Generation of antigen specific T suppressor cells for treatment of rejection
a technology of t suppressor cells and antigens, which is applied in the field of antigen specific t suppressor cells for treatment of rejection, can solve the problems of difficult generation of allospecific human suppressor t cells (ts), increasing the risk of infections and malignancies for recipients, and sub-s /sub, so as to prevent autoimmune diseases, prevent autoimmune diseases, and prevent autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
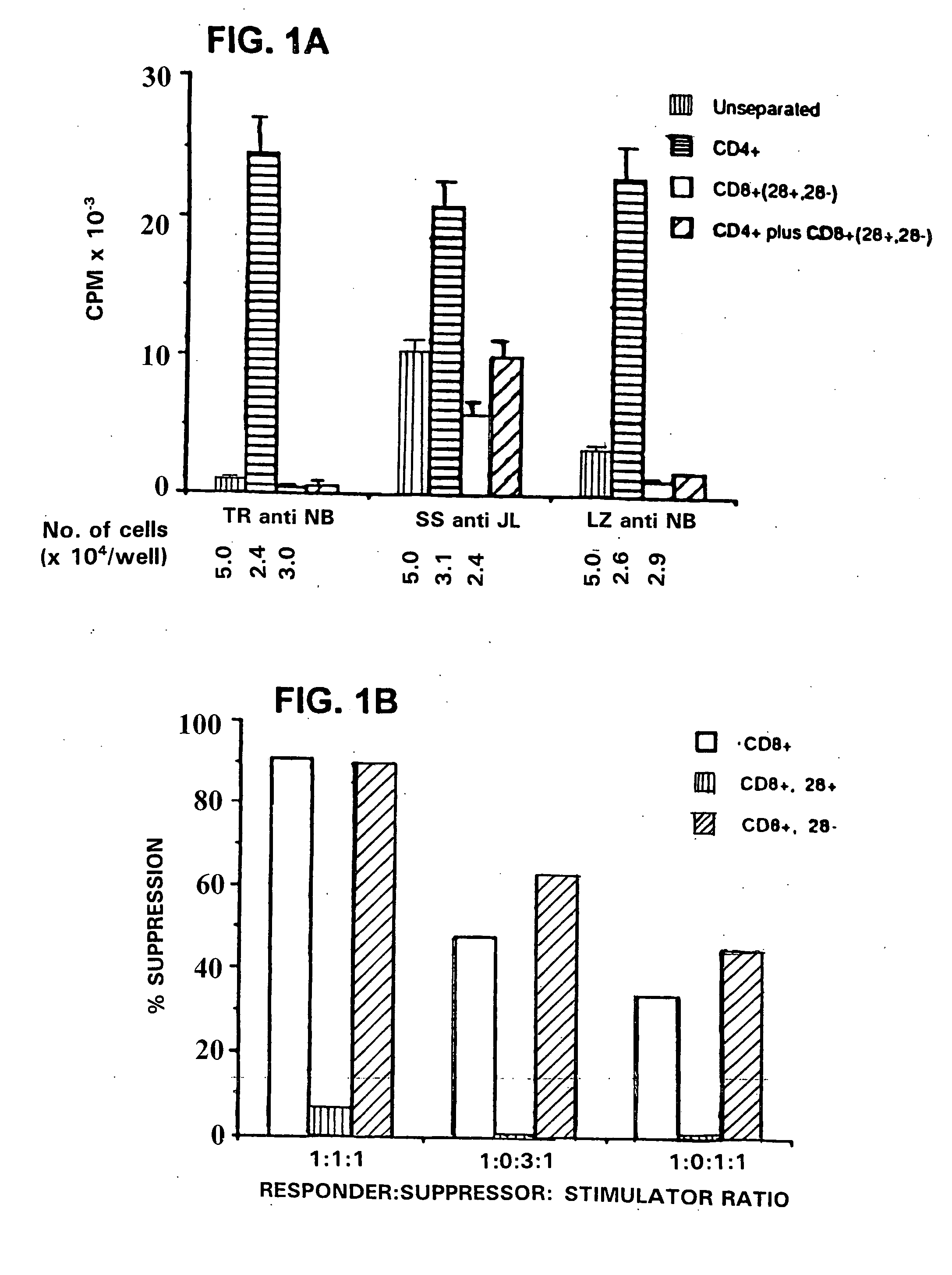
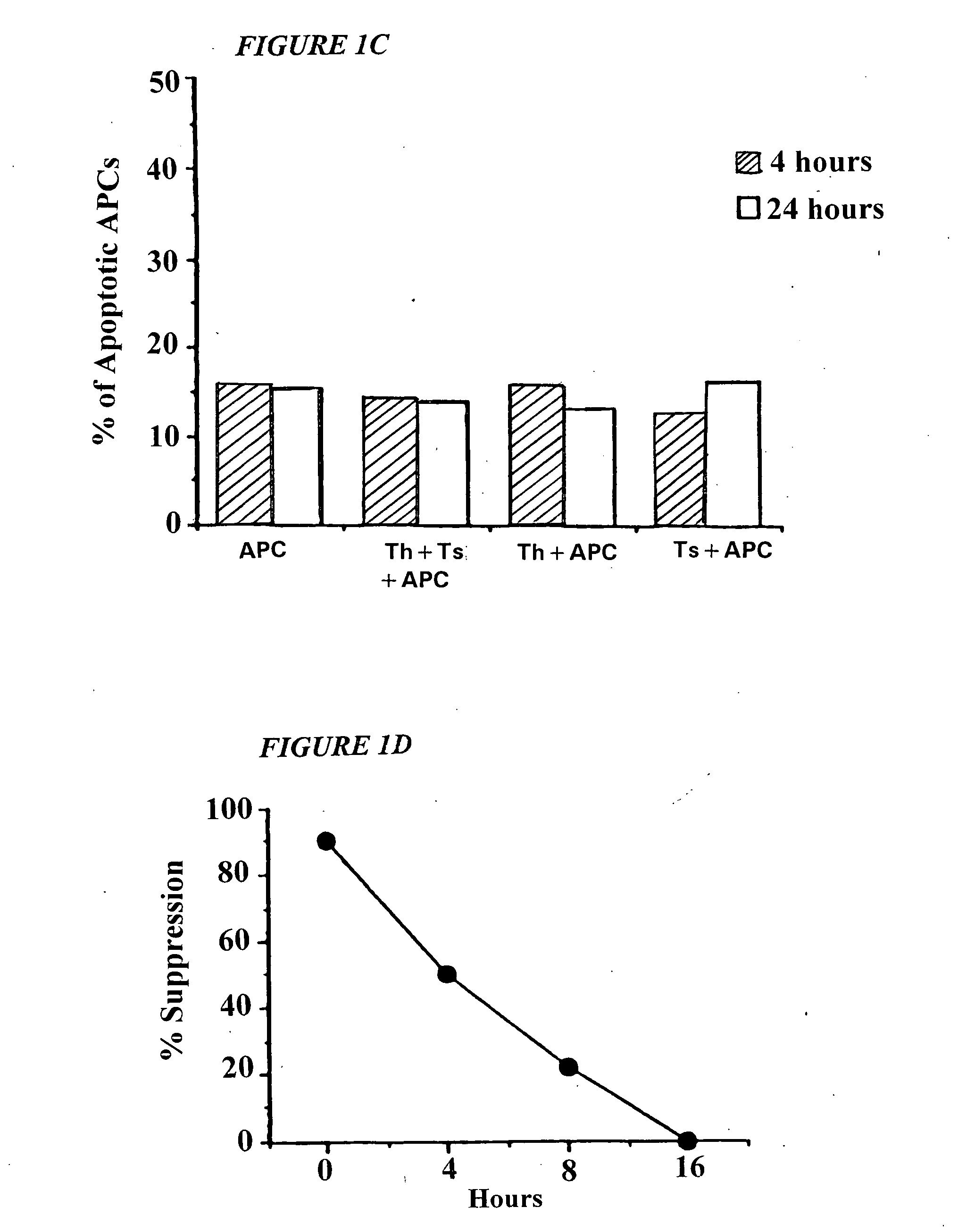
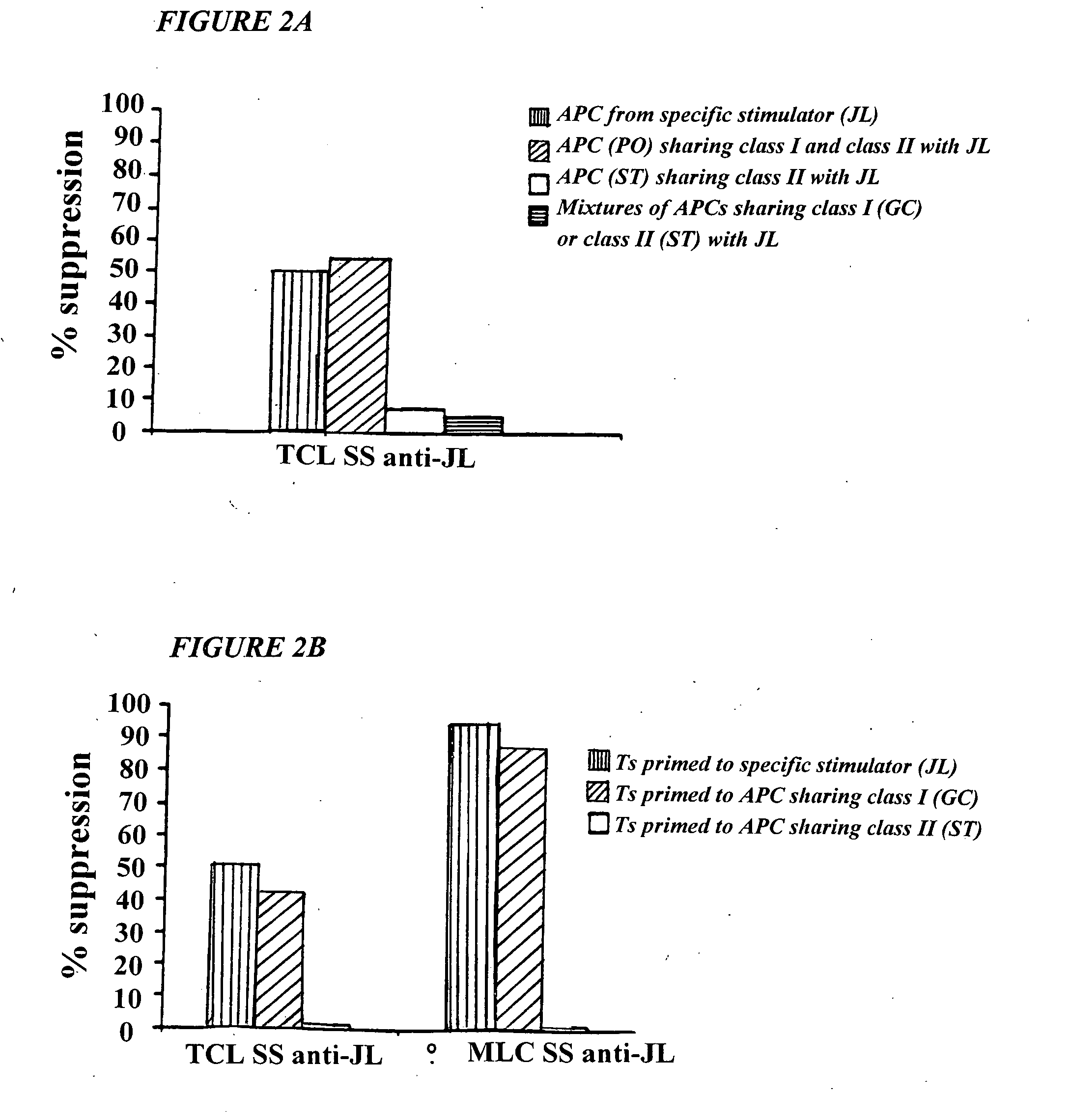
[0081] This invention provides a method of generating antigen specific allospecific human suppressor CD8+CD28− T cells which comprises: a) obtaining peripheral blood T cells from a subject; b) stimulating by multiple priming a T cell line from the T cells obtained in step (a) with allogeneic antigen presenting cells (APCs), said APCs expressing an MHC class I antigen recognized by the primed T cell line and an MHC class II antigen recognized by CD4+ T helper cells from said primed T cell line; c) isolating primed CD8+ T cells and CD4+ T helper cells from the T cell line stimulated in step (b); d) isolating primed CD8+CD28− T cells from the isolated primed CD8+ T cells of step (c); e) detecting suppression by the primed CD8+CD28− T cells isolated in step (d) of interaction between the CD4+ T helper cells isolated in step (c) and allogeneic antigen presenting cells (APCs) expressing the same MHC class I antigen and the same MHC class II antigen expressed by the APCs used to stimulate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com