Preparation method of newcastle disease, avian influenza virus and avian adenovirus trivalent inactivated vaccine
A technology of chicken Newcastle disease virus and avian adenovirus, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of difficult to achieve high-efficiency transfection, unfavorable experimental operation, cumbersome preparation, etc., and achieve complete immune protection and antibody production Fast, long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 liposome complex:
[0042] (1) Weigh 0.2g of phosphatidylcholine, 0.08g of cholesterol, and 0.04g of distearoylphosphatidylethanolamine, mix them, dissolve in 50mL of ethanol, and sonicate for 5min;
[0043] (2) Rotate evaporation at 28°C and 0.1MPa to remove ethanol, let stand for 30min, add 100mL phosphate buffer solution containing 0.5% (m / v) catalase at pH 7.4, and place in a water bath at 35°C Medium shaking hydration reaction for about 1 hour to obtain liposome suspension;
[0044] (3) Adjust the particle size of the liposome suspension to 150 nm using a syringe filter to obtain liposomes containing catalase inside.
Embodiment 2
[0045] Example 2 Preparation of poultry adenovirus type 4 seedling virus solution and determination of virus content
[0046] (1) Preparation of poultry adenovirus type 4 seedling virus liquid:
[0047] ① Cell preparation: Digest and disperse LMH cells with trypsin-0.02% EDTA with a mass volume ratio of 0.025%, add 10% (v / v) newborn bovine serum, 1.0% (v / v) double antibody and 2.0% (m / v) DMEM culture solution of hydroxyethylpiperazineethanesulfonic acid, at 37°C, 5% CO 2 After culturing to a monolayer of cells, wash the cells three times with serum-free DMEM medium for avian adenovirus type 4 virus inoculation;
[0048] ②Inoculation and cultivation of avian adenovirus type 4: inoculate the cells prepared in step ① with the virus seed of avian adenovirus type 4 at a final volume of 1:200, absorb the virus solution at 37°C for 40 minutes, and add 1.0% (v / v) newborn bovine serum, 0.4% (m / v) glutamine, 1.0% (v / v) double antibody, 2.5% (v / v) liposome complex, 0.15% (m / v) Lipoic ...
Embodiment 3
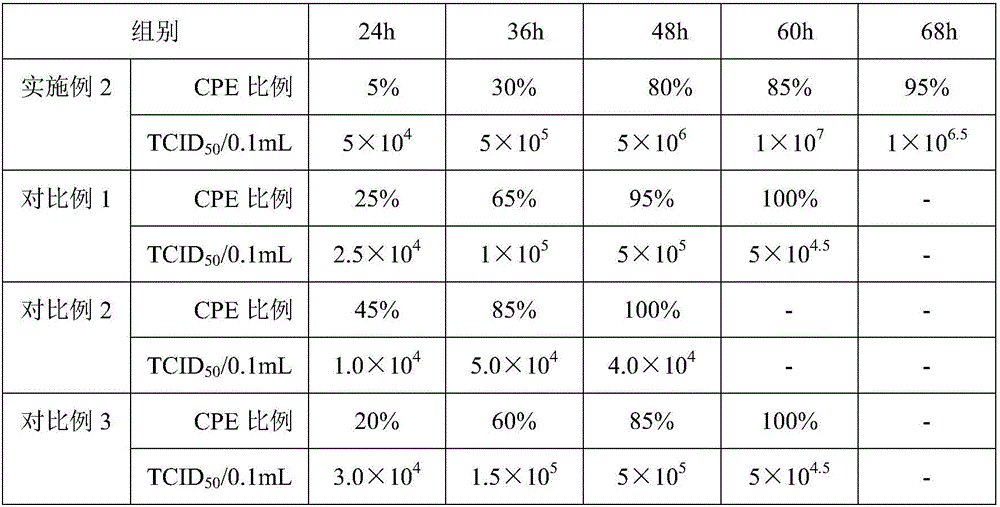
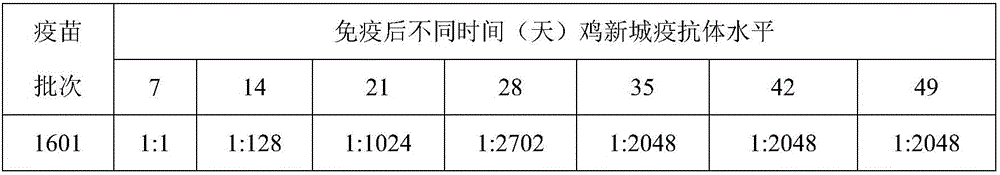
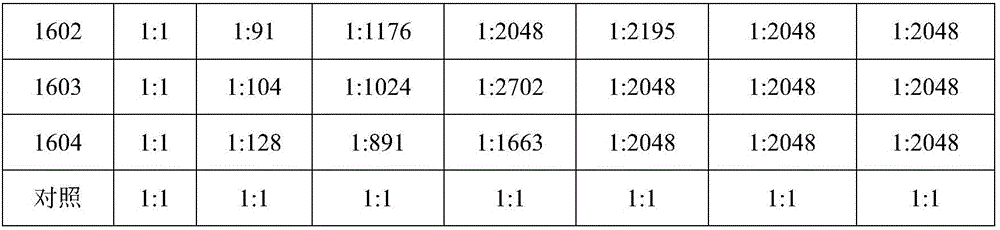
[0060] Example 3 Preparation of virus solution for chicken Newcastle disease vaccine production and avian influenza H9 subtype vaccine production and virus content determination
[0061] (1) Preparation of Newcastle Disease Vaccination Virus Liquid:
[0062] Dilute chicken Newcastle disease virus seed to 10 with sterilized PBS -4 or 10 -5 , inoculate 10-11-day-old chicken embryos, inoculate 0.2ml into the allantoic cavity of each embryo, and continue to incubate at 36-37°C. It is not necessary to turn the embryos, and irradiate the embryos once a day, and incubate them for 96 hours. Take them out, irradiate the embryos, and discard them. Remove the dead embryos, put the air chamber of the living embryos upright, and cool at 2-8°C for 12-24 hours, take out the cooled chicken embryos, harvest the chicken embryo allantoic fluid, absorb the chicken embryo allantoic fluid and place it in a sterilized container, Take a sample to measure the agglutination value of red blood cells. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com