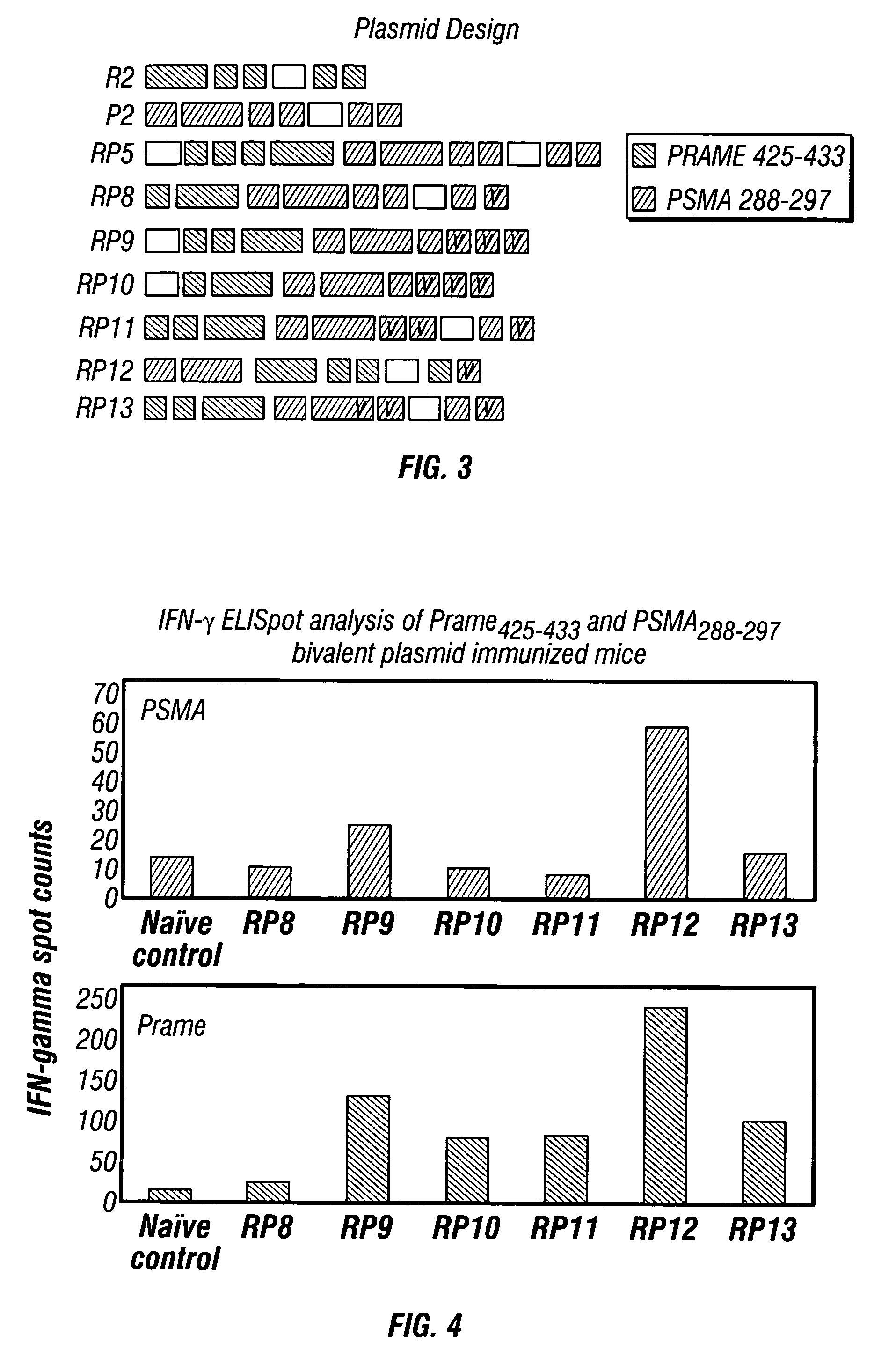
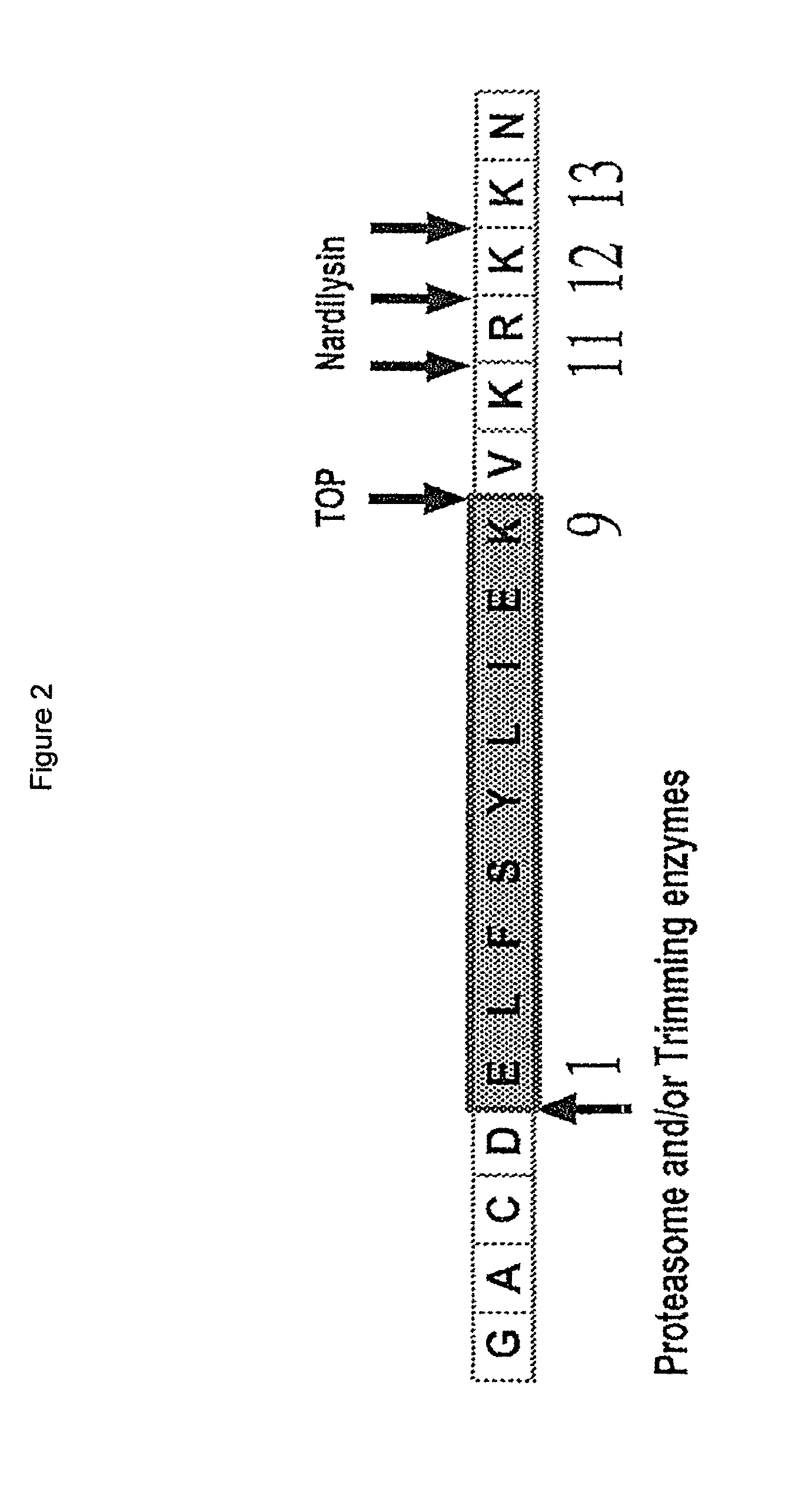
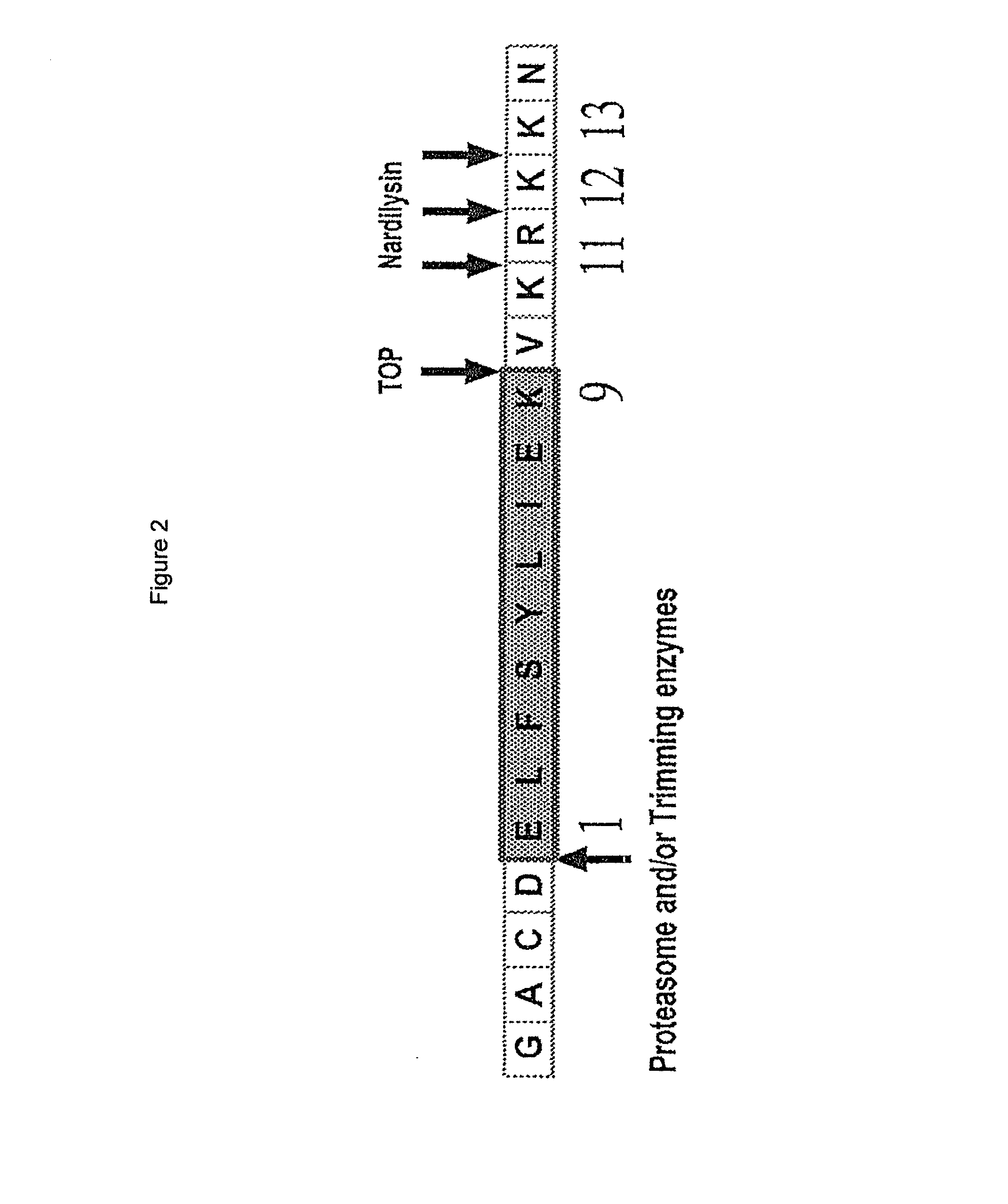
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "PRAME" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
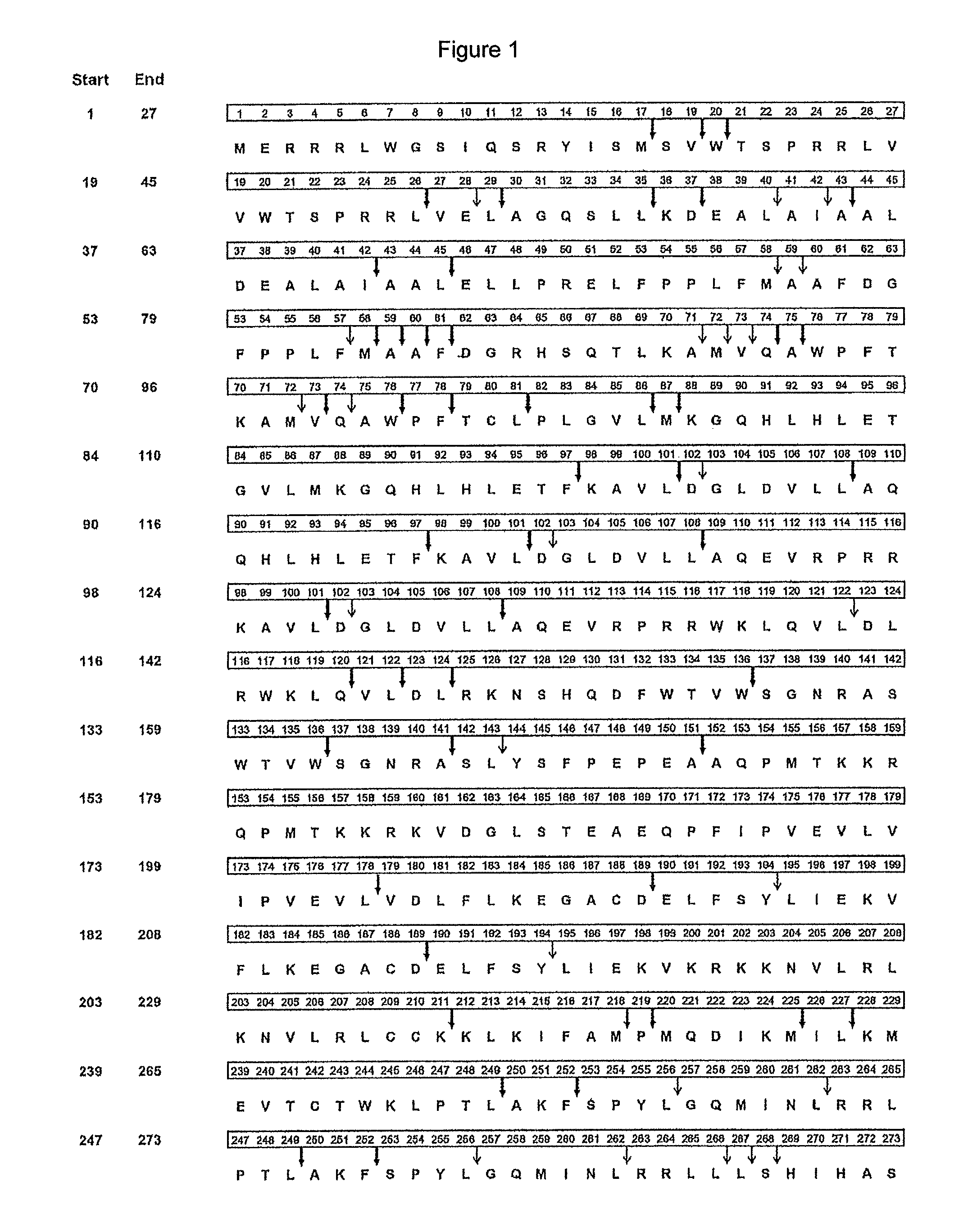
Melanoma antigen preferentially expressed in tumors is a protein that in humans is encoded by the PRAME gene. Five alternatively spliced transcript variants encoding the same protein have been observed for this gene.
Biomarkers for differentiating melanoma from benign nevus in the skin
Disclosed is a method for diagnosing melanoma in a human subject, as well as a method for providing a prognosis to a human subject who is at risk of developing melanoma recurrence, and a method for determining the stage of melanoma in a human subject, comprising the step of determining the level of expression of phosphatase and actin regulator 1 (PHACTR1) gene, or fragments thereof, either alone or in combination with the level of expression of secreted integrin-binding phosphoprotein (SPP1), preferentially expressed antigen in melanoma (PRAME), growth differentiation factor 15 (GDF 15), and chemokine C-X-C motif ligand 10 (CXCL10) genes. Further, the invention relates to a diagnostic kit, comprising at least one substance for detection of the expression of PHACTR1, or fragments thereof, either alone or in combination with the detection of SPP1, PRAME, GDF15, and CXCL10, for the diagnosis or prognosis of melanoma.
Owner:ADVANCED CELL DIAGNOSTICS INC
Methods and compositions to elicit multivalent immune responses against dominant and subdominant epitopes, expressed on cancer cells and tumor stroma
InactiveUS20070004662A1Effective controlBeneficial implicationTumor rejection antigen precursorsGenetic material ingredientsMHC class IEpitope
The present invention provides a method of treating cancer by providing to a subject in need thereof an immunogenic composition comprising a nucleic acid construct encoding a polypeptide comprising CTL epitopes PSMA288-297 and PRAME425-433, or a cross-reactive analogue. In embodiments of the present invention there is provided methods and compositions for inducing, entraining, and / or amplifying the immune response to MHC class-I restricted epitopes of carcinoma antigens to generate an effective anti-cancer immune response.
Owner:MANNKIND CORP
TCR for identifying PRAME antigen
ActiveCN106519019AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenPRAME
The invention provides a T cell receptor (TCR) capable of specifically binding with a short peptide GLSNLTHVL derived from a PRAME antigen. The short peptide GLSNLTHVL can form a compound with HLA A0201 and is presented on the cell surface together with the HLA A0201. The invention further provides a nucleic acid molecule encoding the TCR and a carrier including the nucleic acid molecule. In addition, the invention further provides a cell transducing the TCR.
Owner:XLIFESC LTD
Combined Use of Prame Inhibitors and Hdac Inhibitors
ActiveUS20080119424A1Inhibit cell growthInduced differentiationBiocideHydroxy compound active ingredientsAntigenRetinoid
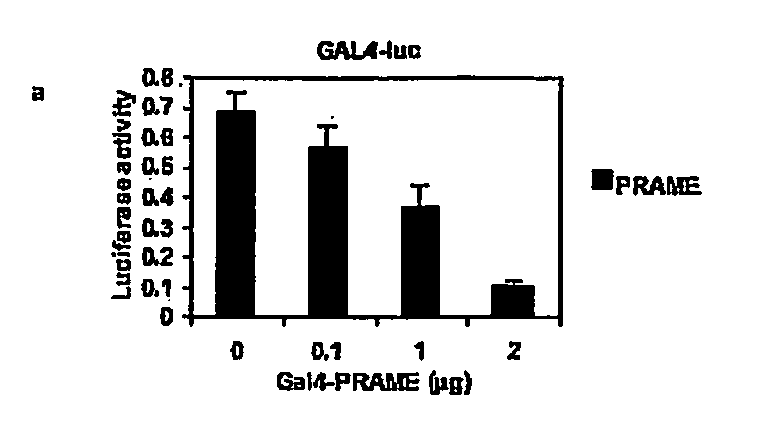
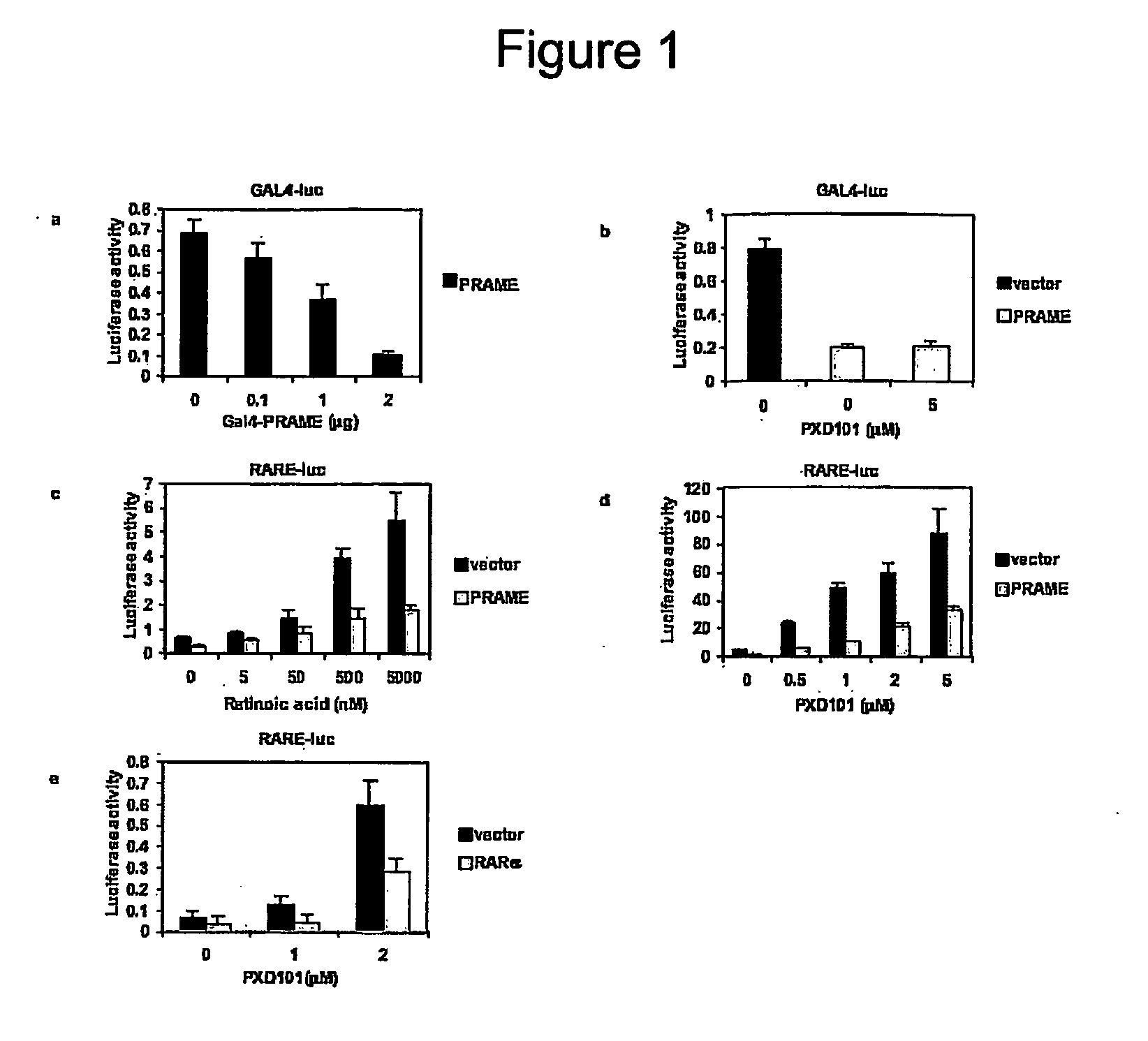
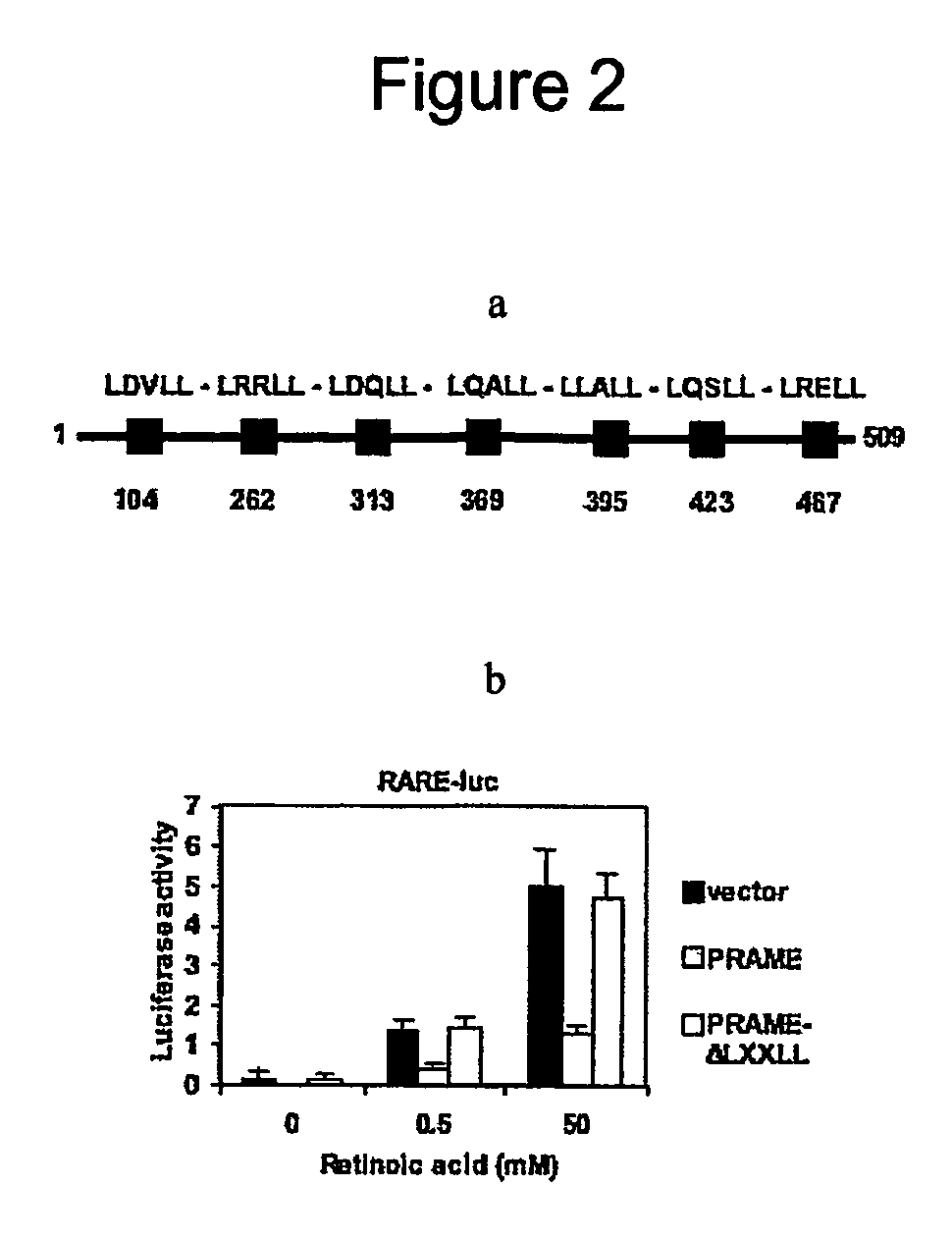
The invention relates to the cancer antigen PRAME (PReferentially expressed Antigen in MElanoma) and its use in a method of treatment of a tumour which comprises administering to a subject in need of treatment an effective amount of an inhibitor of PRAME, in combination with a second agent selected from the group of an inhibitor of HDAC (an HDACi) and a retinoid.
Owner:BERNARDS RENE +2
T cell receptor for identifying PRAME antigen
ActiveCN106831978AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenCellular receptor
The invention provides a TCR (T cell receptor) capable of specifically binding with oligopeptide PYLGQMINL derived from a PRAME (preferentially expressed antigen of melanoma) antigen. The antigen oligopeptide PYLGQMINL and HLA A2402 can form a compound, and be transferred to the cell surface. The invention also provides a nucleic acid molecule for encoding the TCR, and a carrier containing the nucleic acid molecule. The invention also provides a cell for transducing the TCR.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
T cell receptor identifying PRAME antigenic short-peptide
ActiveCN106699874AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenT-Cell Receptor Gene
The invention provides a T cell receptor (TCR) capable of specifically combining short-peptide PYLGQMINL derived from a PRAME antigen, and the antigenic short-peptide PYLGQMINL and HLA A2402 can form a compound and are together delivered to the cell surface. The invention further provides nucleic acid molecules encoding the TCR and a carrier comprising the nucleic acid molecules. The invention further provides a cell transducing the TCR.
Owner:XLIFESC LTD
PRAME derived peptides and immunogenic compositions comprising these
ActiveUS8492514B2Strong immune responseBroad HLA haplotype coverageTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeHla class ii
The invention relates to a peptide having a length of no more than 100 amino acids and comprising at least 19 contiguous amino acids from the amino acid sequence of the human PRAME protein, wherein the peptide comprises at least one HLA class II epitope and at least one HLA class I epitope from the amino acid sequence of the human PRAME protein and to its use as such or in a composition as a medicament for the treatment and / or prevention of cancer.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Prame derived peptides and immunogenic compositions comprising these
ActiveUS20100120683A1Strong immune responseBroad HLA haplotype coverageTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeHla class ii
The invention relates to a peptide having a length of no more than 100 amino acids and comprising at least 19 contiguous amino acids from the amino acid sequence of the human PRAME protein, wherein the peptide comprises at least one HLA class II epitope and at least one HLA class I epitope from the amino acid sequence of the human PRAME protein and to its use as such or in a composition as a medicament for the treatment and / or prevention of cancer.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
TCP identifying short chain polypeptide from PRAME antigen
ActiveCN108264550AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenPRAME
The invention provides a T cell receptor (TCR) capable of being specifically bound to short chain polypeptide VLDGLDVLL derived from a PRAME antigen. The antigen short chain polypeptide VLDGLDVLL andHLA A0201 can form a compound and are together presented to the cell surface. The invention further provides nucleic acid molecules encoding the TCR and a carrier containing the nucleic acid molecules. In addition, the invention provides cells transducing the TCR.
Owner:XLIFESC LTD
T cell receptor that recognizes prame antigen
ActiveCN106831978BGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenCellular receptor
The invention provides a TCR (T cell receptor) capable of specifically binding with oligopeptide PYLGQMINL derived from a PRAME (preferentially expressed antigen of melanoma) antigen. The antigen oligopeptide PYLGQMINL and HLA A2402 can form a compound, and be transferred to the cell surface. The invention also provides a nucleic acid molecule for encoding the TCR, and a carrier containing the nucleic acid molecule. The invention also provides a cell for transducing the TCR.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Protein tyrosine phosphatase, non-receptor type 11 (ptpn11) and triple-negative breast cancer
The present invention relates to a method for treating breast cancer in a subject having a breast cancer of the triple-negative type, which method comprises the step of administering to said subject a therapeutically effective amount of a modulator of the protein tyrosine phosphatase, non-receptor type 11 (PTPN11) gene or of its gene product (Shp2). The present invention also relates to a method for treating breast cancer in a subject having a breast cancer over-expressing the “SHP2 signature” genes, as compared to normal breast tissue samples, which method comprises the step of administering to said subject a therapeutically effective amount of a modulator of the protein tyrosine phosphatase, non-receptor type 11 (PTPN11) gene or of its gene product (Shp2), wherein said “SHP2 signature” genes consist of the genes SGCB, ZSCAN12, ID4, ZIC3, CPVL, HLA-A, MCOLN3, SPATA18, TMEM45A, GNAL, CYBRD1, TSPAN7, ZEB1, CNTLN, NEFL, CENPV, ARL6, HPRT1, LRRC34, PDPN, BEND7, SLC16A10, FAM27E1, PLEKHA1, HERC5, CHIC1, PHF6, ELOVL4, ANTXR1, PRAME, SCML1, CLIP4, CECR2, CNOT10, IGF2BP3, NAP1L3, GPC3, KIAA1804, DGKE, FAS, EPHA6, KDELC1, CRISPLD1, DOCK3, ACSL4, CNTNAP3, PLEKHM3, RDX, TBX18, RRAGD, HOXB5, SNCA, FUNDC2, ITGA8, HFM1, IGF2BP2, CCND2, SGTB, MKX, CRYBG3, WBP5, LPHN3, BEX4, CPNE8, GLDC, SLC35F1, HOXA13, SERPINF1, NEFM, SYCP2L, FHL1, APOBEC3C, CALD1, FKBP10, HOXD11, DENND2C, LRRC49, FAM55C, KIAA0408, HOXB9, C160RF62, ACN9, TUSC3, ELOVL2, SPOCK3, HOXB6, WDR35, MPP1, FBX038, PRKAA2, SLAIN1, NPHP3, KIAA1524, PRPS1, GJC1, AMOT, SLC9A6, KCTD12, NUP62CL, DZIP3, JAM3, HOXA9, ANKRD19, CDKN2A, BCAT1, OAT, LPHN2, CCDC82, HSD17B11, SAMHD1, WDR17, STK33, GSTP1, TRPC1, CKB, LIN28B, ALDH1L2, SACS, CLGN, MY03A, EPB41L3, SLC25A27, VCAN, GPX8, GALNT13, PVRL3, MOXD1, HEY1, MAP7D3, ESD, MPP6, EYA4, SPG20, ZDBF2, ZNF204, IFT57, AKR1B1, ADAT2, ZNF717, CCDC88A, ZNF215, MIDI, FBN2, LOC100130876, TCEAL8, IGF2BP1, ANKRD18B, PLAGL1, PM20D2, LDHB, C150RF51, PTPN11, EPB41L2, TLE4, GOLM1, C60RF192, HOXD13, SLIT2, UCHL1, DYNC2H1, CPS1, GPR180, PYGL, NRN1, PRTFDC1, SLC16A1, DSC3, TMC01, LRCH2, SLC6A15, DZIP1, HOXA5, HSPA4L, CDR1, PLS3, ECHDC1, SMARCA1, CXORF57, HOXD10, and IRS4.
Owner:NOVARTIS FORSCHUNGSSTIFTUNG ZWEIGNIEDERLASSUNG FRIEDRICH MIESCHER INSTITTUE FOR BIOMEDICAL RES
Prame derived peptides and immunogenic compositions comprising these
ActiveUS20140348862A1Strong immune responseBroad HLA haplotype coverageTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeHla class ii
The invention relates to a peptide having a length of no more than 100 amino acids and comprising at least 19 contiguous amino acids from the amino acid sequence of the human PRAME protein, wherein the peptide comprises at least one HLA class II epitope and at least one HLA class I epitope from the amino acid sequence of the human PRAME protein and to its use as such or in a composition as a medicament for the treatment and / or prevention of cancer.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
TCR (T Cell Receptor) for recognizing PRAME (Preferentially Expressed Antigen of Melanoma) antigen oligopeptide
ActiveCN109400696AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenBiology
The invention provides a TCR (T Cell Receptor) which is specifically combined with oligopeptide AVLDGLDVLL which derives PRAME (Preferentially Expressed Antigen of Melanoma) antigen, and the antigen oligopeptide AVLDGLDVLL and HLA A0201 form a compound and are represented to the surfaces of cells together. The invention further provides a nucleic acid molecule for encoding the TCR and a vector with the nucleic acid molecule. In addition, the invention further provides a cell for transducing the TCR provided by the invention.
Owner:XLIFESC LTD
T cell receptor capable of recognizing short peptide derived from PRAME antigen
ActiveCN108948184AGood killing effectImmunoglobulin superfamilyMammal material medical ingredientsAntigenPRAME
The invention provides a T cell receptor (TCR) capable of specifically binding a short peptide LYVDSLFFL derived from a preferentially expressed antigen of melanoma (PRAME) antigen. The antigen shortpeptide LYVDSLFFL and HLA A2402 can form a complex and can be presented to the cell surface together. The invention also provides a nucleic acid molecule for encoding the TCR and a carrier containingthe nucleic acid molecule. In addition, the invention also provides a cell for transducing the TCR provided by the invention.
Owner:XLIFESC LTD
TCR (T Cell Receptor) for recognizing PRAME (Preferentially Expressed Antigen of Melanoma) antigen oligopeptide and related composition of TCR
ActiveCN109400697AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenCellular receptor
The invention provides a TCR (T Cell Receptor) which is specifically combined with oligopeptide LYVDSLFFL which derives PRAME (Preferentially Expressed Antigen of Melanoma) antigen, and the antigen oligopeptide LYVDSLFFL and HLA A2402 form a compound and are represented to the surfaces of cells together. The invention further provides a nucleic acid molecule for encoding the TCR and a vector withthe nucleic acid molecule. In addition, the invention further provides a cell for transducing the TCR provided by the invention.
Owner:XLIFESC LTD
Method for implementing high-grade link control procedure prame treatment
InactiveCN1477885AProcessing speedWireless network protocolsRadio/inductive link selection arrangementsData memoryByte
The method for implementing high-grade link control protocol frame treatment includes framing and deframing processes. The described framing process includes the following steps: receiving a piece ofdata transferred from exterior and identification number of said user; temporarily storing the data into data buffer memory; fetching a byte of data from data buffer memory by data processing machine; escaping said byte data and resolving frame correction sequence (FCS); judging that the processed data byte number can meet some set threshold value y or not, if it can meet said threshold value, feeding the data byte number into the next data buffer memory, if it can not meet, judging that the piece of data is processed completely or not, if it is not, returning to third step, if it is processed, executing next step, the data in the second data buffer memory can be outputted by means of channel dispatcher.
Owner:ZTE CORP
TCR capable of recognizing preferentially expressed antigen of melanoma (PRAME) short chain polypeptide and coding sequence of TCR
ActiveCN109575120AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenPRAME
The invention provides a T cell receptor (TCR) capable of specifically combining short chain polypeptide VLDGLDVLL derived from preferentially expressed antigen of melanoma (PRAME), the antigen shortchain polypeptide VLDGLDVLL and HLA A0201 can form a compound and be presented to the cell surface together. The invention further provides a nucleic acid molecule encoding the TCR and a carrier containing the nucleic acid molecule. In addition, the invention further provides cells transducing the TCR.
Owner:XLIFESC LTD
Short peptide derived from self tumor antigen PRAME
ActiveCN108203460ATumor rejection antigen precursorsTumor specific antigensTumor antigenWilms' tumor
The invention relates to a short peptide derived from self tumor antigen PRAME, a compound formed from the short peptide and MHC molecule, and applications of the short peptide and the compound, and also relates to molecules capable of combining the short peptide and the compound, and applications of the molecules.
Owner:XLIFESC LTD
Method for implementing high-grade link control procedure prame treatment
InactiveCN1305323CProcessing speedWireless network protocolsRadio/inductive link selection arrangementsData memoryByte
The method for implementing high-grade link control protocol frame treatment includes framing and deframing processes. The described framing process includes the following steps: receiving a piece of data transferred from exterior and identification number of said user; temporarily storing the data into data buffer memory; fetching a byte of data from data buffer memory by data processing machine; escaping said byte data and resolving frame correction sequence (FCS); judging that the processed data byte number can meet some set threshold value y or not, if it can meet said threshold value, feeding the data byte number into the next data buffer memory, if it can not meet, judging that the piece of data is processed completely or not, if it is not, returning to third step, if it is processed, executing next step, the data in the second data buffer memory can be outputted by means of channel dispatcher.
Owner:ZTE CORP
Cancer vaccines targeting prame and uses thereof
ActiveUS20190175713A1Elicit immune responseTumor rejection antigen precursorsPeptide/protein ingredientsAntigenTumor therapy
Disclosed herein are nucleic acid molecules comprising one or more nucleic acid sequences that encode a mutated consensus PRAME antigen. Vectors, compositions, and vaccines comprising one or more nucleic acid sequences that encode a mutated consensus PRAME antigen are disclosed. Methods of treating a subject with a PRAME-expressing tumor and methods of preventing a PRAME-expressing tumor are disclosed. Mutated consensus PRAME antigen is disclosed.
Owner:INOVIO PHARMA
A short peptide derived from the tumor antigen prame
The present invention relates to a tumor antigen short peptide derived from PRAME, a complex formed between the short peptide and MHC molecules and the use of the short peptide and the complex. At the same time, the present invention also relates to molecules combined with the above-mentioned short peptides or complexes, and uses of these molecules.
Owner:XLIFESC LTD
T cell receptors
ActiveUS20220177541A1Reduce the amount requiredReduce the binding forceTumor rejection antigen precursorsPeptide/protein ingredientsAntigenDisease
Owner:IMMUNOCORE LTD
Tumor antigen short peptide derived from prame
The present invention relates to a newly discovered short peptide derived from tumor antigen PRAME, a complex formed between the short peptide and MHC molecules and the use of the short peptide and the complex. At the same time, the present invention also relates to molecules combined with the above-mentioned short peptides or complexes, and uses of these molecules.
Owner:XLIFESC LTD
Improved targeted t-cell therapy for treatment of multiple myeloma
PendingUS20220062342A1Improve abilitiesLong responseMammal material medical ingredientsCancer antigen ingredientsXBP1Plasma cell
Provided herein are activated adoptive T-cell compositions targeting plasma cell dyscrasias such as multiple myeloma and methods of treating plasma cell dyscrasias such as multiple myeloma using such compositions. The T-cell compositions of the present disclosure are activated against a select group of antigens associated with multiple myeloma (MMAAs) and, in certain embodiments, in combination with more widely expressed tumor associated antigens (TAAs). In particular, the T-cell compositions of the present disclosure are directed to the MMAAs selected from B-cell maturation antigen (BCMA), X box Protein 1 (XBP1), CS1, and Syndecan-1 (CD 138), or a combination thereof. In certain embodiments, the T-cell composition includes T-cells activated to a TAA selected from preferentially expressed antigen of melanoma (PRAME), Survivin, Wilms' Tumor 1 protein (WT1), and melanoma antigen 3 (MAGE-A3), or a combination thereof.
Owner:CHILDRENS NAT MEDICAL CENT
Multigene assay
Owner:AGENCY FOR SCI TECH & RES +1
Cancer vaccines targeting PRAME and uses thereof
ActiveUS11338029B2Tumor rejection antigen precursorsPeptide/protein ingredientsAntigenNucleic acid sequencing
Disclosed herein are nucleic acid molecules comprising one or more nucleic acid sequences that encode a mutated consensus PRAME antigen. Vectors, compositions, and vaccines comprising one or more nucleic acid sequences that encode a mutated consensus PRAME antigen are disclosed. Methods of treating a subject with a PRAME-expressing tumor and methods of preventing a PRAME-expressing tumor are disclosed. Mutated consensus PRAME antigen is disclosed.
Owner:INOVIO PHARMA
Short-chain peptide derived from tumor antigen PRAME
ActiveCN107266552ATumor rejection antigen precursorsPeptide/protein ingredientsTumor antigenWilms' tumor
The invention relates to a newly discovered short peptide derived from a tumor antigen PRAME, a complex formed by the short-chain peptide and aMHC molecules, and application of the short-chain peptide and complex. At the same time, the invention also relates to molecules combined with the short-chain peptide or the complex above, and application of the molecules.
Owner:XLIFESC LTD
A T-cell receptor that recognizes short peptides derived from the prame antigen
ActiveCN108948184BGood killing effectImmunoglobulin superfamilyMammal material medical ingredientsAntigenCellular receptor
Owner:XLIFESC LTD
Prame purification
InactiveUS20140234424A1Reduce aggregationReduce protein aggregationPowder deliveryTumor rejection antigen precursorsPurification methodsDiluent
Methods and processes for the purification of PRAME are provided. In particular, methods for reducing the aggregation of PRAME during a diluent exchange from diluent A to diluent B comprising: (i) adding a polyanionic compound to diluent A prior to or contemporaneously with the exchange; and (ii) exchanging protein from diluent A to diluent B are provided. Compositions produced by the method are also provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Combined use of prame inhibitors and HDAC inhibitors
ActiveUS20130123327A1Inhibit cell growthInduced differentiationBiocideHydroxy compound active ingredientsAntigenStage melanoma
The invention relates to the cancer antigen PRAME (PReferentially expressed Antigen in MElanoma) and its use in a method of treatment of a tumour which comprises administering to a subject in need of treatment an effective amount of an inhibitor of PRAME, in combination with a second agent selected from the group of an inhibitor of HDAC (an HDACi) and a retinoid.
Owner:BERNARDS RENE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com