Subcutaneously administered Anti-il-6 receptor antibody
a technology of il-6 receptor and antibody, which is applied in the field of subcutaneous administration of anti-il-6 receptor antibody, can solve the problems of inconclusive data to date, and certain histocompatibility antigens are associated with poorer outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Studies Identifying Fixed Dose of Anti-IL-6R Antibody for Subcutaneous (SC) Administration
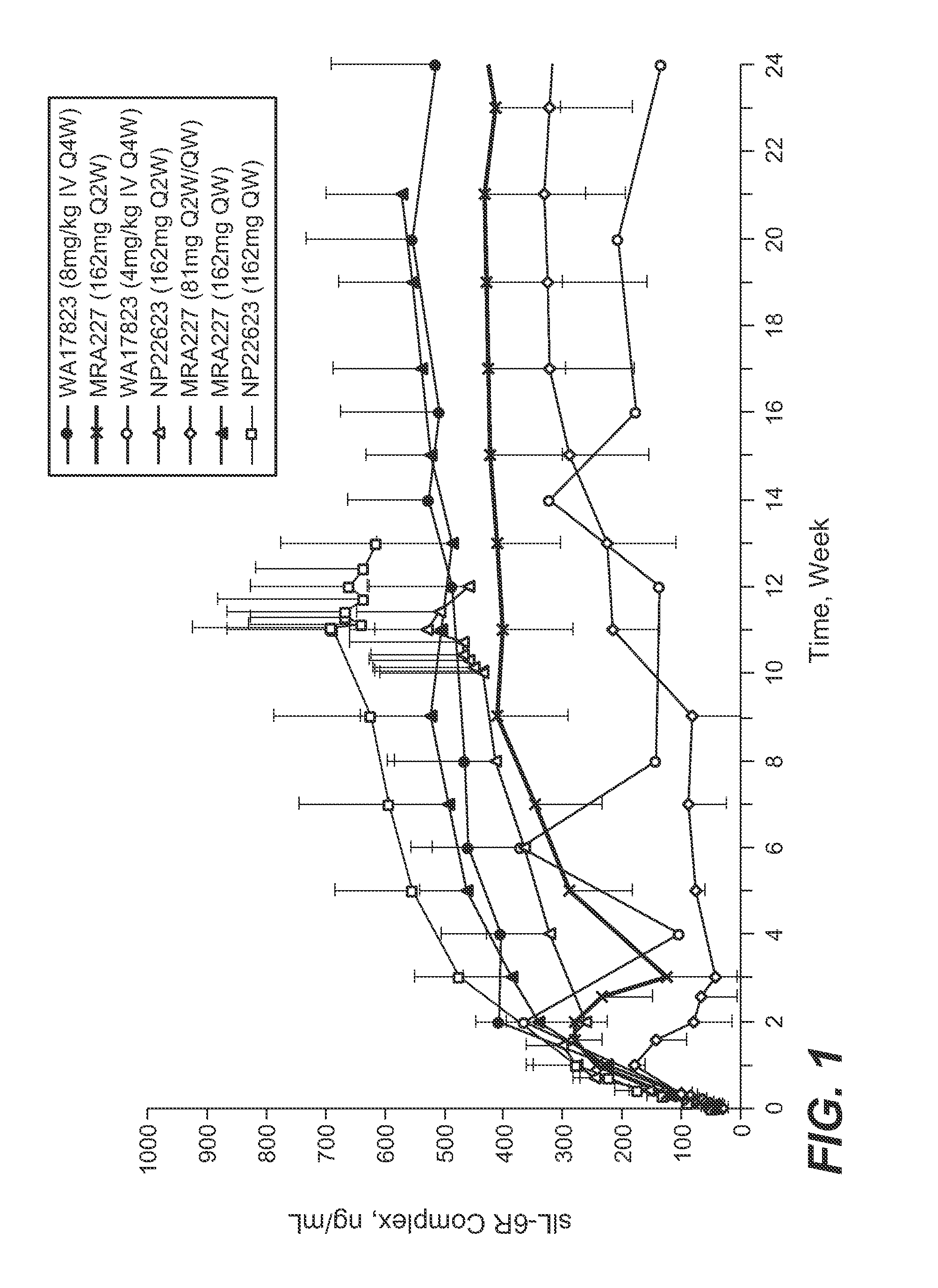
[0180]The selection of 162 mg anti-IL-6R antibody (tocilizumab, TCZ) subcutaneously administered every week (SC QW) was based on results from four phase 1 / 2 studies, including two phase 1 studies in healthy subjects (WP18097 and BP22065), one phase 1 / 2 study in Japanese RA patients (MRA227), and one phase 1b study in Caucasian RA patients (NP22623). Further details regarding these SC studies and the four studies from which data are drawn for comparison are provided in Table 1.
TABLE 1Clinical Pharmacology Studies Following SC Administration of TCZ in Healthy Subjects and RA PatientsStudy No.TCZ Treatment / CountryObjectivesDesign and PopulationDoseDurationSample SizeStatusWP18097PK from a pilot SCSingle center, single-Group 1: 160 mgSingle dose, PKN = 20 total andCompletedFranceformulation,blind, placebo-SCsampling up to Day 14,n = 12 for SCabsolutecontrolled, randomized,Group 2: 160 mgon...
example 2
Clinical Study of Sc Anti-IL-6 Receptor Antibody in RA
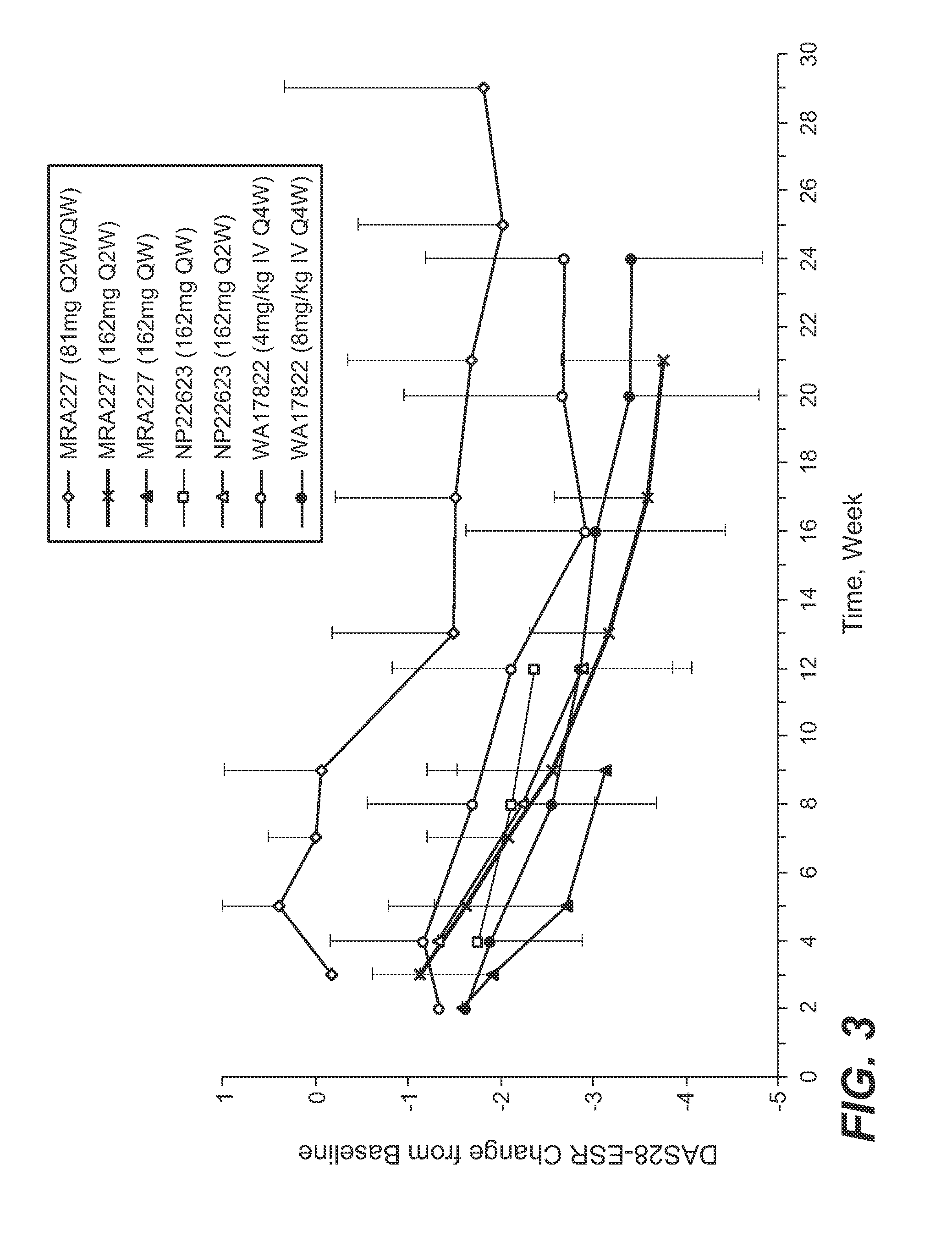
[0200]This is a Phase 3, 2-arm, 2-year, randomized, double-blind, double-dummy, active controlled, parallel group multicentre trial in patients with moderate to severe, active RA who currently have an inadequate response to a stable dose of DMARDs that may include one or more anti-TNF biological agent. The primary endpoint will be evaluated at 24 weeks. The overall design of this example is shown in FIG. 5. The formulation is that as in Example 1.
[0201]The screening visit can occur up to 21 days (or up to 56 days if a washout period is required for longer than 21 days) prior to the baseline randomization visit. Patient eligibility will be determined at the screening and baseline visits, at which time the patient will be randomized. The number of patients that have failed previous anti-TNF treatment will be limited to approximately 20% of the total study population.
Inclusion Criteria
[0202]1. Age ≧18 years[0203]2. Rheumatoid arthri...
example 3
Anti-IL-6R Antibody Sc for Inhibiting Progression of Joint Damage
[0222]This is a Phase 3, 2-arm, 2-year, randomized, double-blind, placebo-controlled, parallel group multicenter trial in patients with moderate to severe, active RA who currently have an inadequate response to DMARD(s) that may include one or more anti-TNF-α agent. The primary endpoint will be evaluated at Week 24.
[0223]The overall design is shown in FIG. 6. The screening visit can occur up to 21 days (or up to 56 days if a washout period is required for longer than 21 days) prior to the baseline randomization visit. Patient eligibility will be determined at the screening and baseline visits. At baseline, patients will be randomized. The number of patients that have failed previous anti-TNF-α treatment will be limited to approximately 20% of the total study population. The formulation is that as in Example 1. The formulation is administered using a pre-filled syringe (PFS) or auto-injector (AI) device.
TCZ Q2W Dosing
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com