Fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting influenza A virus subtype H7N9
A type of influenza virus, fluorescent quantitative technology, applied in the direction of fluorescence/phosphorescence, microbial-based methods, microbial measurement/inspection, etc., can solve the problems of lack of antigens and antibodies, long cycle, high technical requirements, etc., to save reagent consumables , high specificity and sensitivity, and the effect of shortening the detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
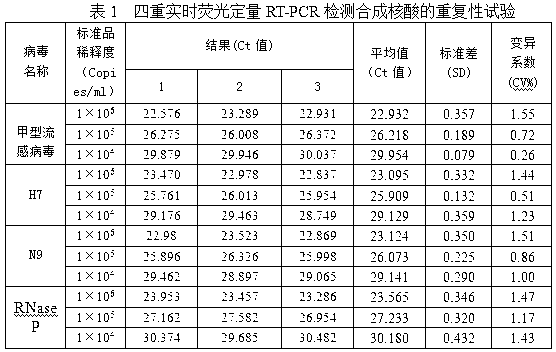
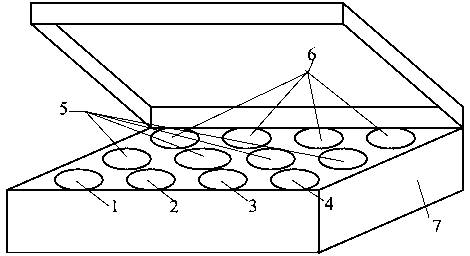
[0059] see figure 1 , a real-time fluorescent quantitative RT-PCR detection kit for detecting influenza A virus H7N9 subtype, which consists of quantitative RT-PCR reaction solution tube 1, enzyme mixing solution tube 2, primer-probe mixing solution tube 3, and standard product tube 4 (4 tubes in total, containing influenza A virus, H7, N9, RNase P), positive control tube 5 (4 tubes in total, containing influenza A virus, H7, N9, RNase P), negative control Tube 6 and box body 7 are formed. Quantitative RT-PCR reaction solution tube 1 is equipped with quantitative RT-PCR reaction solution, enzyme mixed solution tube 2 is equipped with enzyme mixed solution, primer-probe mixed solution tube 3 is equipped with primer-probe mixed solution, and 4 standard tubes 4 are respectively equipped with Influenza A virus, H7, N9, RNase P, 4 positive control tubes 5 are equipped with positive plasmid samples of influenza A virus, H7, N9, RNase P respectively.
[0060] Quantitative RT-PCR r...
Embodiment 2
[0063] 1 Materials and methods
[0064] 1.1 Clinical specimens and viral nucleic acids:
[0065] The clinical samples of influenza A virus and influenza A virus H7N9 subtype come from the nasopharynx of confirmed and suspected patients with influenza A virus H7N9 subtype in the First Affiliated Hospital of Zhejiang University School of Medicine and several other hospitals in Zhejiang Province Specimens such as swabs or sputum are transported to the P3 laboratory after the samples are collected. In addition, other influenza A viruses such as human seasonal H1N1, new A H1N1, human seasonal H3N2, H5N1, and influenza B virus, respiratory adenovirus, human metapneumovirus, human coronavirus-HKU1, human coronavirus- The positive nucleic acids of NL63, respiratory syncytial virus, and Boca virus were provided by the State Key Laboratory of Diagnosis and Treatment of Infectious Diseases.
[0066] 1.2 Primers and probes
[0067]Multiple gene sequences covering domestic and foreign i...
Embodiment 3
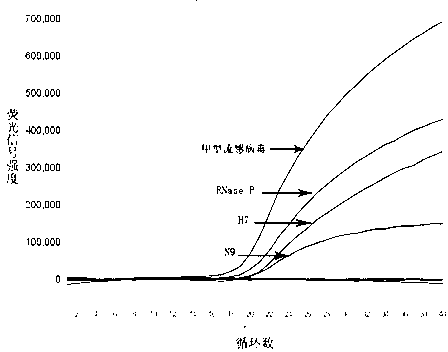
[0103] The detection of clinical samples using this kit is mainly based on the "Twelfth Five-Year Plan" major project - infectious disease pathogen detection technology platform project (2012ZX10004-210). The collected clinical samples were mainly from the First Affiliated Hospital of Zhejiang University School of Medicine and several other hospitals in Zhejiang Province between March 2013 and April 2013. A total of 1039 samples from the recent fever clinics: nasopharyngeal swab samples (971 ), sputum samples (68); a total of 143 samples from inpatients diagnosed with H7N9 in the First Affiliated Hospital of Zhejiang University School of Medicine: nasopharyngeal swab samples (95), sputum samples (48) and stool samples (10 share). The collected samples were verified by quadruple real-time fluorescent quantitative RT-PCR in this method, and the test results are as follows: 112 samples sent to the fever clinic were positive for influenza A virus, and the H7N9 subtype of influenza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com