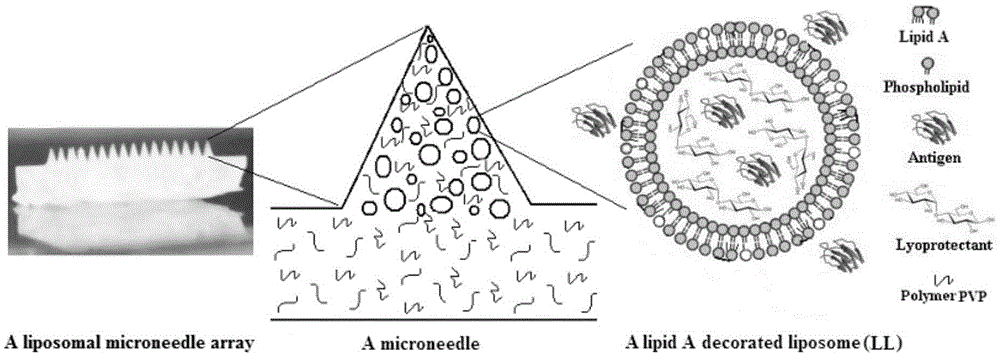
Microneedle array vaccine adjuvant transmission system built by using lipid modifying carrier
A technology of microneedle array and vaccine adjuvant, which can be used in antibody medical ingredients, medical preparations containing active ingredients, allergic diseases, etc. Efficiency of uptake and utilization, elimination of hindrance, effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] With OVA as antigen, SPC / LA (100:1, mole ratio) as membrane material, total lipid / OVA (20:1, mass ratio), 10% trehalose, 20% PVPk30 (excipient) solution as In the water phase, liposomes were prepared by a film dispersion method to form OVA / Lipid A-liposomes (OVA / LL) with an average particle diameter of 250 nanometers, a zeta potential of 6 mV, and an encapsulation efficiency of 10%. Then mix LL with aluminum phosphate (average particle size 500 nm) (1:5, W / W), and fill it into the pinholes (5×5 holes) of the microneedle array mold prepared by polydimethoxysilane by decompression , then covered with 10% trehalose, 20% PVPk30 (excipient) solution, freeze-dried to remove water, and peeled off to obtain OVA / LL-MAV (6 × 6 microneedles, substrate 0.65 × 0.65cm 2 , fixed on the substrate per microneedle: 250×250×500 μm 3 square cone). After OVA / LL-MAV was stored for 2 weeks, H1sAg-MLL was recovered after hydration, and the above indicators had no significant changes...
Embodiment 2
[0037]
[0038] The surface antigen (hemagglutinin1 antigen, H1sAg) of influenza A (H1N1) virus (influenza A (H1N1) virus) was used as the antigen, and SPC / MPC / MPLA / DOTAP (20:1:0.05:1, mole ratio) was used as the membrane material. Lipid / H1sAg (20:1, mass ratio), using 10% trehalose, 20% PVPk30 (excipient) solution as the water phase, prepared liposomes by reverse evaporation method, forming an average particle size of 320 nanometers, zeta potential MPC / Lipid A double modified liposome (MLL) with 13mV and 59% encapsulation efficiency. Afterwards, the MLL was filled into the pinholes of the microneedle array mold prepared by polydimethoxysilane (6×6 holes) by decompression, and then covered with 10% trehalose, 20% PVPk30 (excipient) solution, and then The mold was placed in an anhydrous calcium chloride drying dish to dry for 8 hours, and peeled off to obtain H1sAg-MLL-MAV (6×6 microneedles, substrate 0.65×0.65cm 2 , fixed on the substrate per microneedle: 100×100×3.14×500 / 3...
Embodiment 3
[0040]
[0041] HBsAg was used as antigen, SPC / GMO / LPS (20:4:0.05, mole ratio) was used as membrane material, total lipid / HBsAg (20:1, mass ratio), 10% sucrose, 30% PVPk17 (excipient ) solution is an aqueous phase, and multivesicular liposomes are prepared by emulsification-evaporation method to form LPS-modified multivesicular liposomes (LML) with an average particle diameter of 520 nanometers, a zeta potential of -12mV, and an encapsulation efficiency of 72%. Afterwards, the MLL is filled into the pinholes of the microneedle array mold prepared by polydimethoxysilane by decompression (6 × 6 holes), and then covered with 10% sucrose, 30% PVPk17 (excipient) solution, and then the mold Place in anhydrous calcium chloride drying dish to dry for 8 hours, and peel off to obtain HBsAg-LML-MAV (8×8 microneedles, substrate 0.75×0.75cm 2 , fixed on the substrate per microneedle: 250×250×500 μm 3 Square column needle body +250×250×50 / 3 micron 3 square cone tip). After HBsAg-LML-MA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com