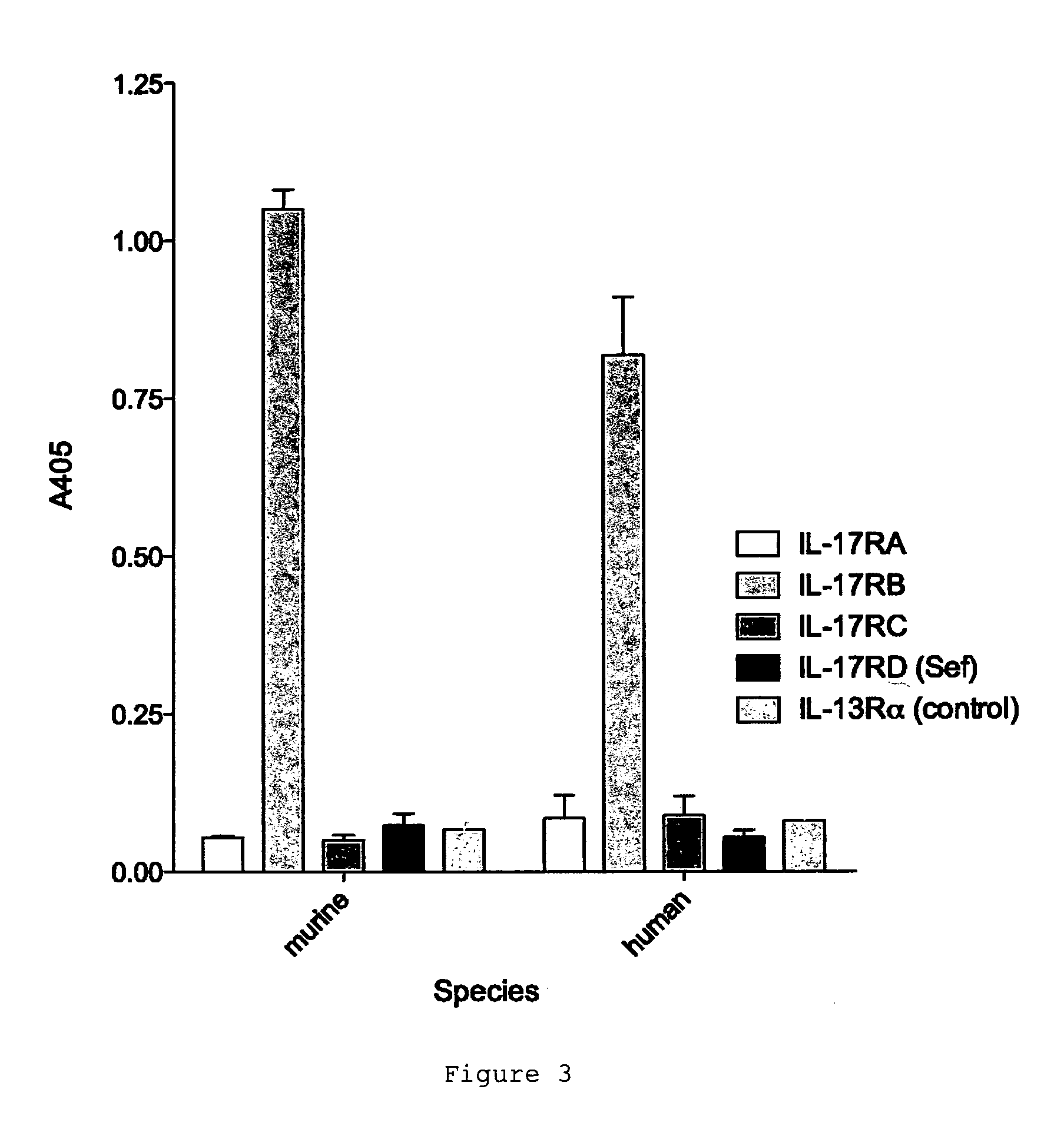
Antibodies against il-17br
ActiveUS20120020985A1Lower Level RequirementsReduce expansionFungiBacteriaCrohn's diseaseIL-17 Receptor B
The invention provides the antibody D9.2 and antibody molecules based on D9.2 which bind interleukin-17 receptor B. These may be useful in therapy, e.g. the treatment of asthma, ulcerative colitis or Crohn's disease.
Owner:UK RES & INNOVATION LTD
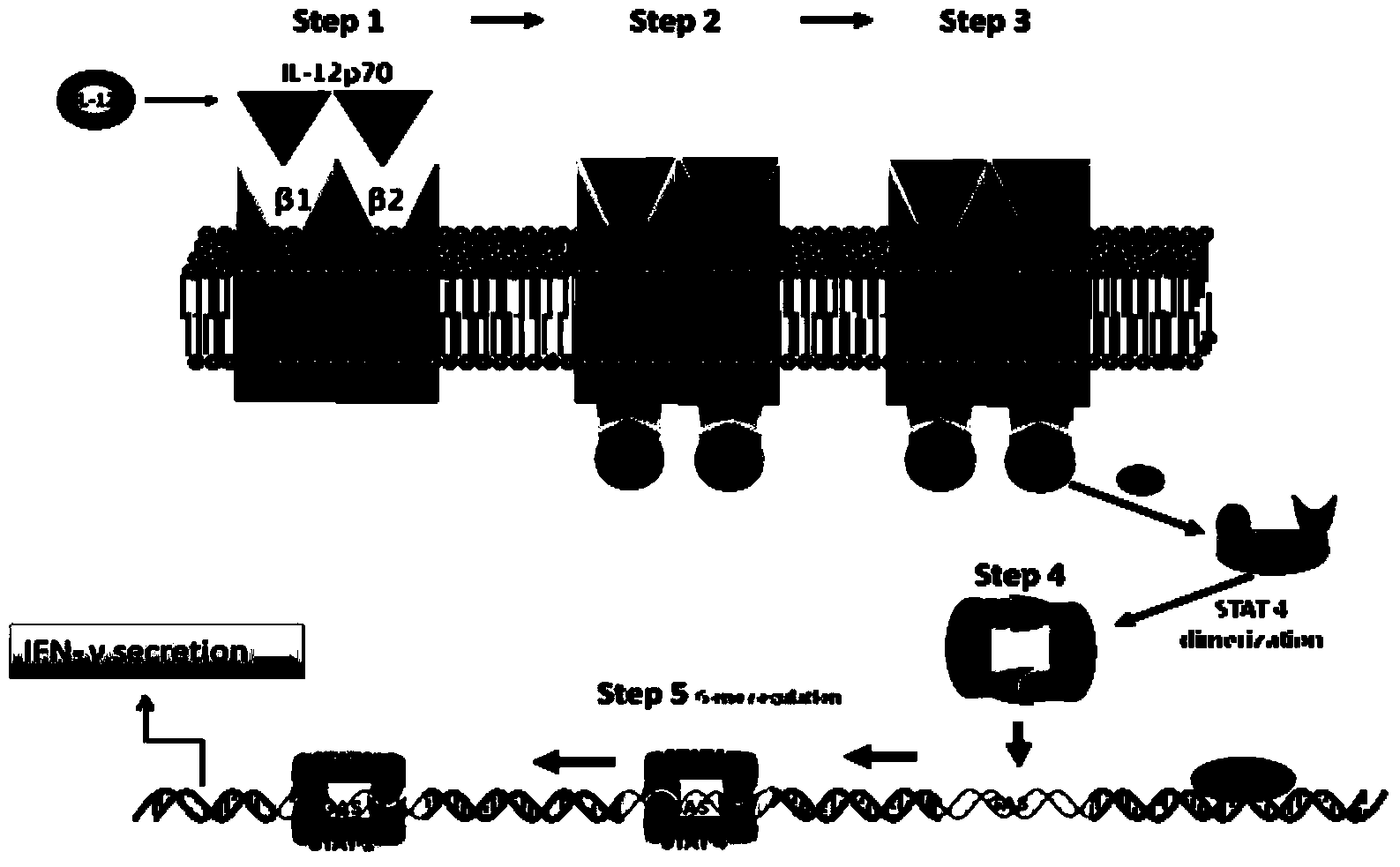
Method for detecting in-vitro activity of recombined human interleukin-12 by using cell with interleukin-12 receptor and application
InactiveCN103837687AUnlimited number of passagesEasy to trainBiological material analysisBiological testingProtein insertionWhite blood cell
The invention discloses a method for detecting the in-vitro activity of recombined human interleukin-12 by using a cell with an interleukin-12 receptor and application. The method comprises the following steps of (1) taking out the frozen cell with the interleukin-12 receptor from a liquid nitrogen tank, and performing recovery activation cultivation till the logarithm growth period is reached; (2) inducing the cell with the interleukin-12 receptor through a recombined human interleukin-12 standard substance under different concentrations to generate gamma interferon to obtain the concentration value ED50s of the recombined human interleukin-12 standard substances; (3) inducing the cell with the interleukin-12 receptor through a recombined human interleukin-12 protein to be detected under different concentrations to generate the gamma interferon to obtain a concentration value ED50r of the recombined human interleukin-12 protein to be detected; and (4) obtaining the in-vitro activity Pr of the recombined human interleukin-12 protein to be detected according to a formula Pr=Ps*ED50s / ED50r.
Owner:QINGDAO KANGLITAI PHARMA
Pharmaceutical composition for treating or preventing obstructive sleep apnea
InactiveCN108339118AImprove night sleep snoringImprove apnea frequencyVertebrate antigen ingredientsAntibody ingredientsAntigenAntigen Binding Fragment
The invention discloses an antagonist or antibody of an interleukin-4 receptor (IL-4R) and an antigen-binding fragment thereof and / or a use of an antagonist or antibody of an interleukin-6 (IL-6) andan antigen-binding fragment thereof in preparation of a pharmaceutical composition for treating or preventing obstructive sleep apnea. The invention also discloses a pharmaceutical composition for treating or preventing obstructive sleep apnea. The active ingredients of the drug comprise an antagonist of an interleukin-4 receptor (IL-4R) or its antibody and antigen-binding fragment, or an antagonist or antibody of an interleukin-6 (IL-6) and an antigen-binding fragment thereof.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
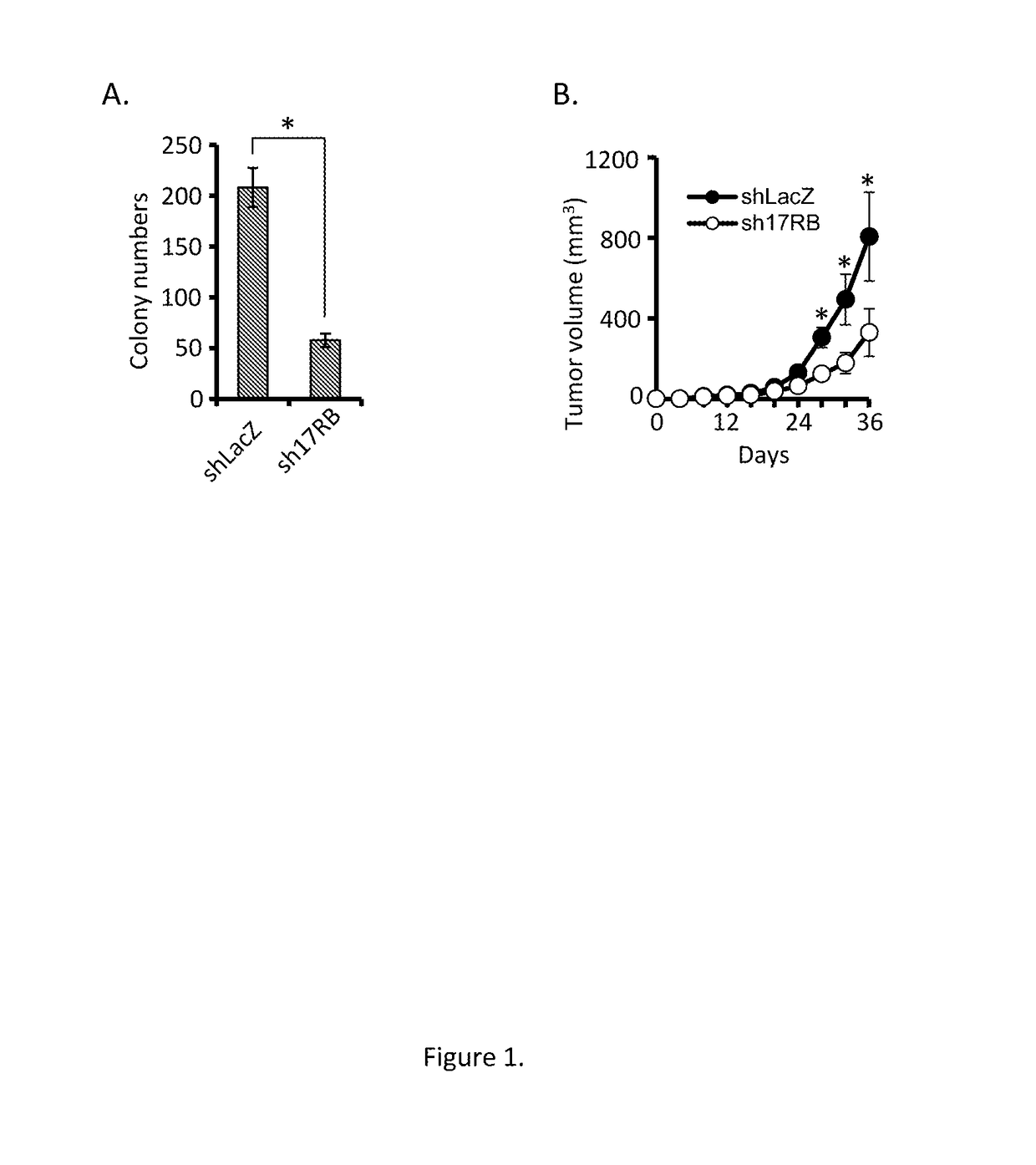
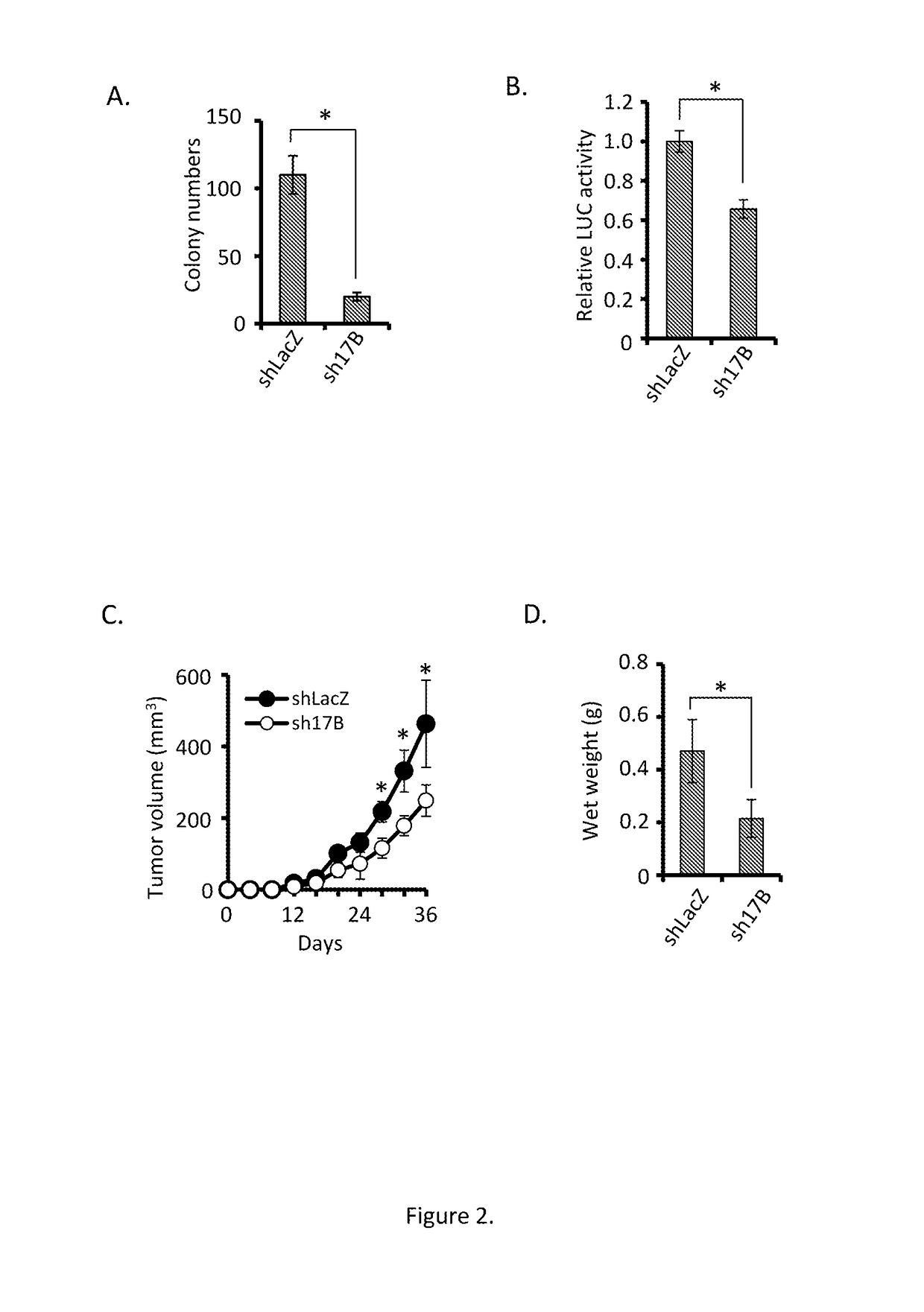
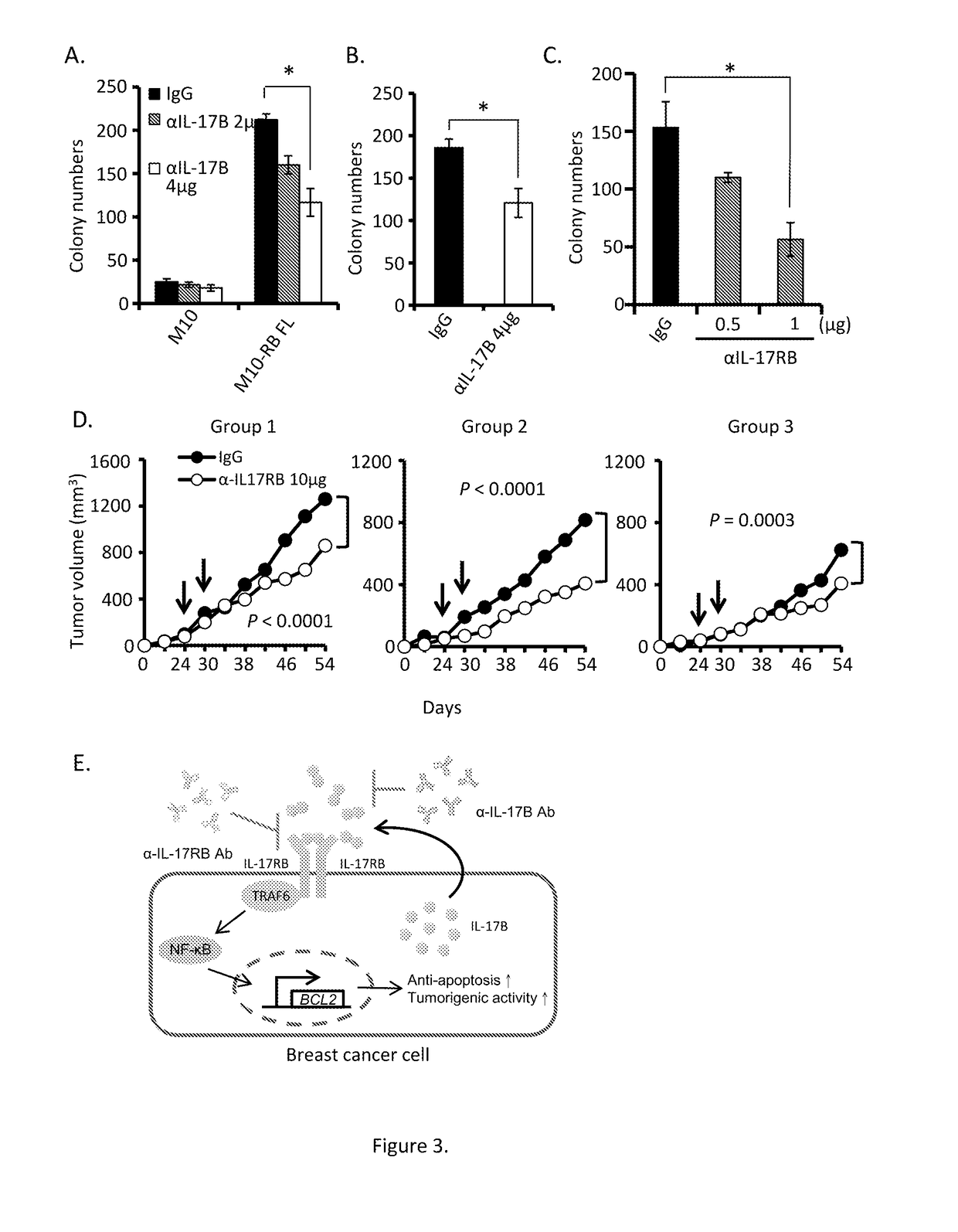
Antagonists for interleukin-17 receptor b (il-17rb) and its ligand il-17b for cancer therapy
ActiveUS20180201672A1Organic active ingredientsPeptide/protein ingredientsWhite blood cellIL-17 Receptor B
Methods and compositions for treatment and therapy of cancer are provided. Specifically, antagonists specific for interleukin-17 receptor B (IL-17RB) and its ligand IL-17B are provided. Potent neutralizing antibodies specific for IL-17RB and methods for their manufacture and use are disclosed. The invention also relates to antisense, RNAi and shRNA compositions for the prevention and treatment of cancer, in particular breast cancer and pancreatic cancer.
Owner:ACAD SINIC
Interleukin-4 receptor-binding fusion proteins and uses thereof
ActiveUS20160215035A1Accelerated deathReduce phosphorylationSenses disorderNervous disorderCell biologyProtein C
The present invention relates to interleukin-4 receptor binding fusion proteins. More specifically, the invention provides, in part, fusion proteins that include an interleukin-4 receptor binding protein moiety joined to a pro-apoptotic Bcl-2 family member protein moiety.
Owner:UNITED STATES OF AMERICA +1
Antibody antagonists of interleukin-13 receptor α1
ActiveUS8207304B2Inhibition releaseSenses disorderSkeletal disorderAntagonistReceptor for activated C kinase 1
Antibody antagonists of human interleukin-13 receptor alpha 1 are disclosed. The antibody molecules are useful in the inhibition of IL-13Rα1-mediated activities and, accordingly, present desirable antagonists for the use in the treatment of conditions associated with hIL-13Rα activity. The present invention also discloses nucleic acid encoding said antibody molecules, vectors, host cells, and compositions comprising the antibody molecules. Methods of using the antibody molecules for inhibiting or antagonizing IL-13Rα1-mediated activities are also disclosed.
Owner:CSL LTD
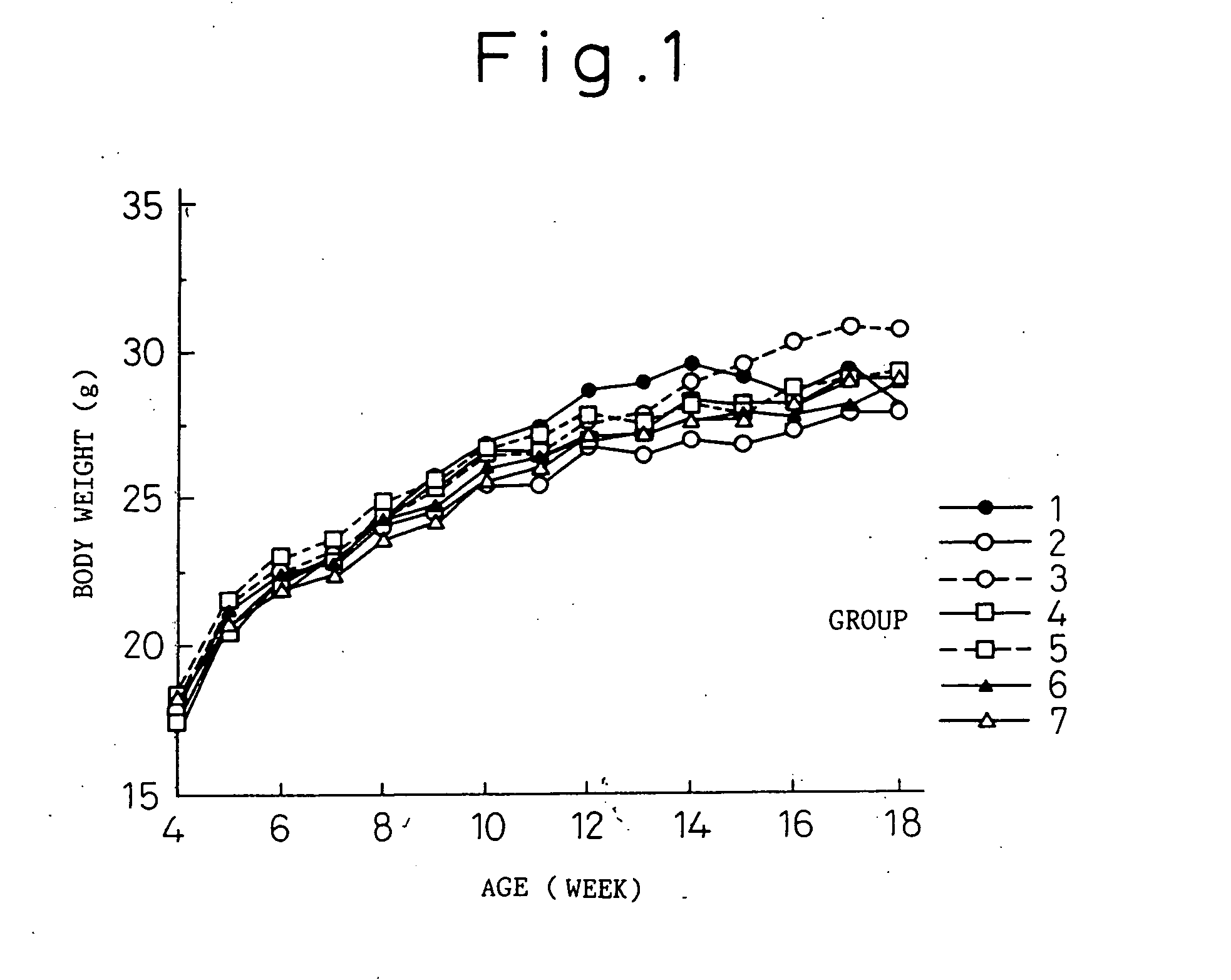
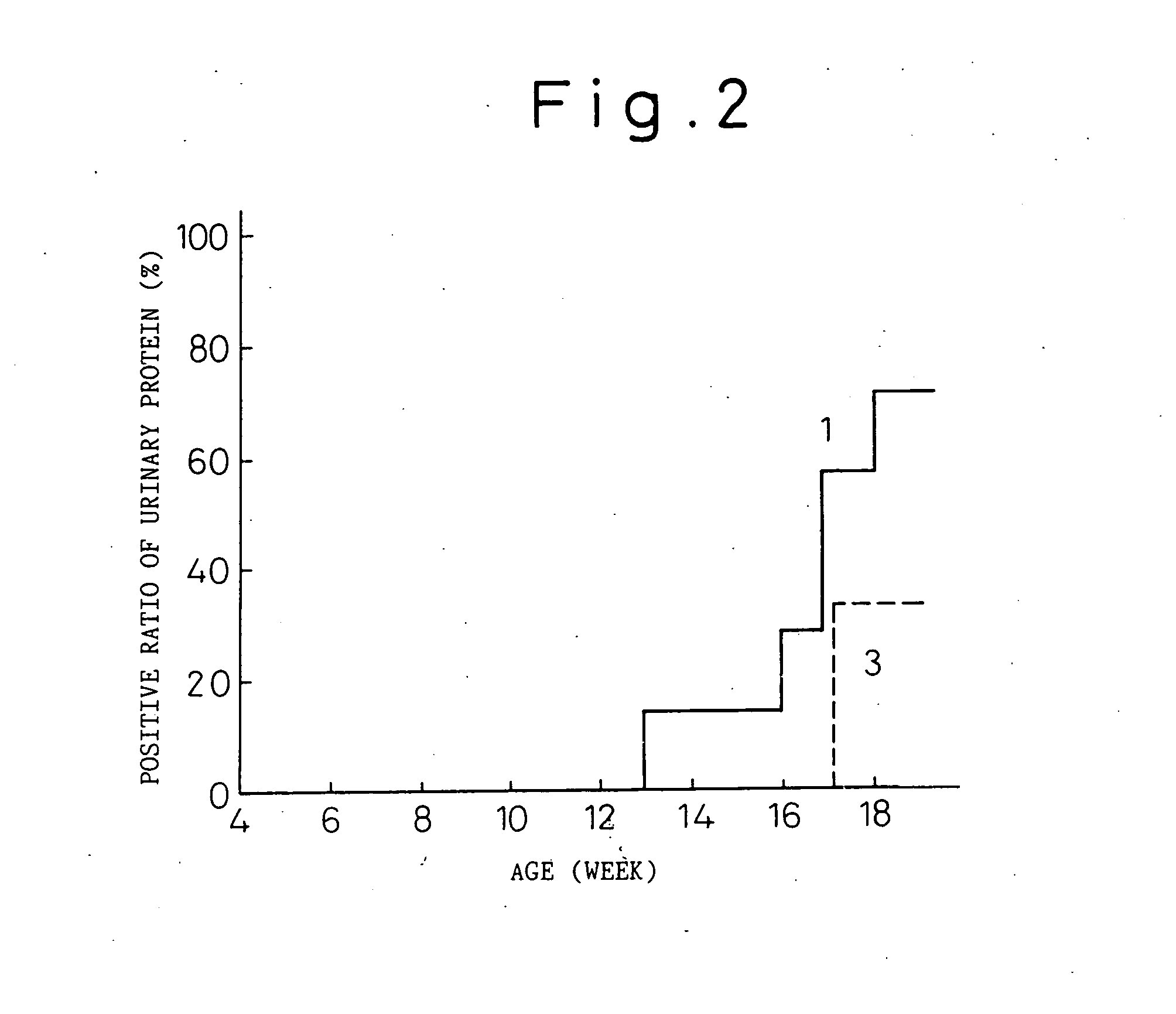
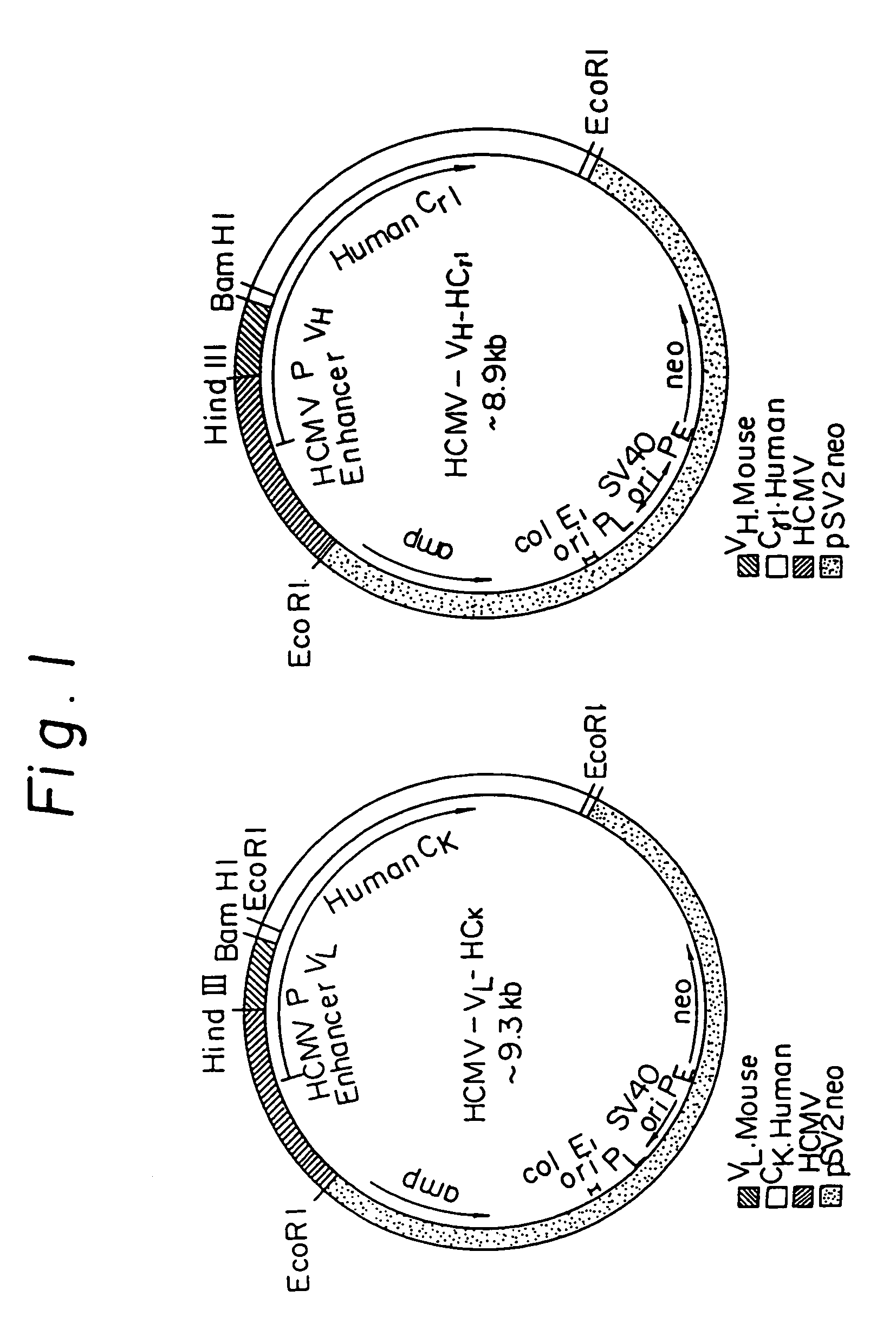
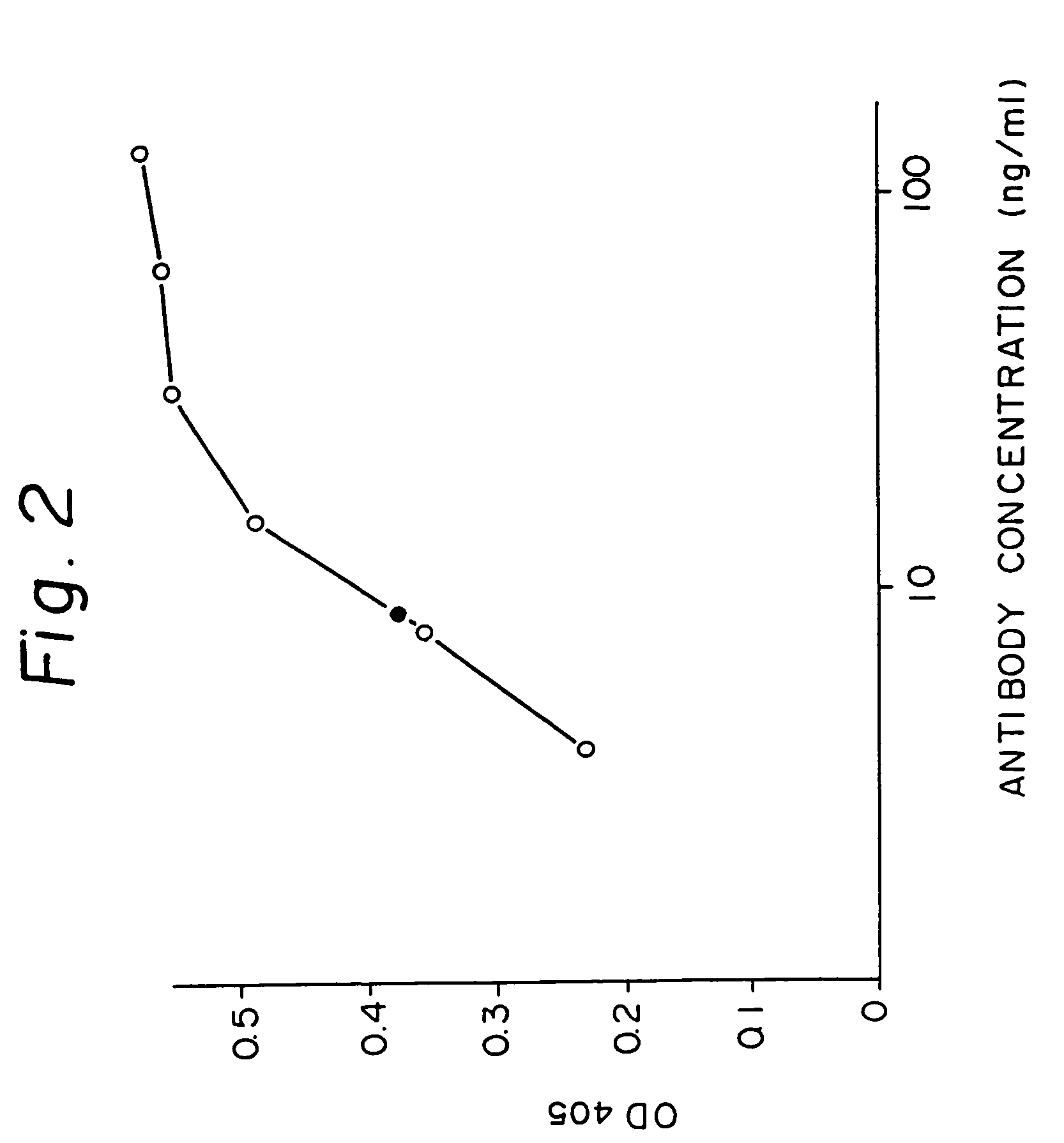
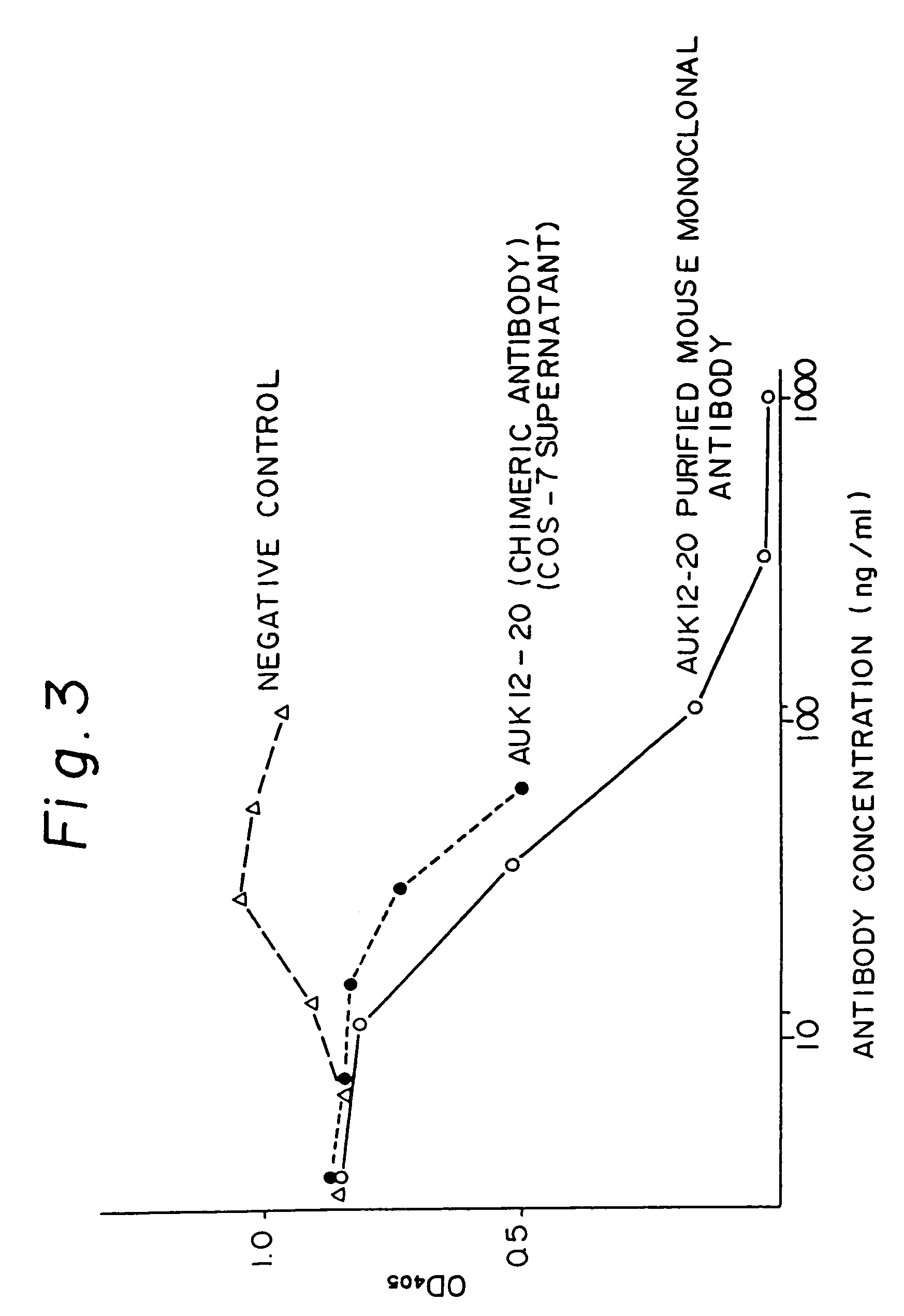
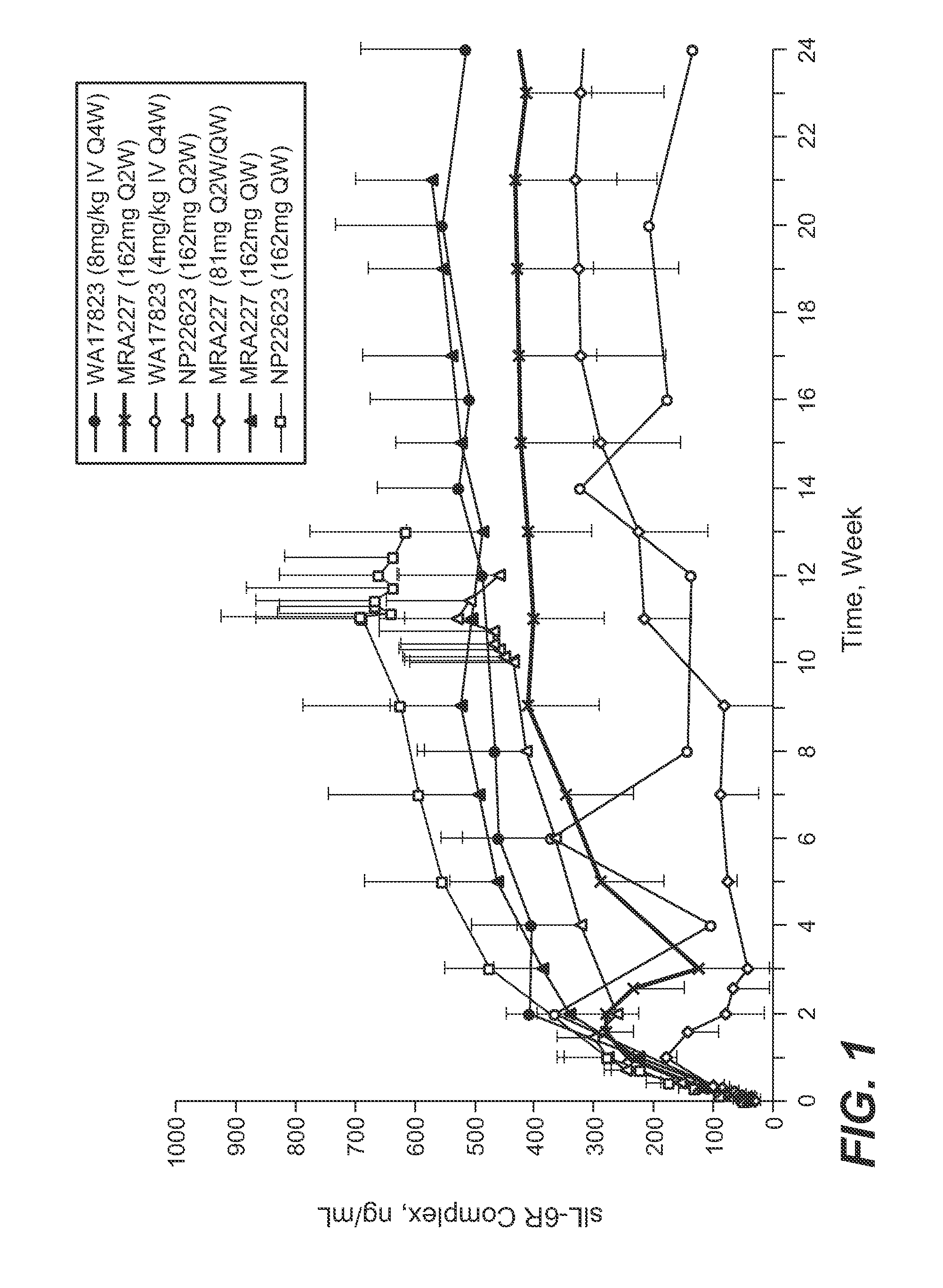
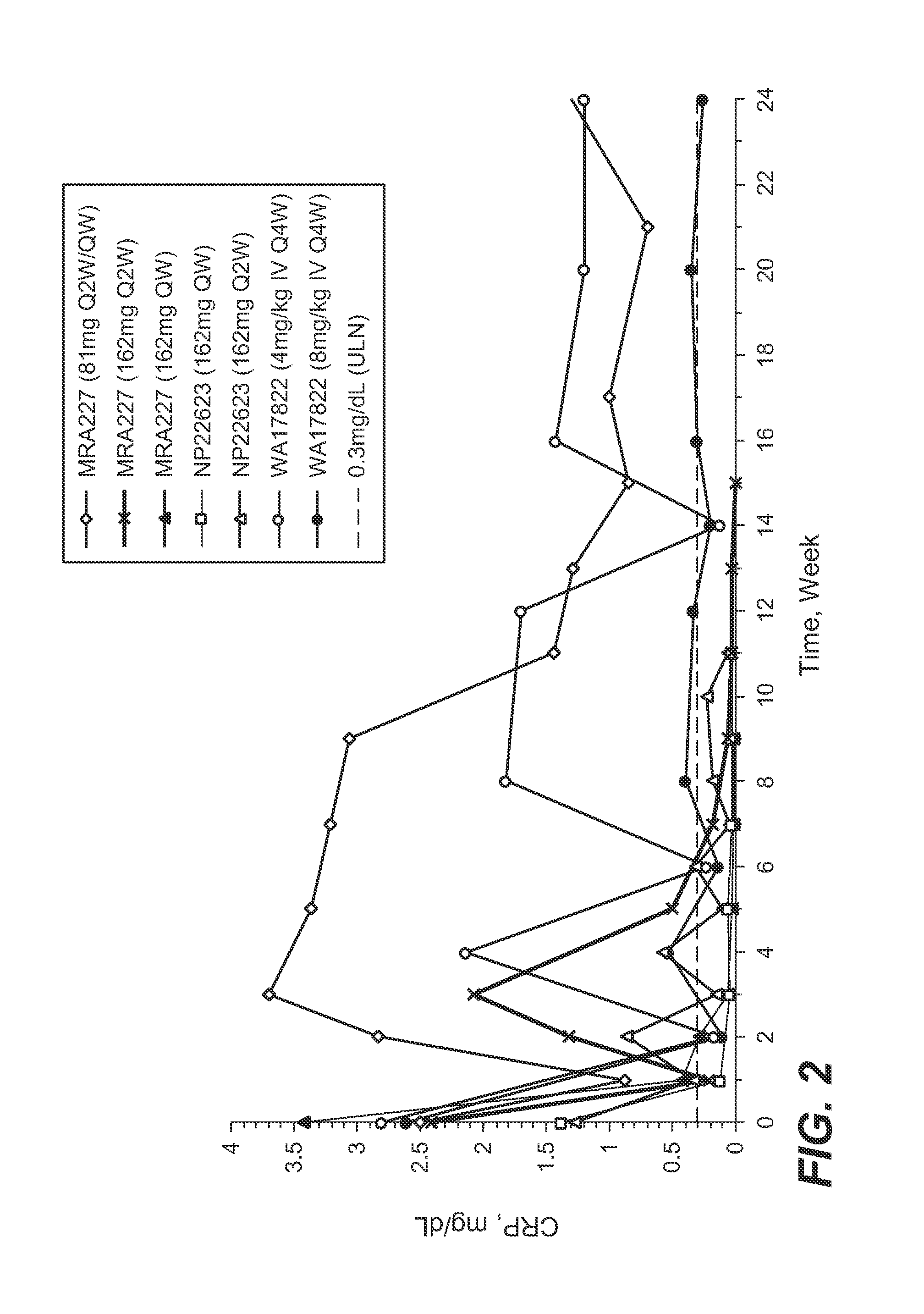
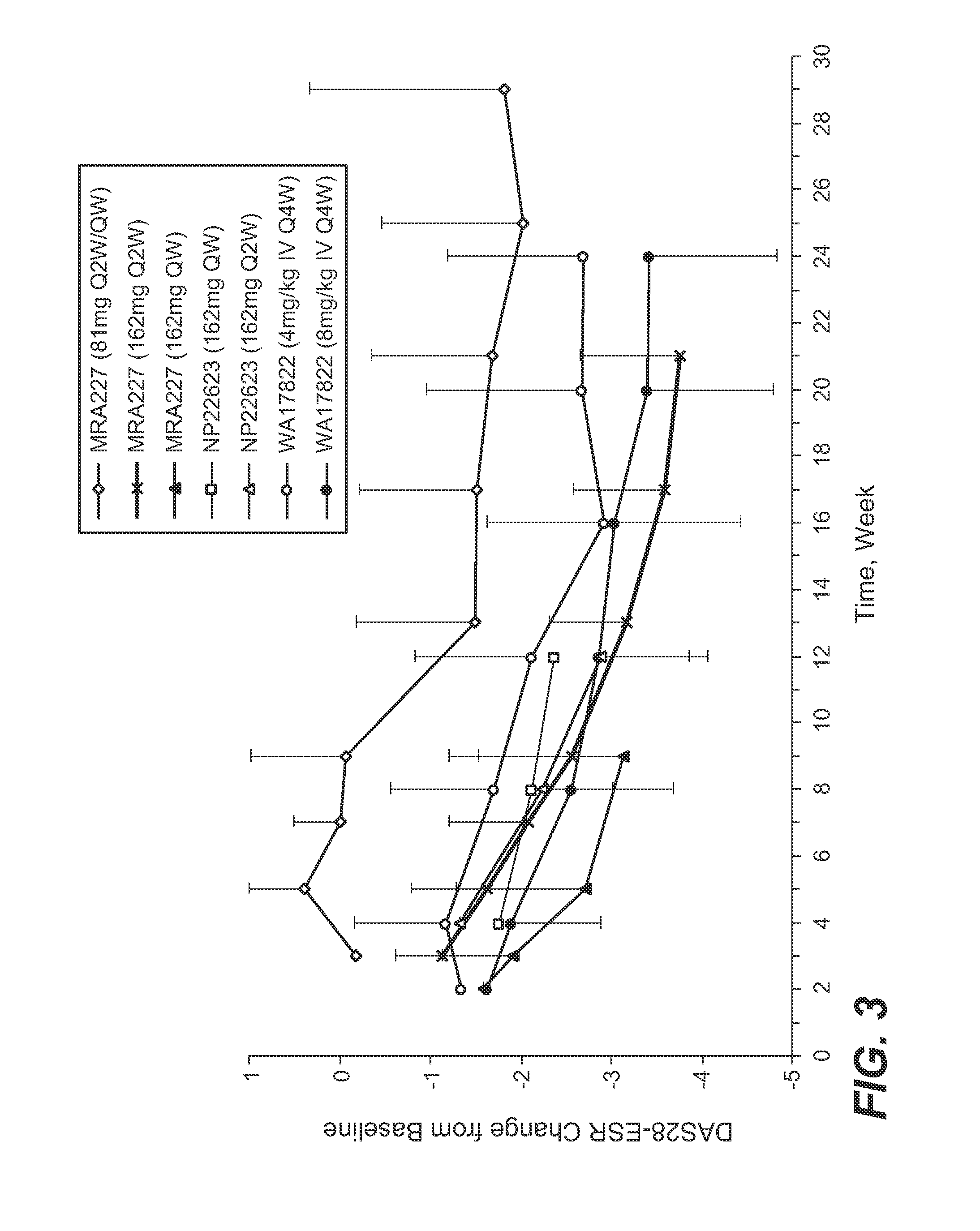
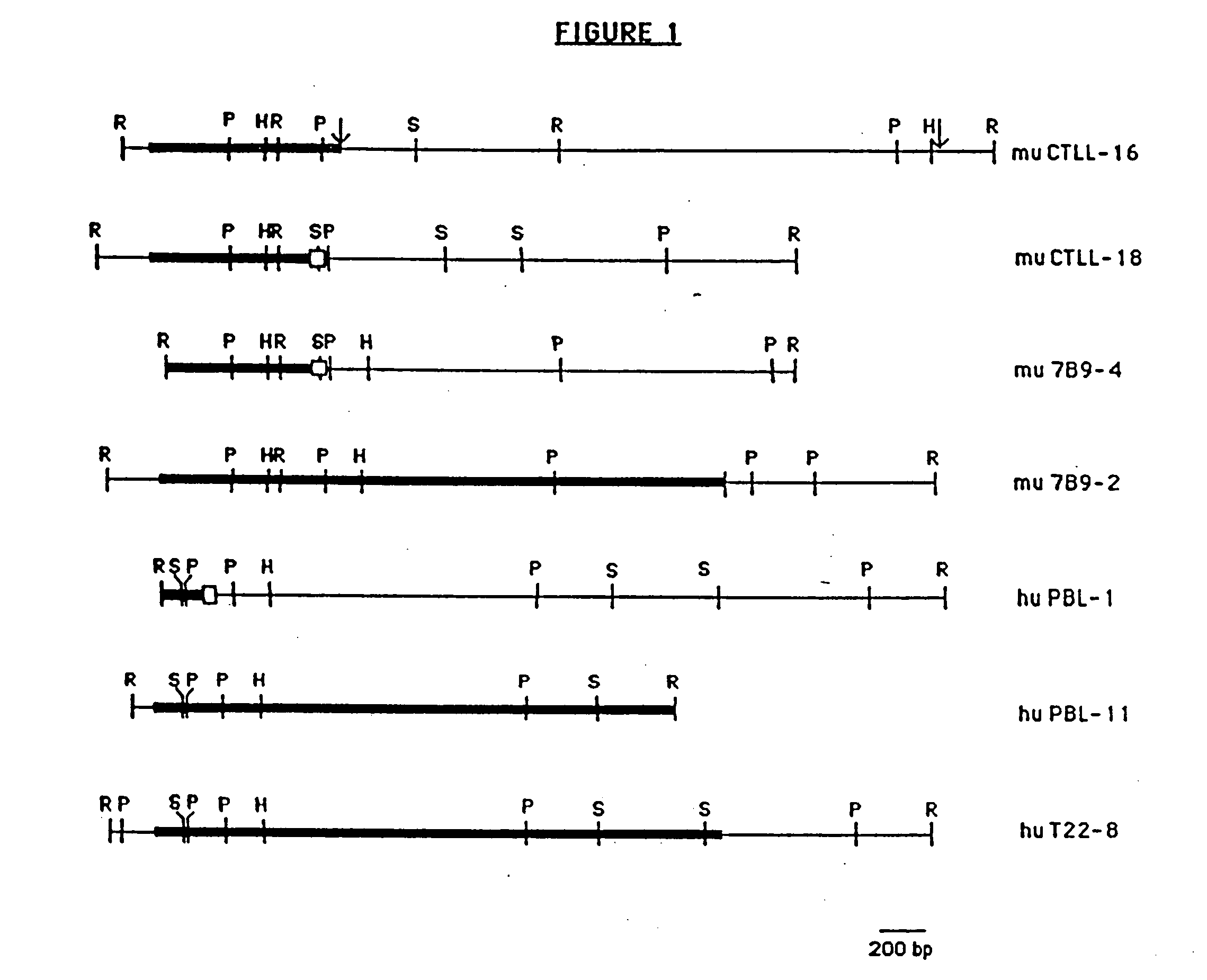
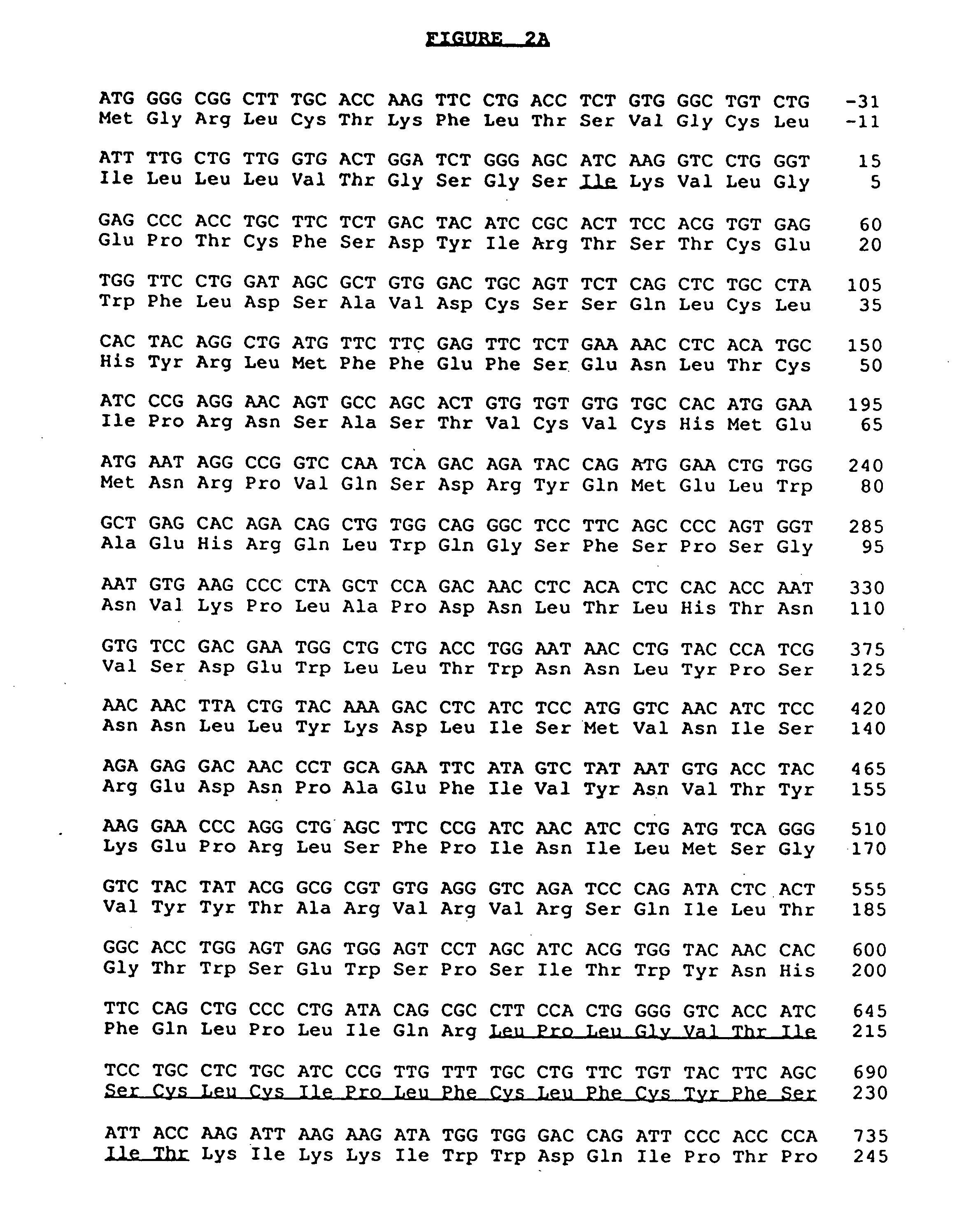
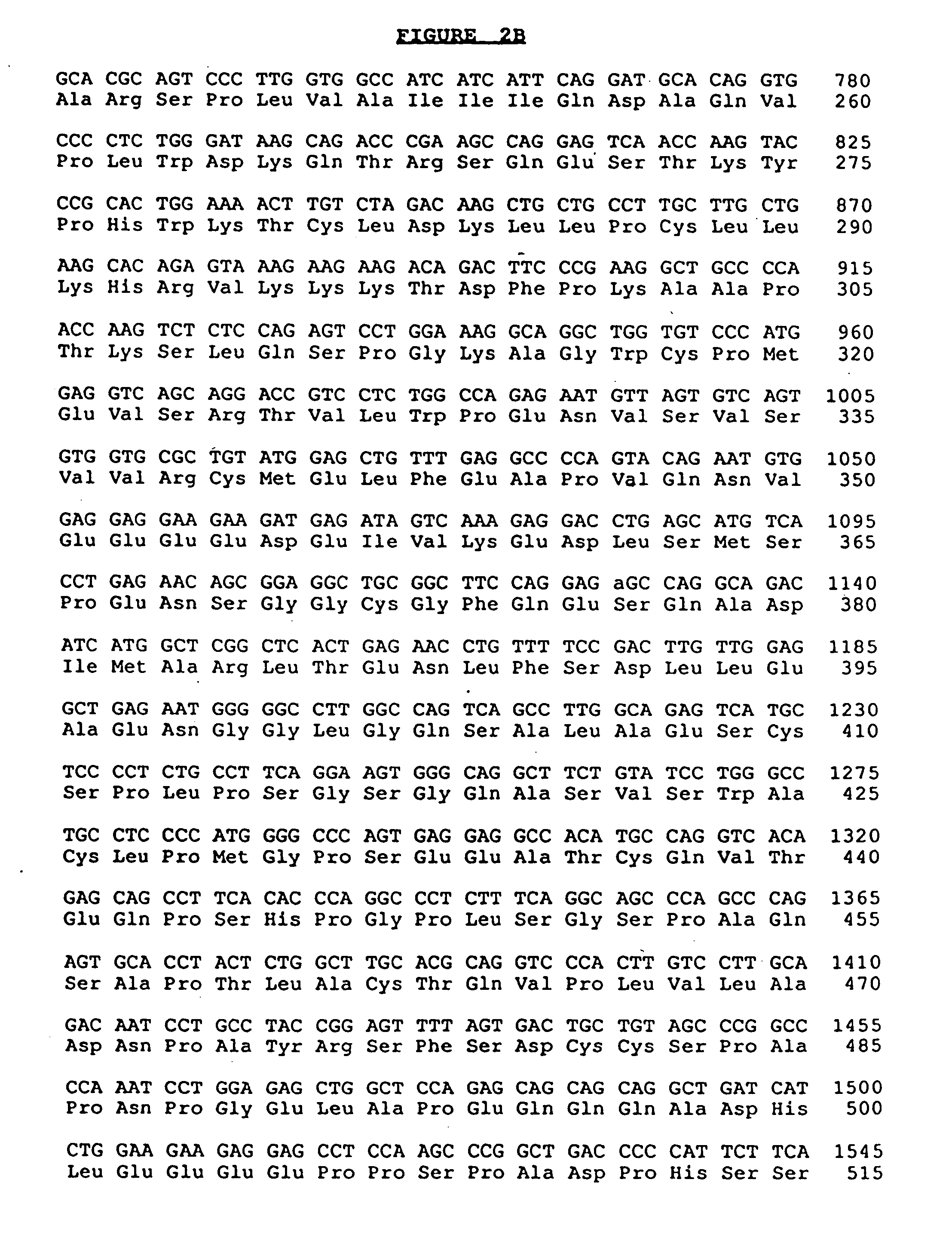
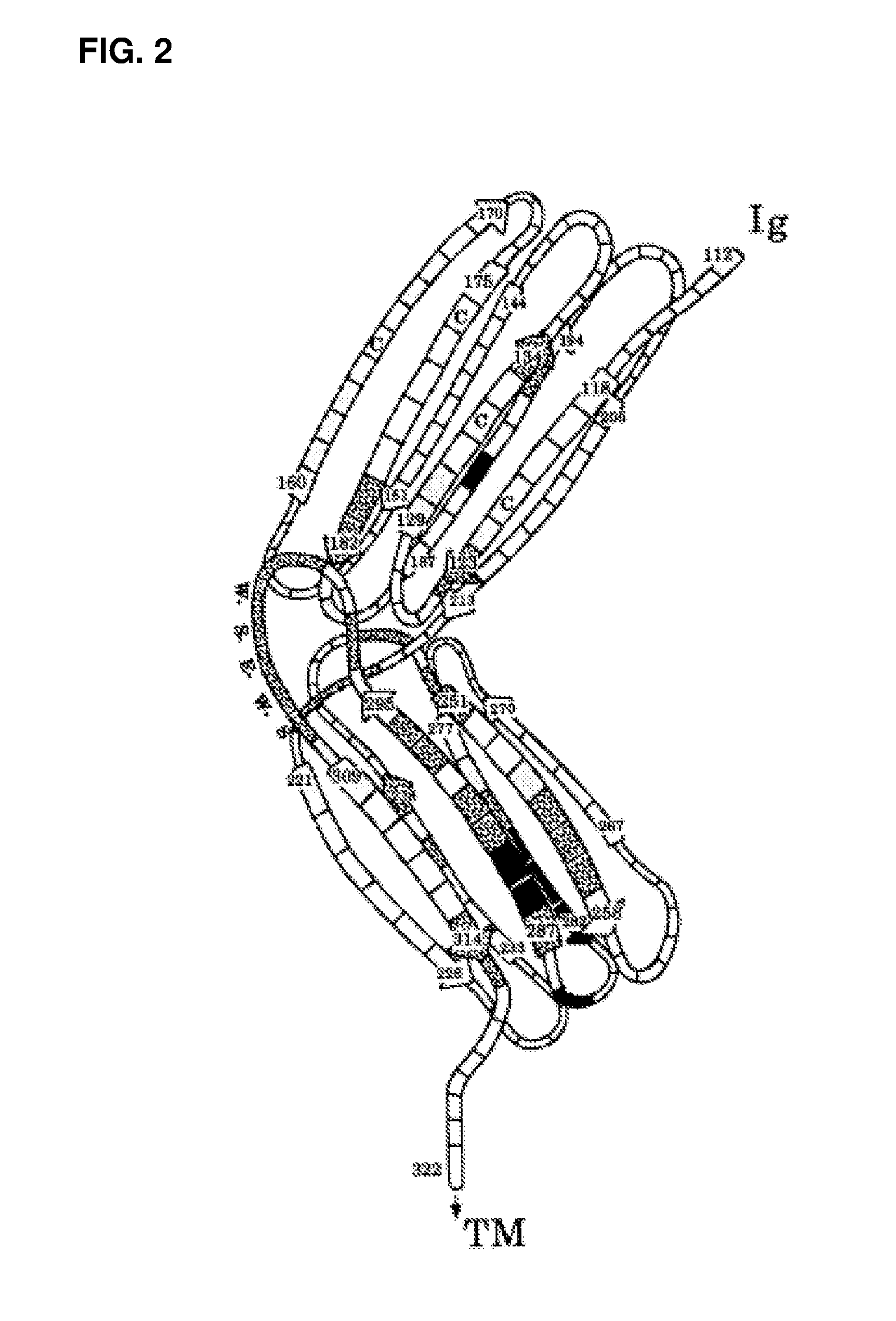
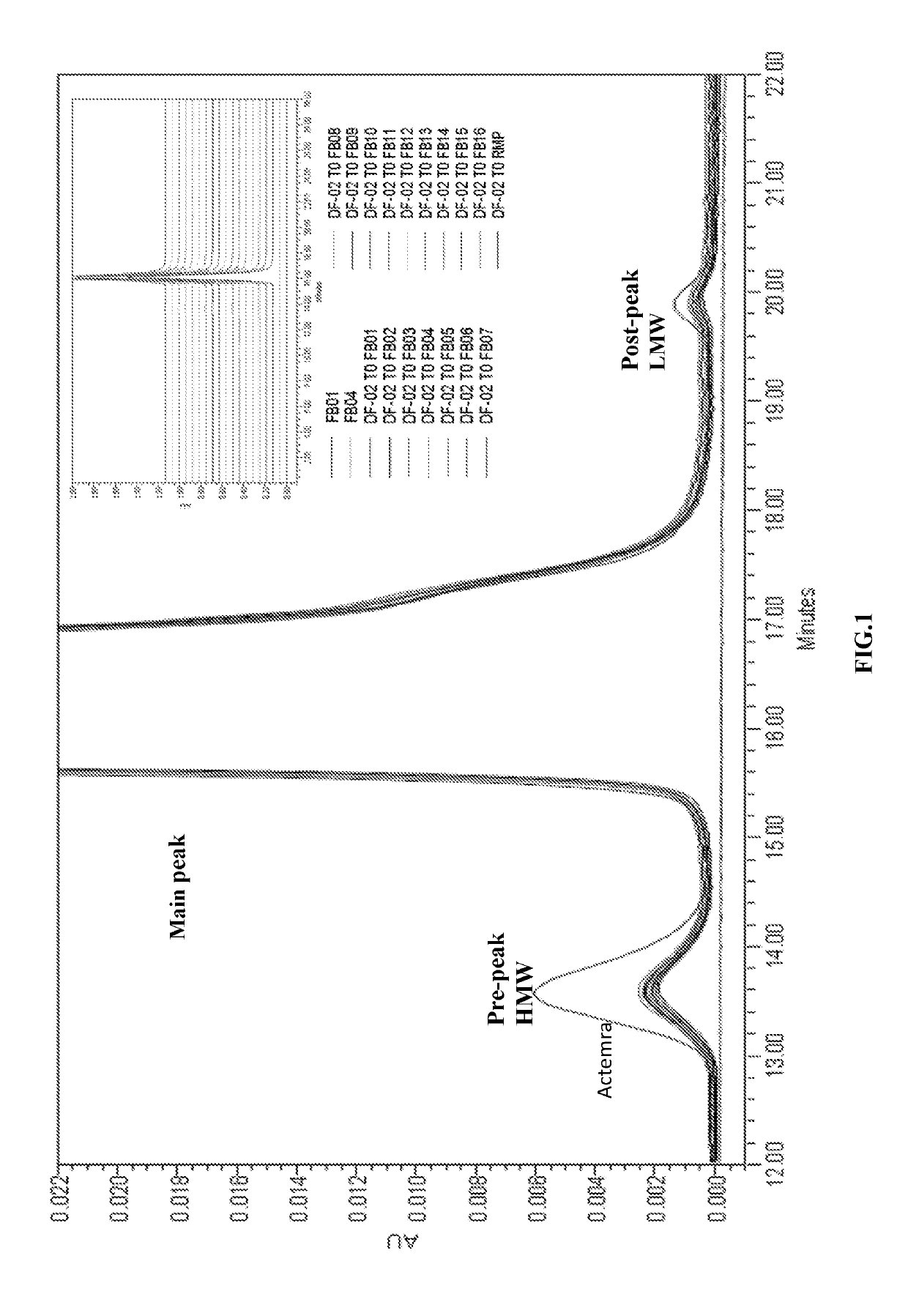
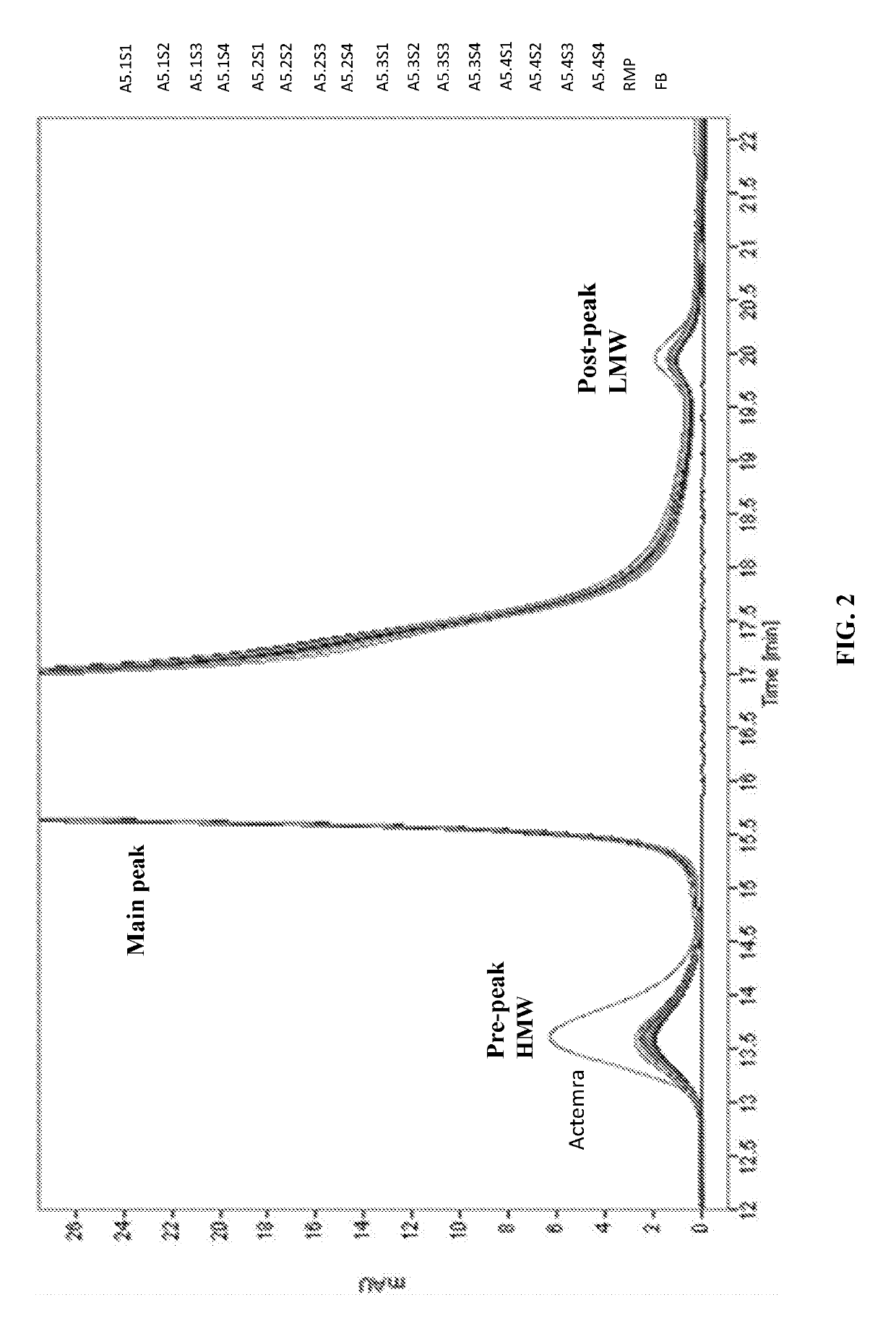
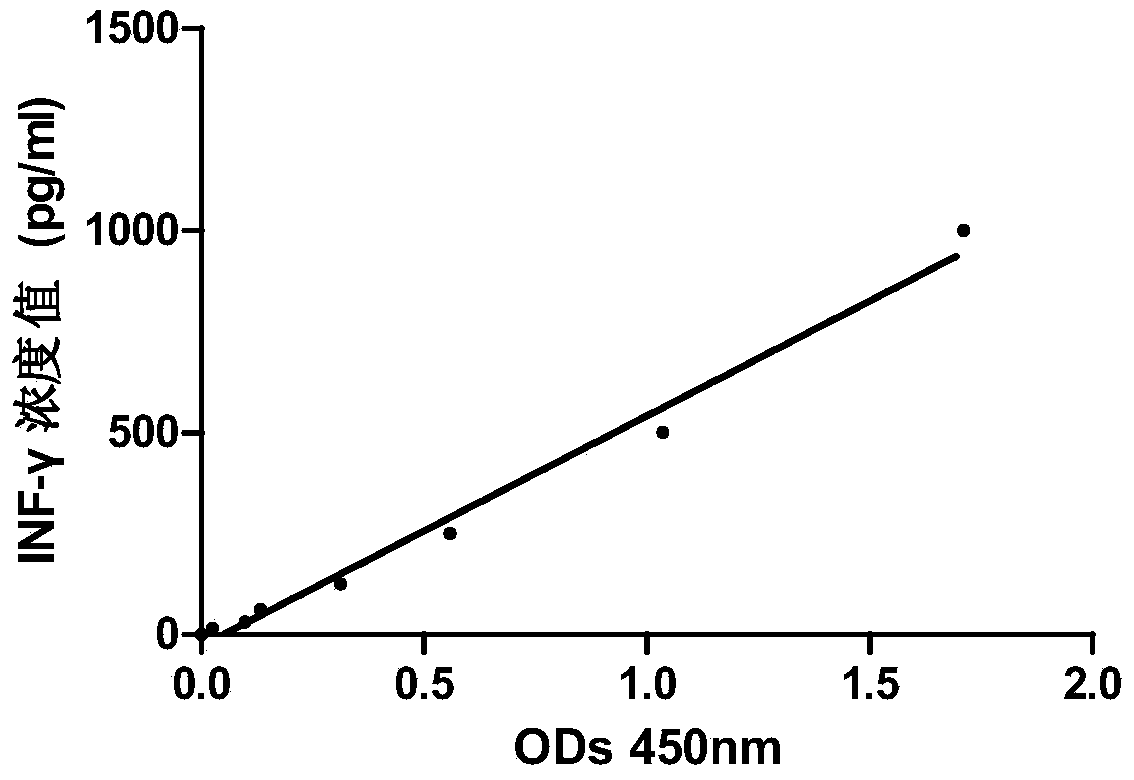
Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof
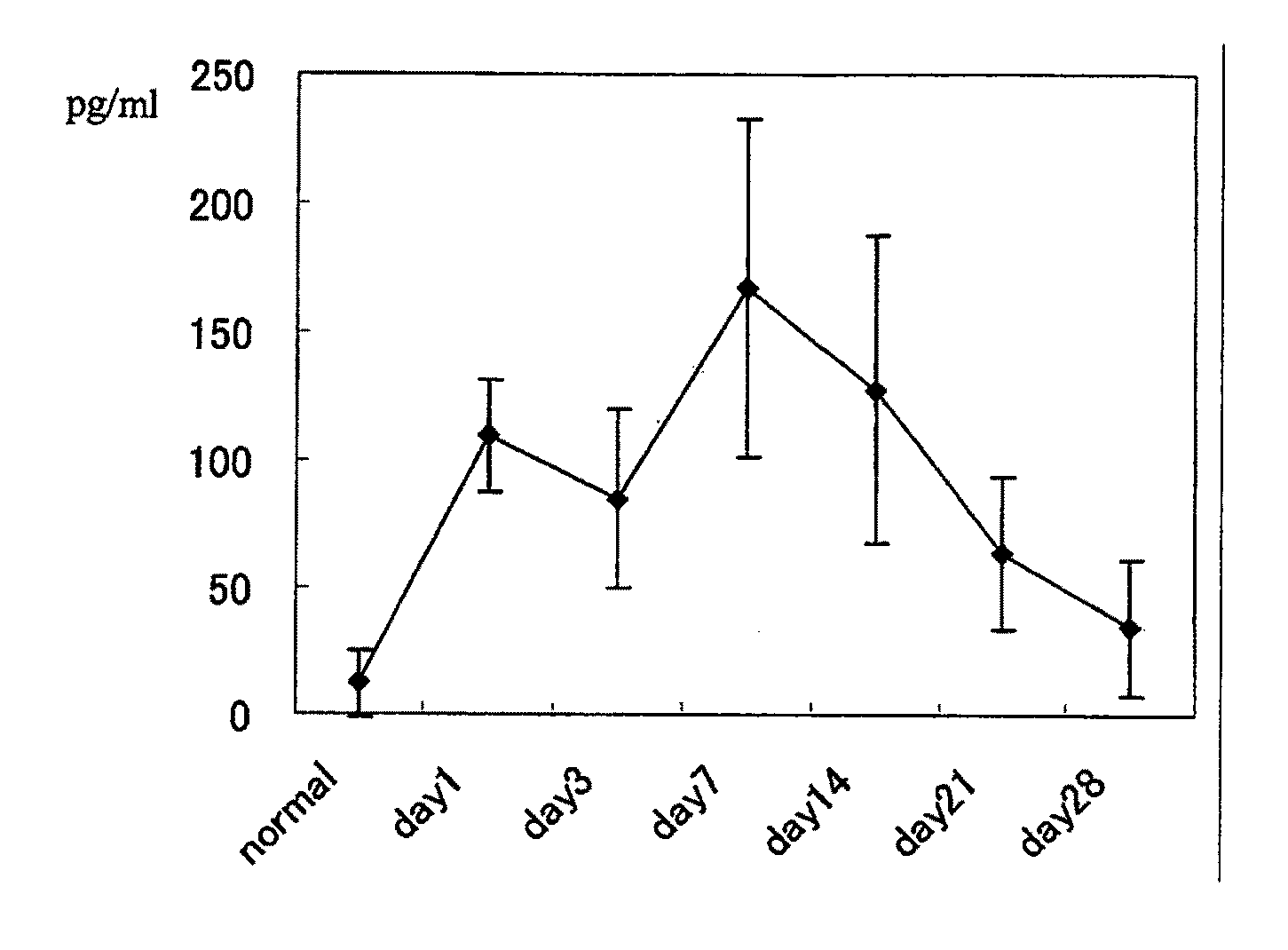
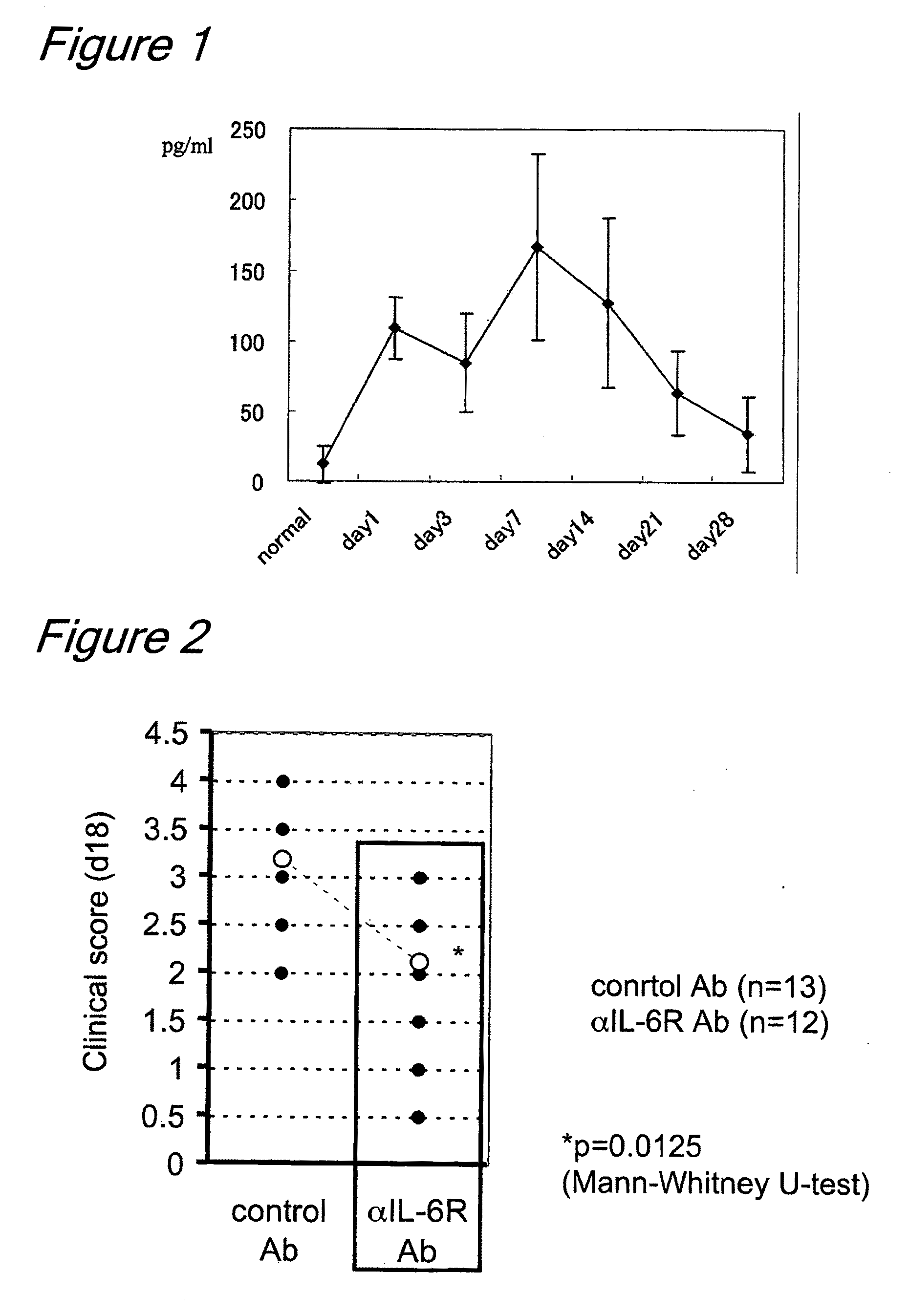
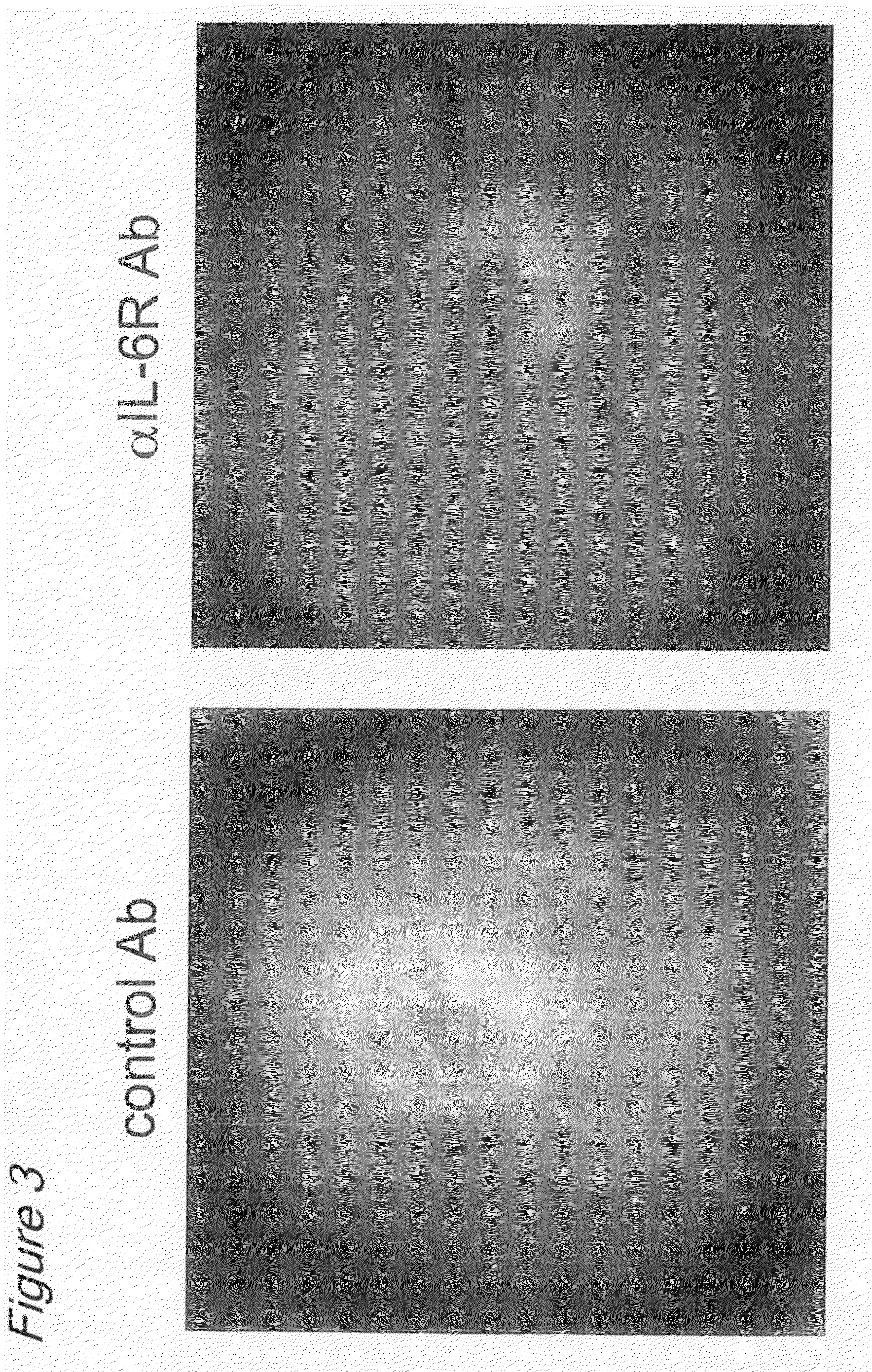
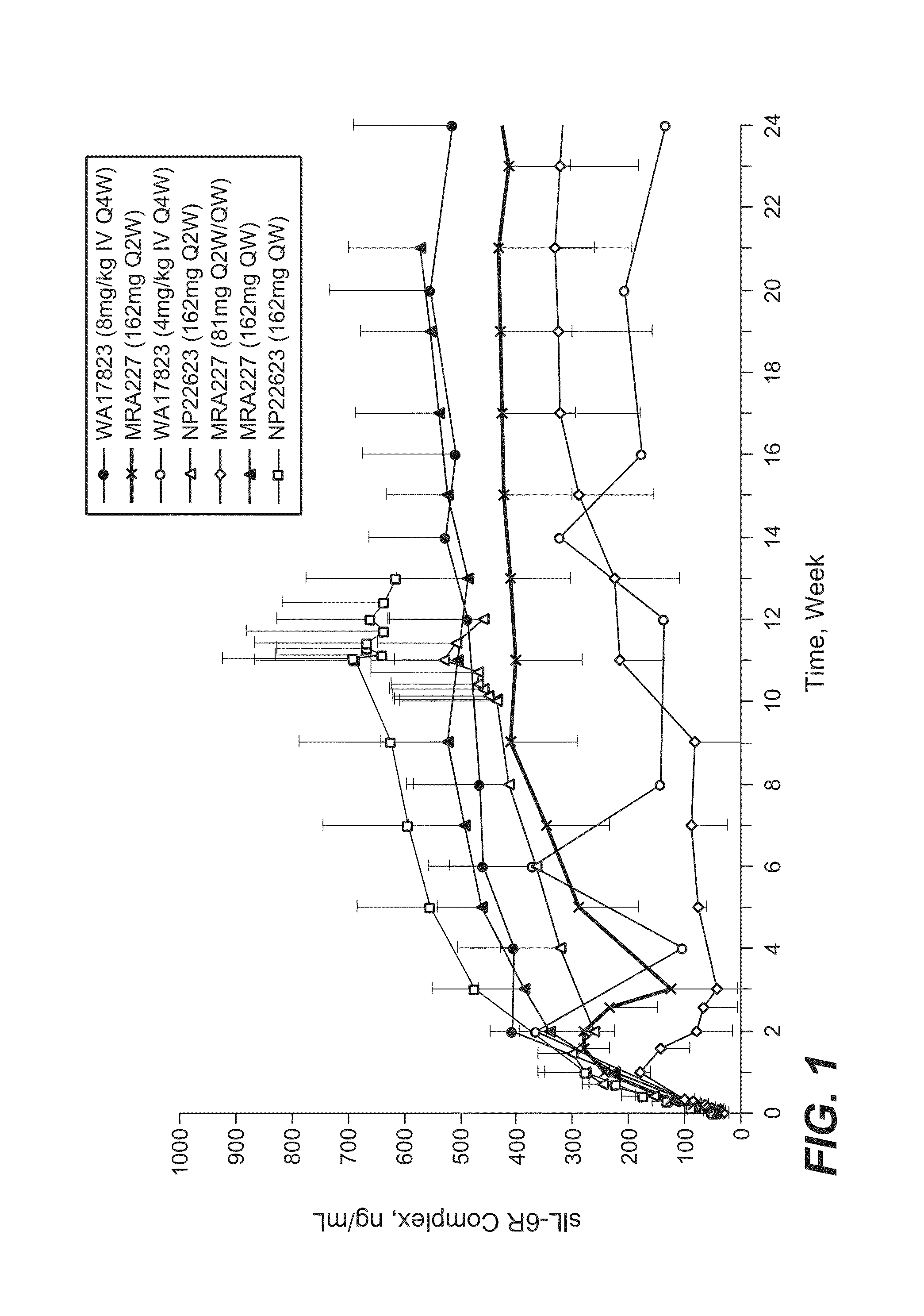
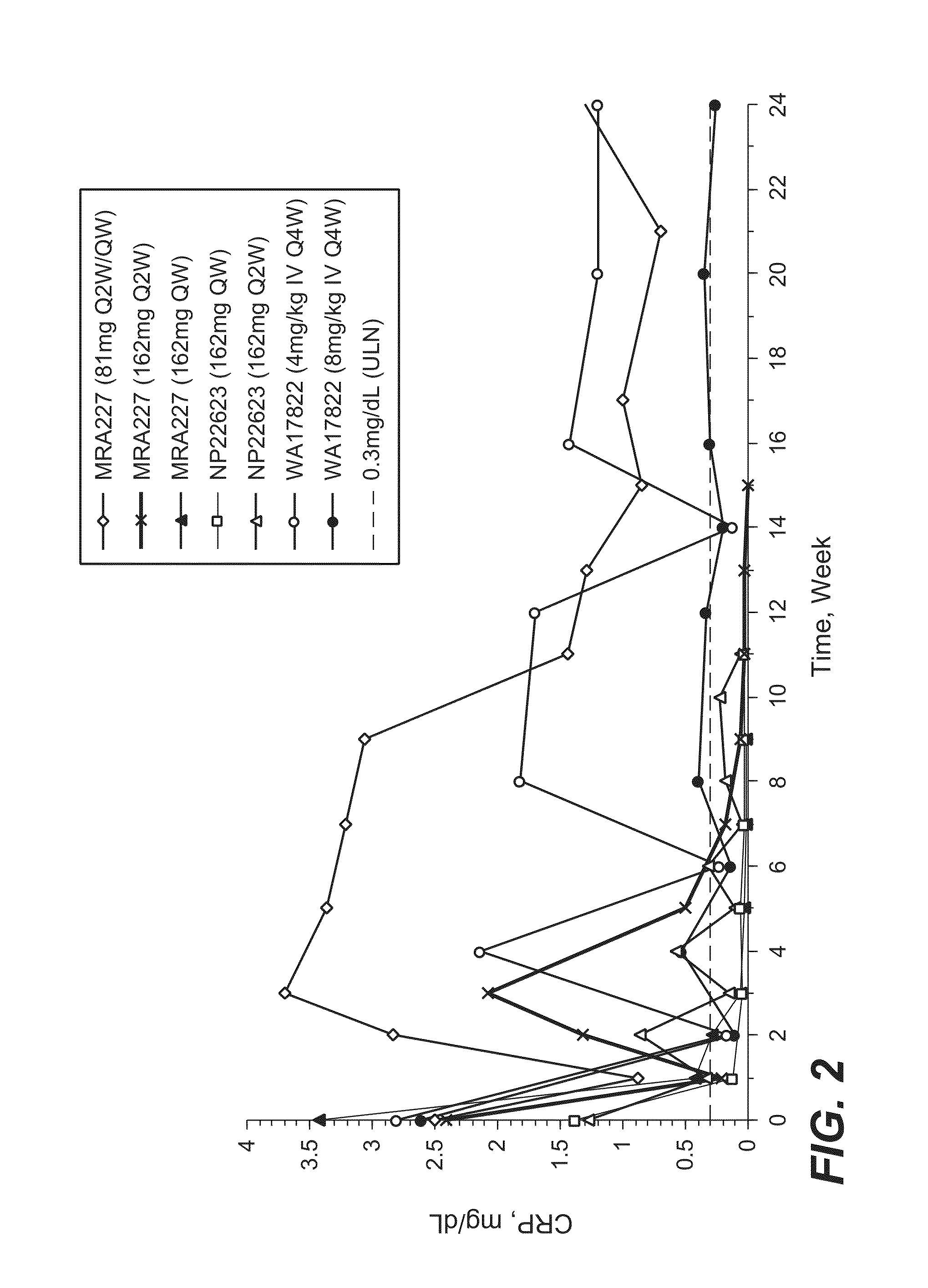
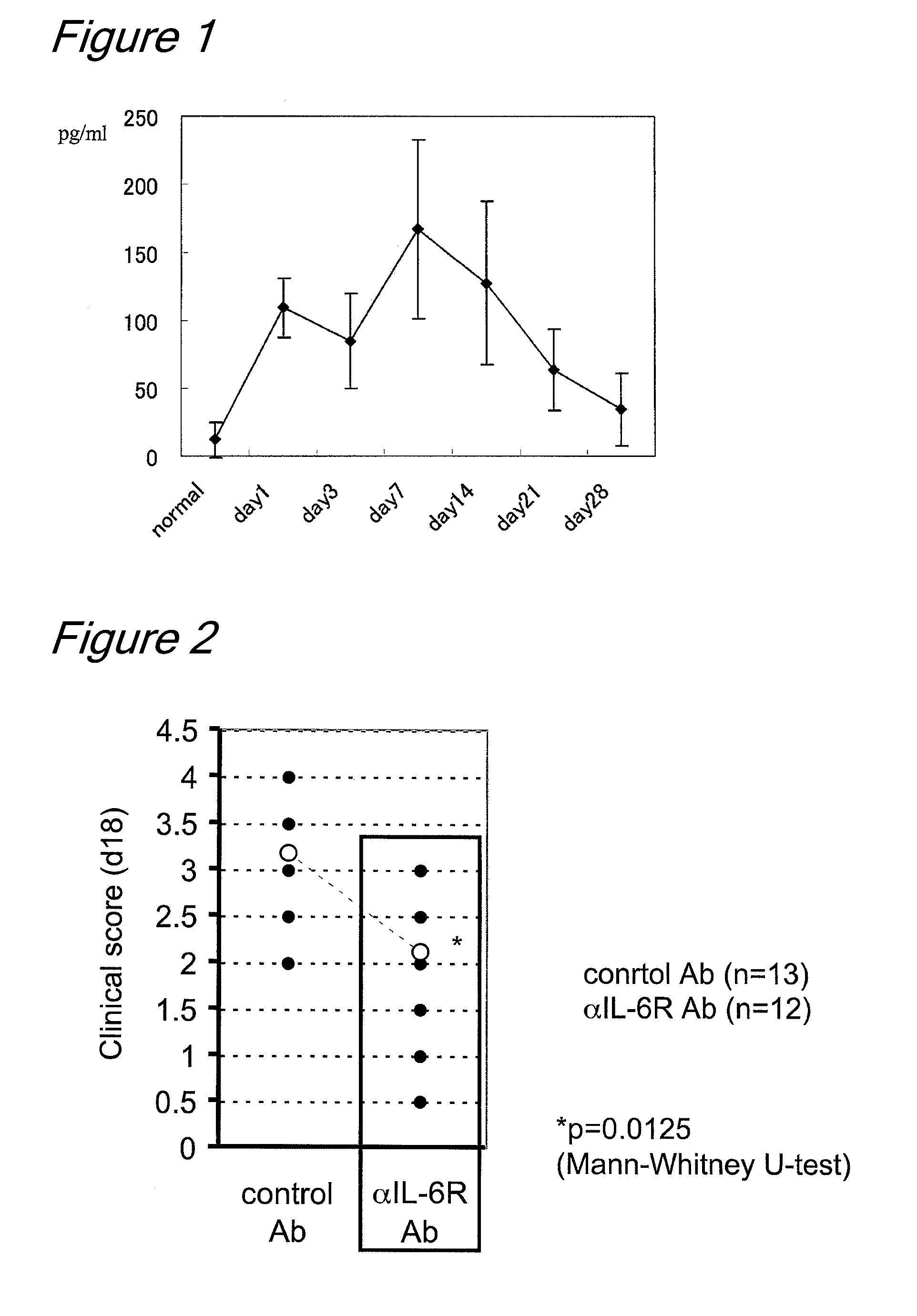
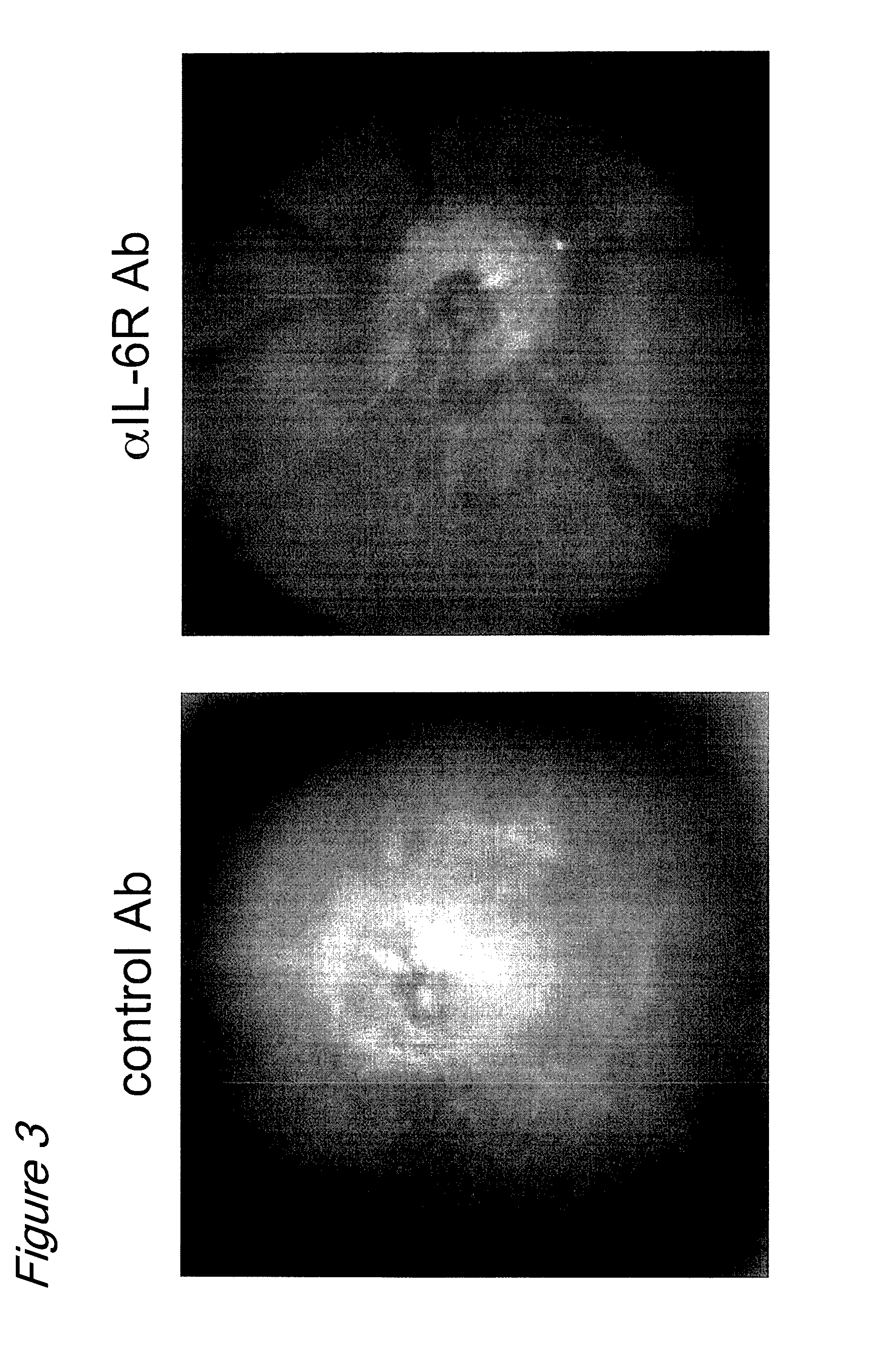
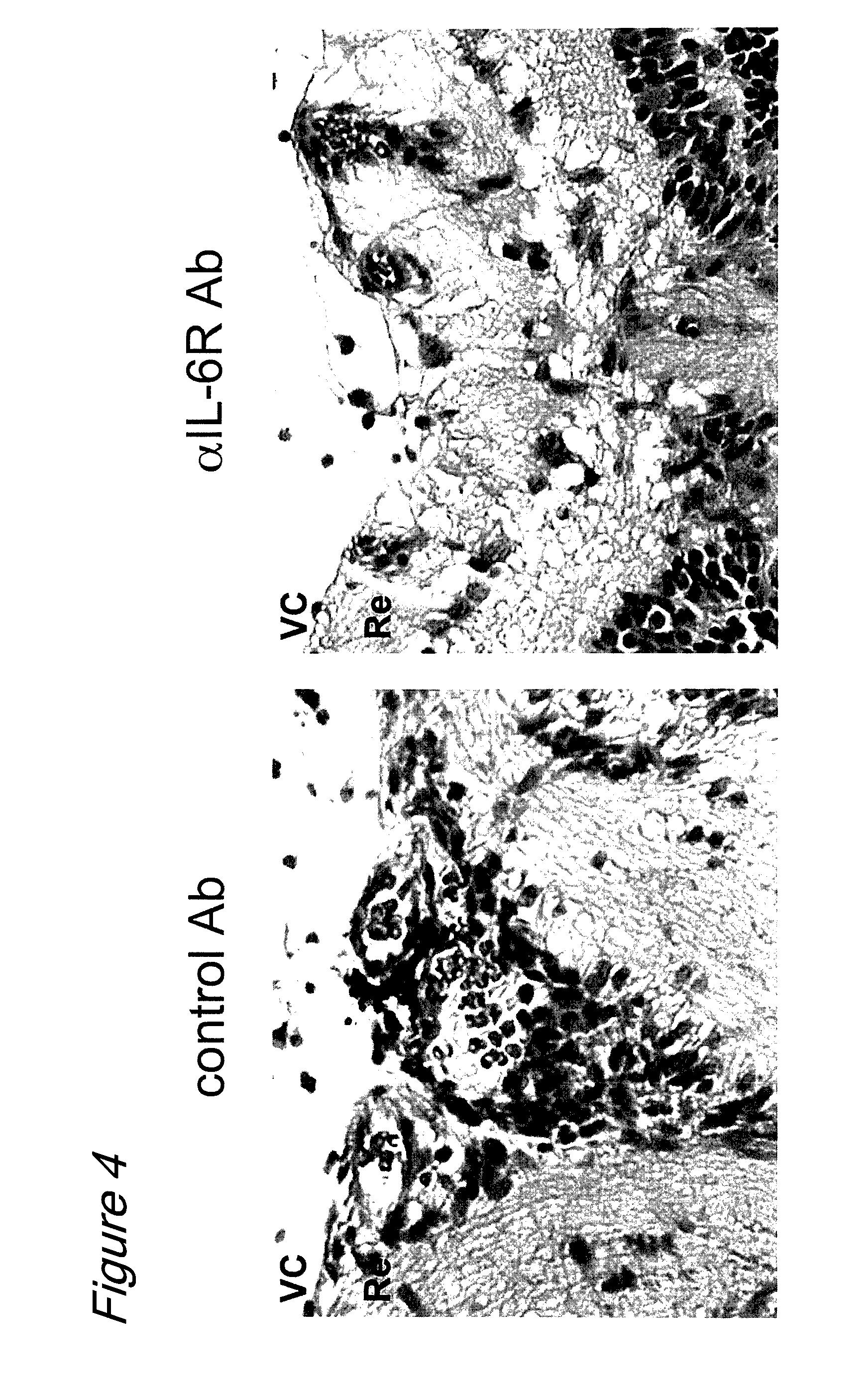
The invention provides an anti-human interleukin-6 (IL-6) receptor [beta]-chain monoclonal antibody. The cDNA and the amino sequence of a light-chain variable region at a Fab-fragment antigen-binding site of the antibody molecule are represented as the Seq ID No.1 and the Seq ID No.2, and the cDNA and the amino sequence of a heavy-chain variable region at the Fab-fragment antigen-binding site of the antibody molecule are represented as the Seq ID No.3 and the Seq ID No.4. The invention also provides a preparation method of the monoclonal antibody, and the applications of the monoclonal antibody in a drug for detecting and intervening in diagnosis and therapy of rheumatoid arthritis. In-vivo and in-vitro tests prove that the monoclonal antibody has a high affinity on positive cells of the IL-6 receptor [beta]-chain and can regulate and control the molecular mechanism of fibroblast-like synoviocytes IL-6 receptor [beta]-chain-RANKL-WNT5A signaling pathway of rheumatoid arthritis patients through antagonism, thereby achieving a result of alleviating immune function disorder and joint injury due to mediated bone metabolic imbalance of the rheumatoid arthritis patients.
Owner:SHANGHAI INST OF IMMUNOLOGY +3
Ciliary neurotrophic factor variants
InactiveUS9187542B2Loss of activityAvoid side effectsPeptide/protein ingredientsAnimals/human peptidesLow affinityNucleotide
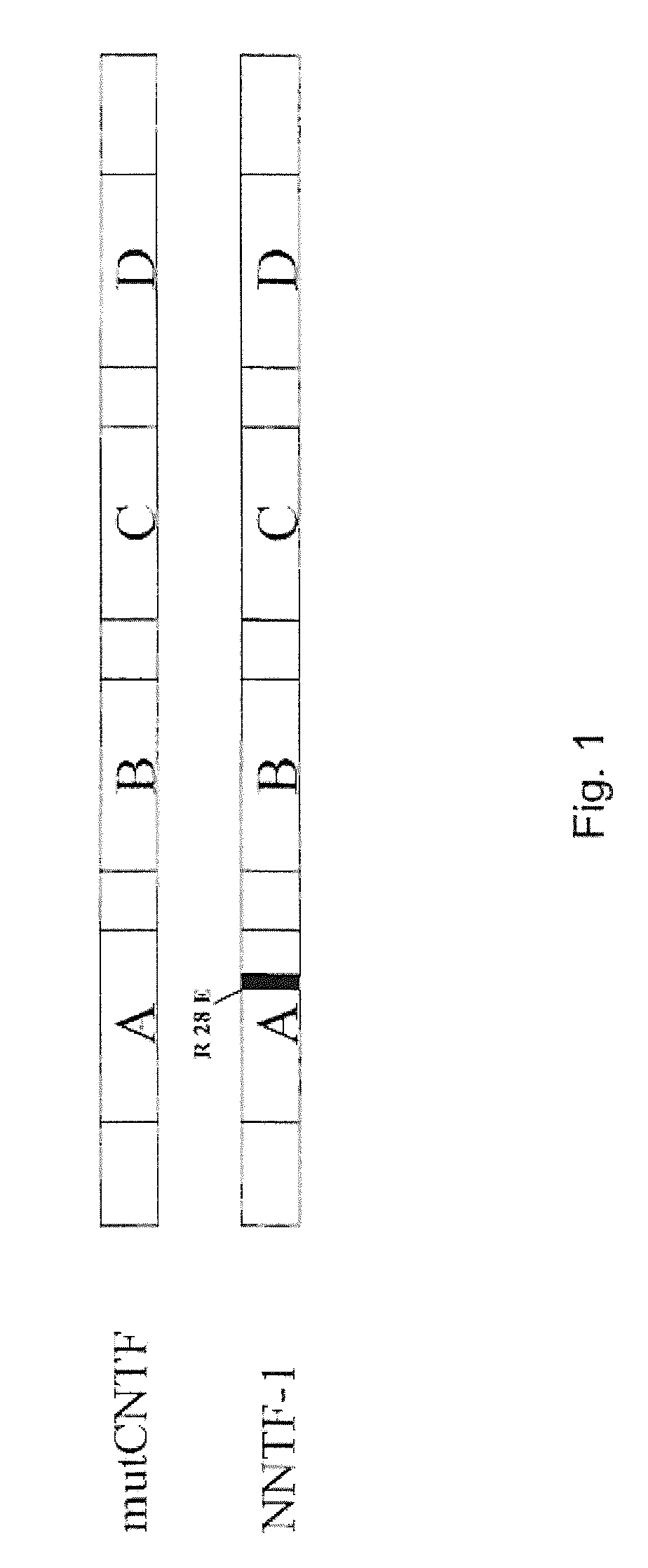
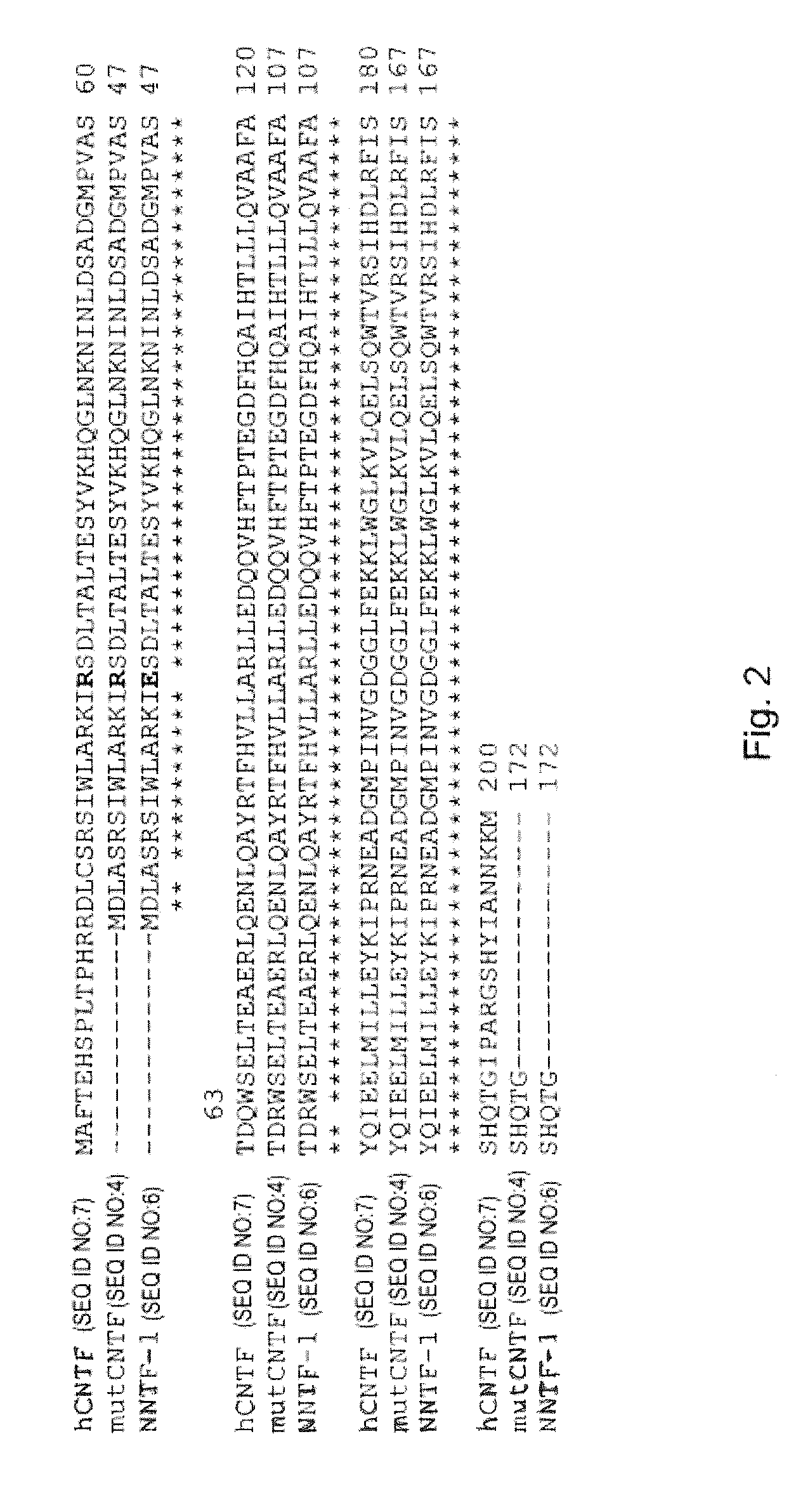
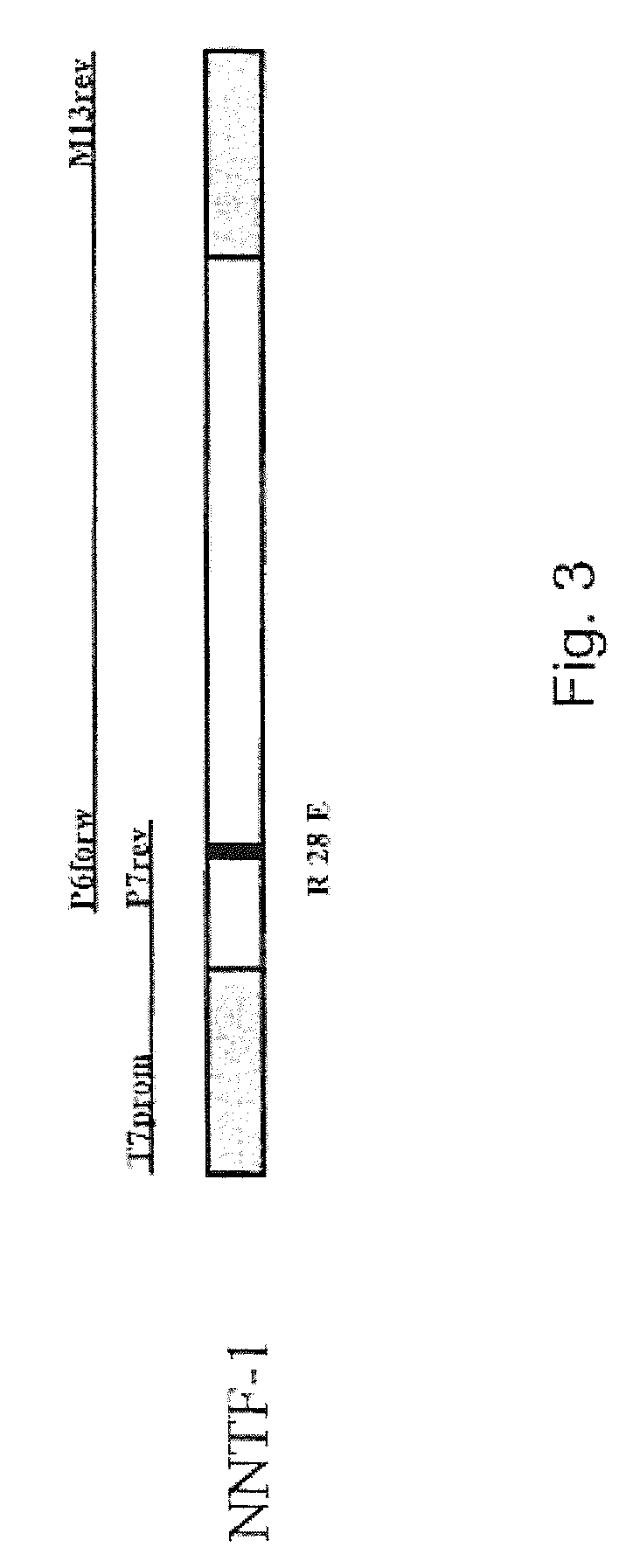
Nucleic acid molecule selected from the group consisting of (a) a nucleic acid molecule having a nucleotide sequence shown in SEQ ID: NO 1, (b) a nucleic acid molecule which encodes a peptide having an amino acid sequence shown in SEQ ID: NO 2, (c) a nucleic acid molecule whose complementary strand hybridizes to a nucleic acid molecule according to (a) or (b) and which codes for a peptide which binds to ciliary neurotrophic factor receptor (CNTFR), the peptide binding with lower affinity than ciliary neurotrophic factor to the interleukin-6 receptor (IL-6R), (d) a nucleic acid molecule whose nucleotide sequence differs from the nucleotide sequence of a nucleic acid molecule according to (c) due to the degenerated genetic code, the codon at positions 82-84 of the nucleic acid molecule according to (a) coding for a non-positively charged amino acid, and the peptide at position 28 shown in SEQ ID: NO 2 having a non-positively charged amino acid residue.
Owner:UNIVERSITY OF KIEL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











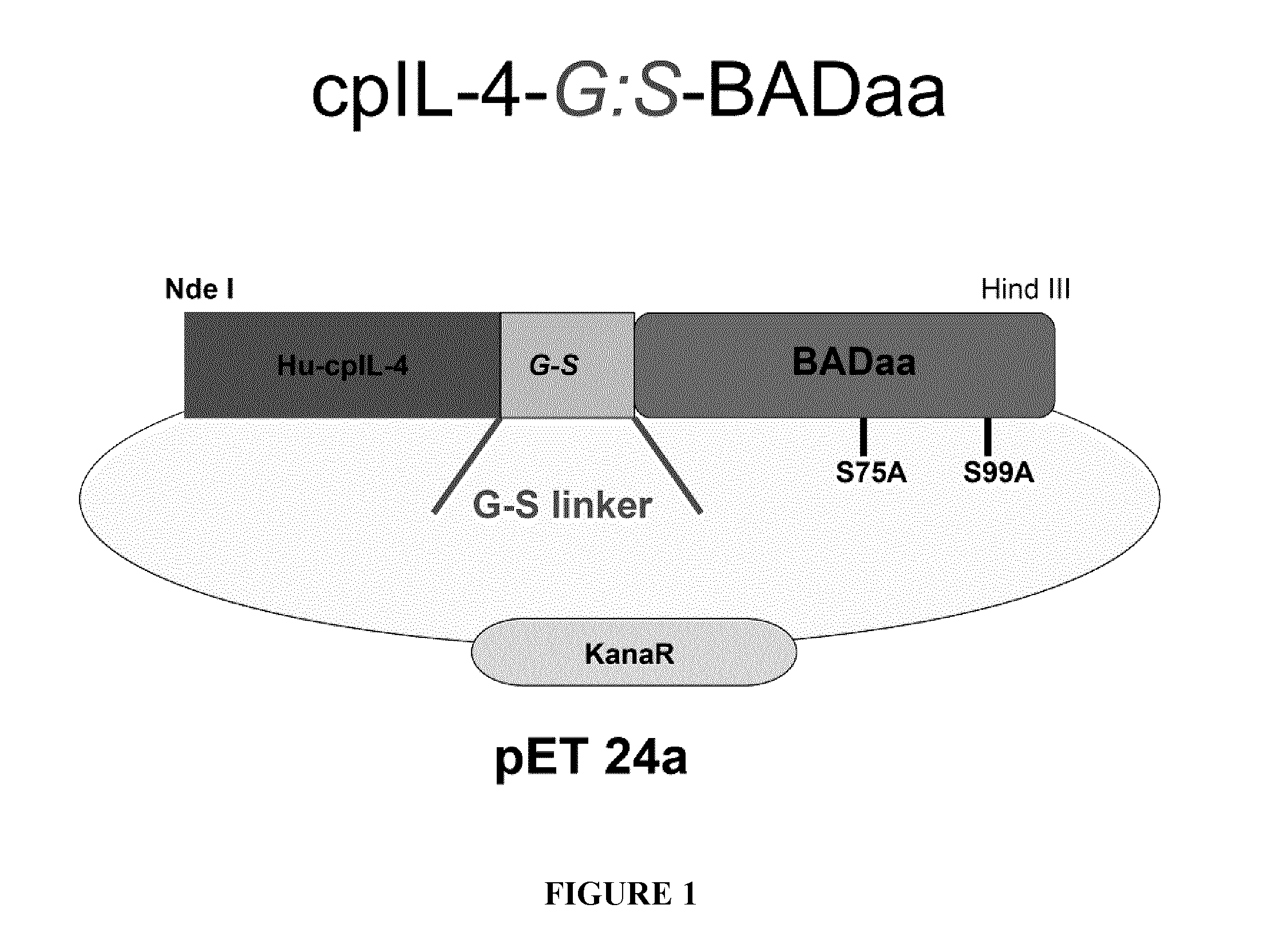

![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000011.PNG)
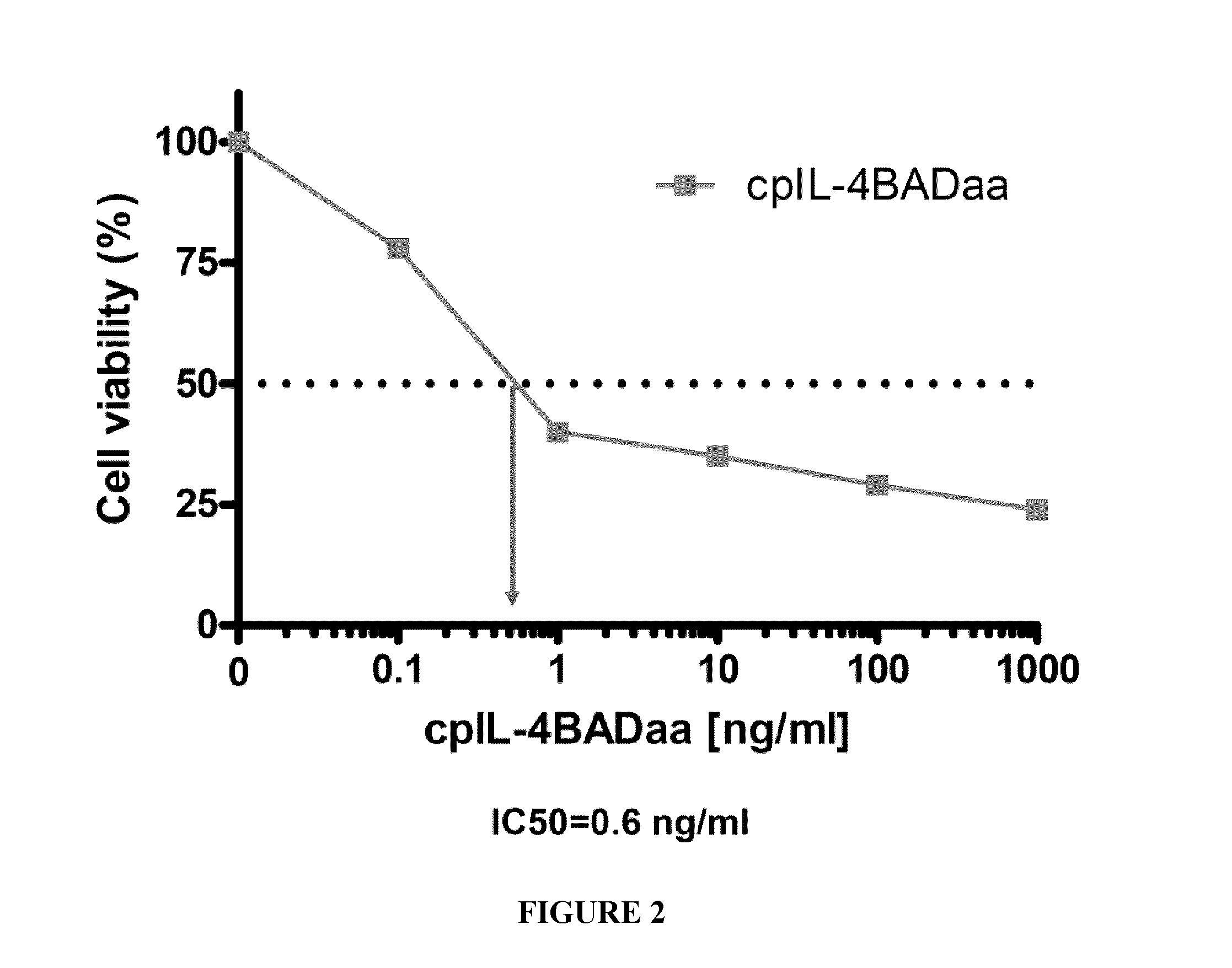
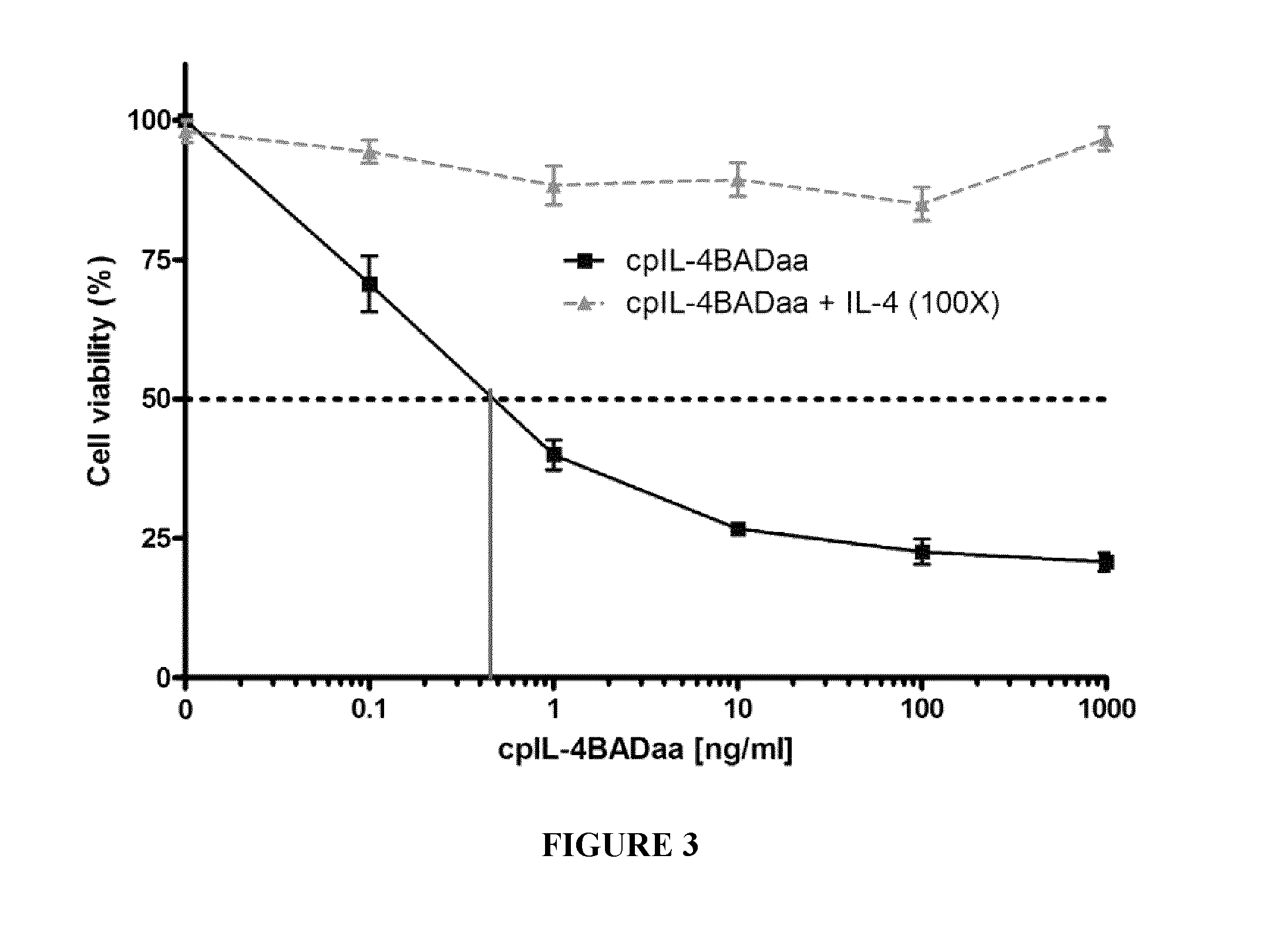
![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000012.PNG)
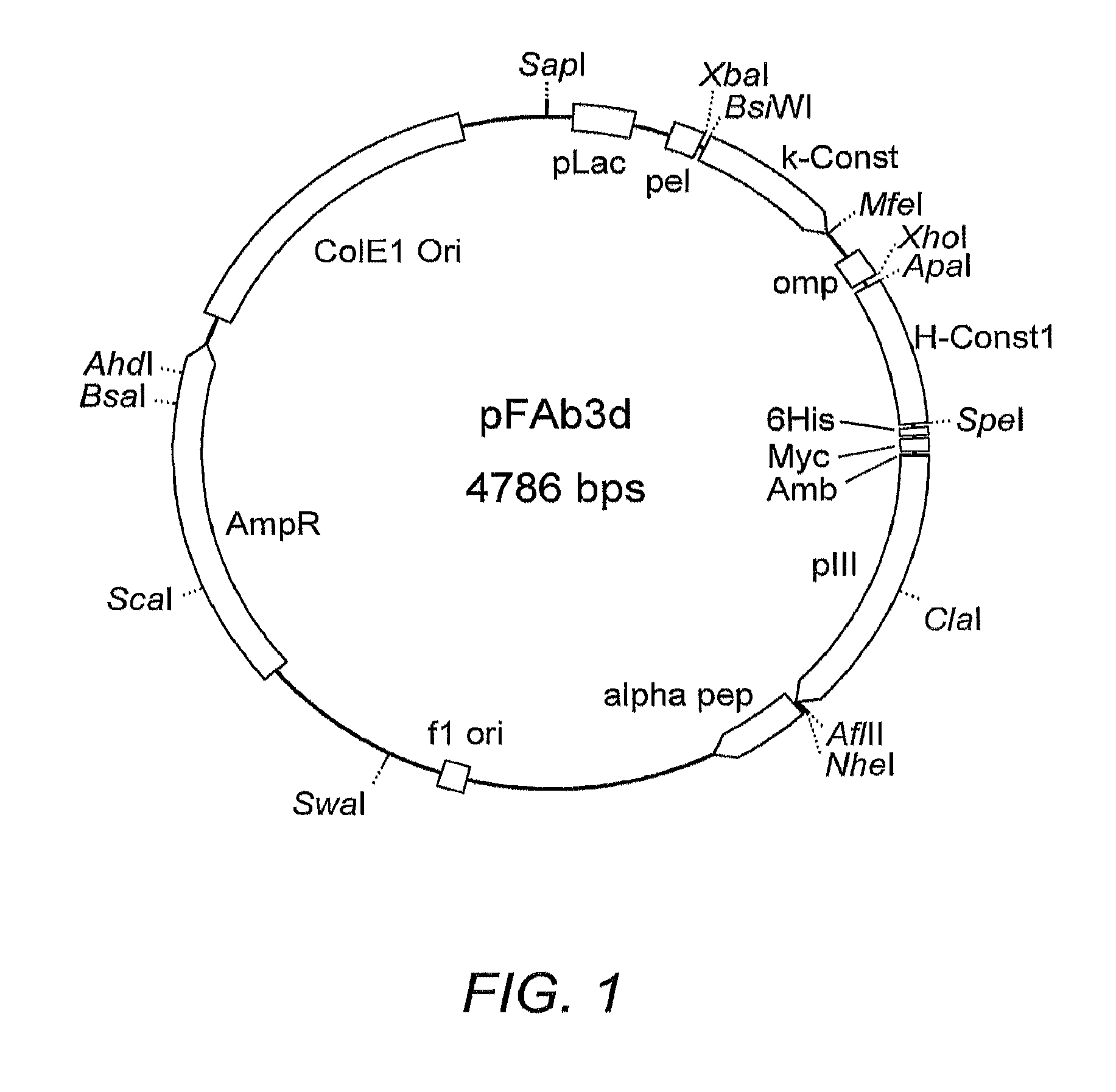
![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000021.PNG)