Recombinant oncolytic vaccinia virus and preparation method and application thereof
A vaccinia virus and oncolytic technology, applied in the field of genetic engineering, can solve the problems of serious immune-related side effects, patients cannot get a complete response, and affect the efficacy of PD-1 monoclonal antibody, and achieve the effect of enhancing killing ability and enhancing safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention is further described in conjunction with accompanying drawings and examples. Unless otherwise specified, the present invention may adopt conventional techniques in the art.
[0026] 1. Synthesis of human full-length PD-1 monoclonal antibody:
[0027] The sequence of the single-chain variable region of the PD-1 monoclonal antibody refers to the sequence of Bristol-Myers Squibb’s PD-1 monoclonal antibody nivolumab, and the sequence of the PD-1 single-chain variable region designed by us is obtained through amino acid deduction and codon optimization. The Fc segment of the monoclonal antibody Referring to the Fc segment sequence of human IgG4, the heavy chain and light chain of human PD-1 antibody were synthesized by chemical synthesis, and the heavy chain and light chain genes were linked by the linker IRES. The full-length gene sequence of the PD-1 monoclonal antibody is shown in SEQ ID NO.1.
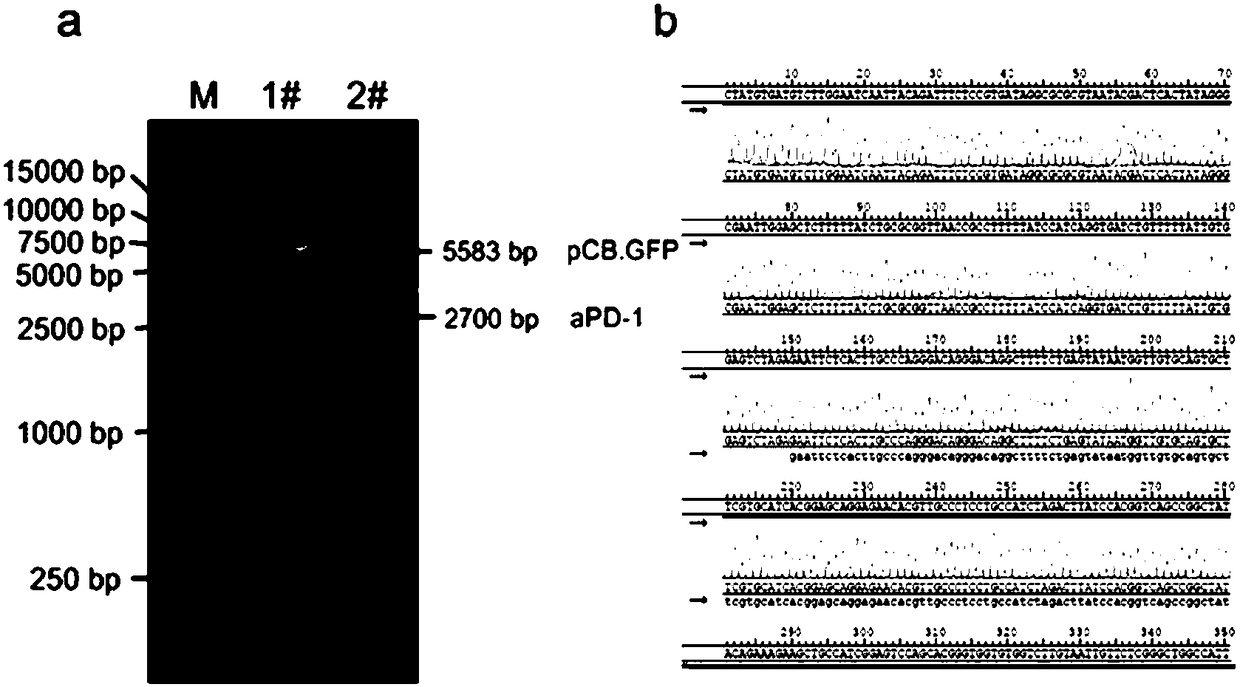
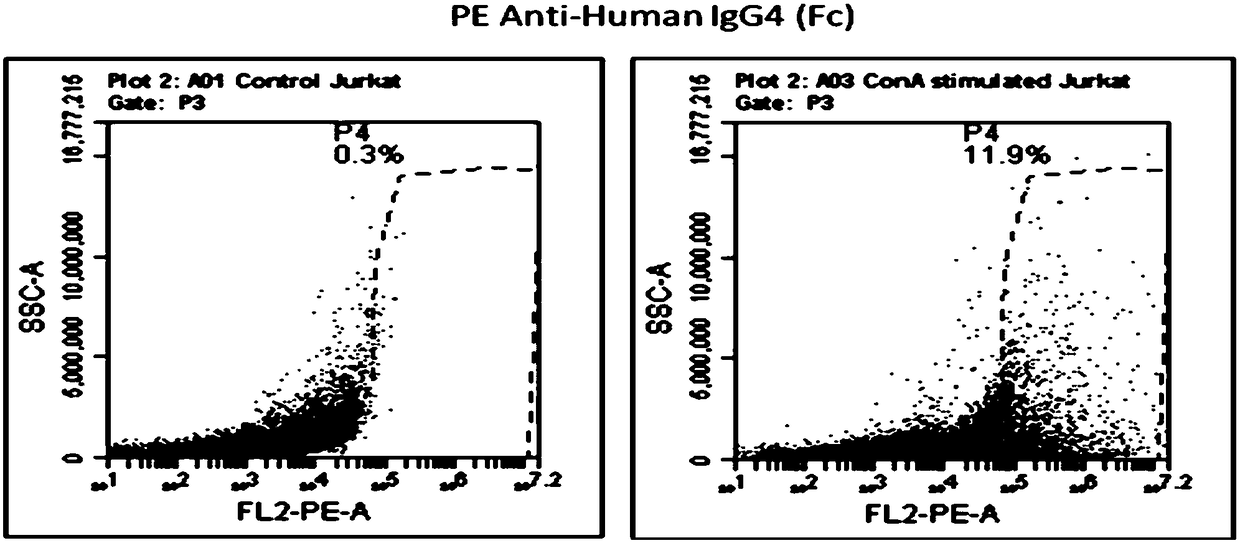
[0028] 2. Construction and identification of oncolyti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com