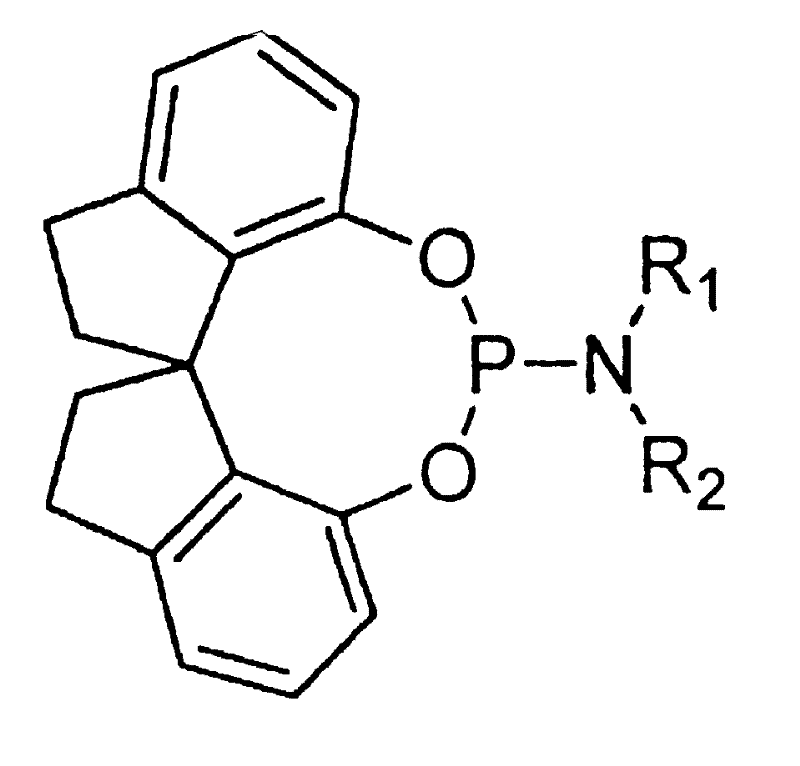
Spirocyclophophorous amine
A technology of spirocyclic phosphoramidites and right-handed spirocyclic phosphoramidites, which is applied in the field of synthesis of chiral ligand compounds, can solve problems such as poor stability and inconvenience, and achieve high stereoselective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of (±)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-dimethylphosphoramidite
[0017] In a 250ml reaction bottle, add 2g (8mmol) (±)-spirocyclic diphenol, 2ml (9mmol) hexamethylphosphoramidite, 20ml anhydrous toluene, and react in a nitrogen stream at 100°C for 2 hours, TLC tracking The response is complete. The resulting reaction solution was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1:16) to obtain white crystals, (±)-O,O'-[7,7'-(1,1'-spiro Dihydroindane)]-N,N-dimethylphosphoramidite 2.37g, yield 92%, melting point 117-118°C.
[0018] 1 H NMR (300MHz, CDCl 3 ): δ=7.24-6.98(m, 4H), 6.93(d, J=8.1Hz, 1H), 6.64(d, J=8.1Hz, 1H), 3.13-3.01(m, 2H), 2.82(dd, J=16.2, 8.1 Hz, 2H), 2.34 (s, 3H), 2.31 (s, 3H), 2.30-2.18 (m, 2H), 2.08-1.80 (m, 2H). 31 P NMR (CDCl 3 ): δ=124.96. Elemental analysis (theoretical value), C 19 h 20 NO 2 P: C69.95 (70.13); H6.06 (6.21); N4.40 (4.30).
Embodiment 2
[0020] Preparation of (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-dimethylphosphoramidite
[0021] Substitute (±)-spirocyclic diphenol in Example 1 with (R)-spirocyclic diphenol, the preparation method, chemical structural formula, spectral data and elemental analysis are the same as in Example 1, with a melting point of 84-85°C, [α] 20 D =+525 (c=1.14×10 -2 , CHCl 3 ).
Embodiment 3
[0023] Preparation of (S)-O, O'-[7,7'-(1,1'-spirodihydroindene)-N,N,N-dimethylphosphoramidite
[0024] The (±)-spirocyclic diphenol in Example 1 is replaced with (S)-spirocyclic diphenol, the preparation method, chemical structural formula, spectral data and elemental analysis are the same as in Example 1, with a melting point of 84.5-85.5°C, [α] 20 D =-519 (c=0.92×10 -2 , CHCl 3 )
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com