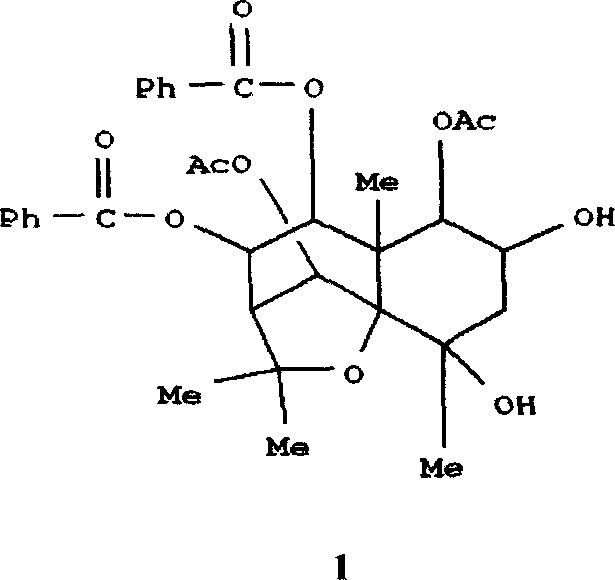
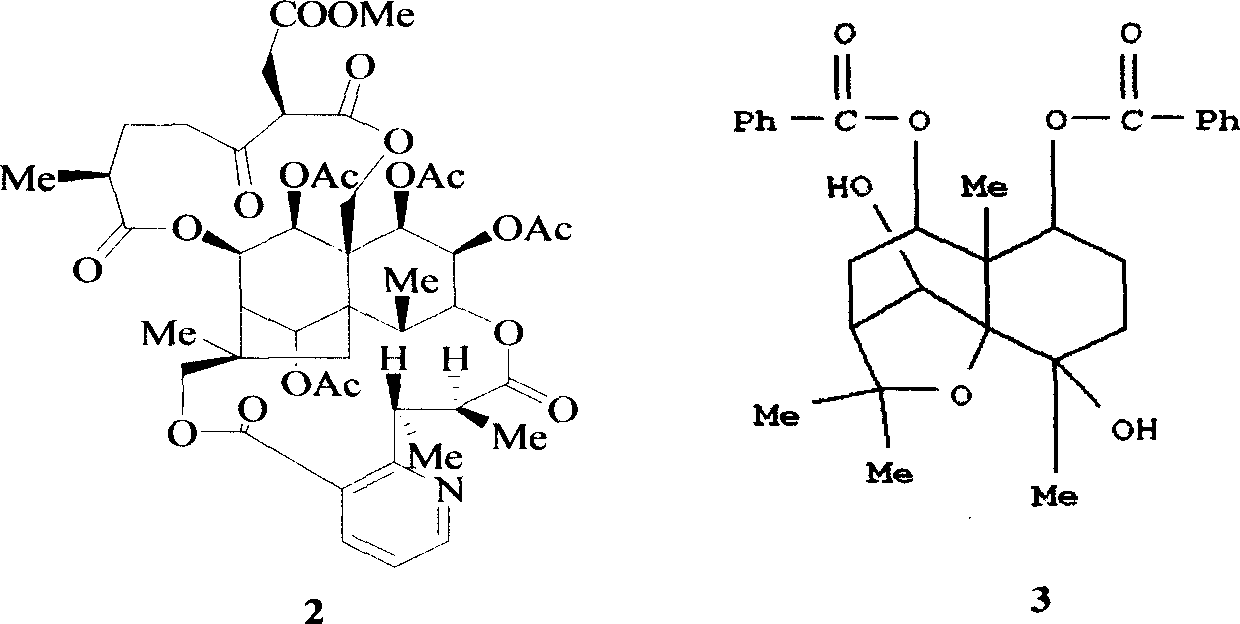
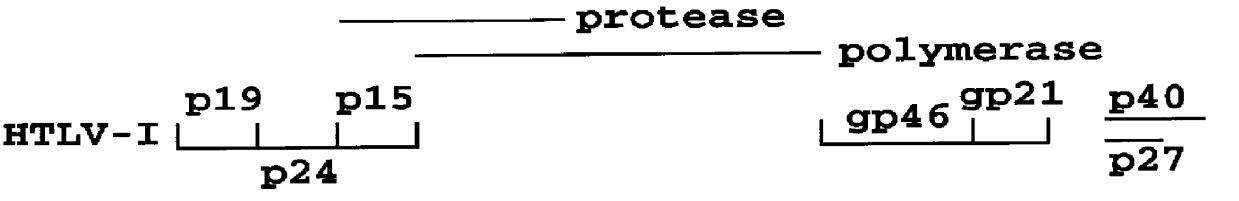
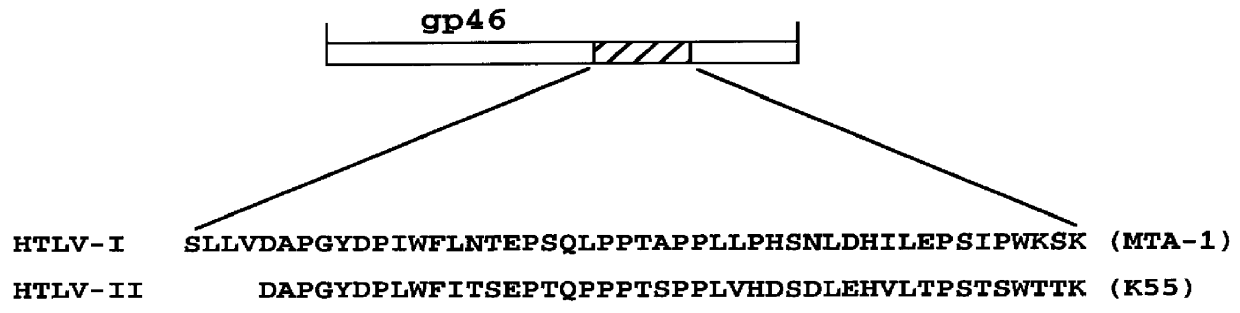
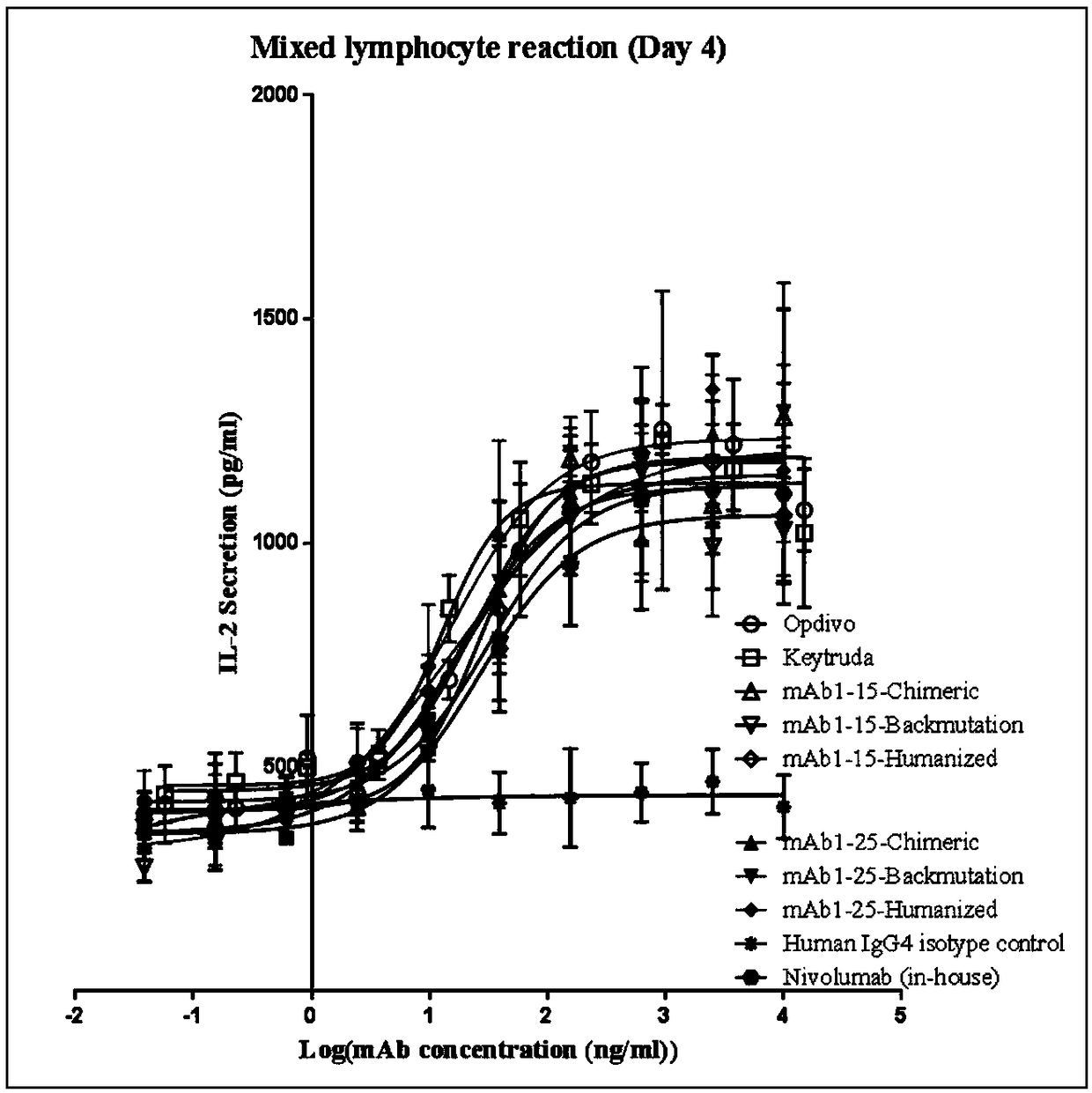
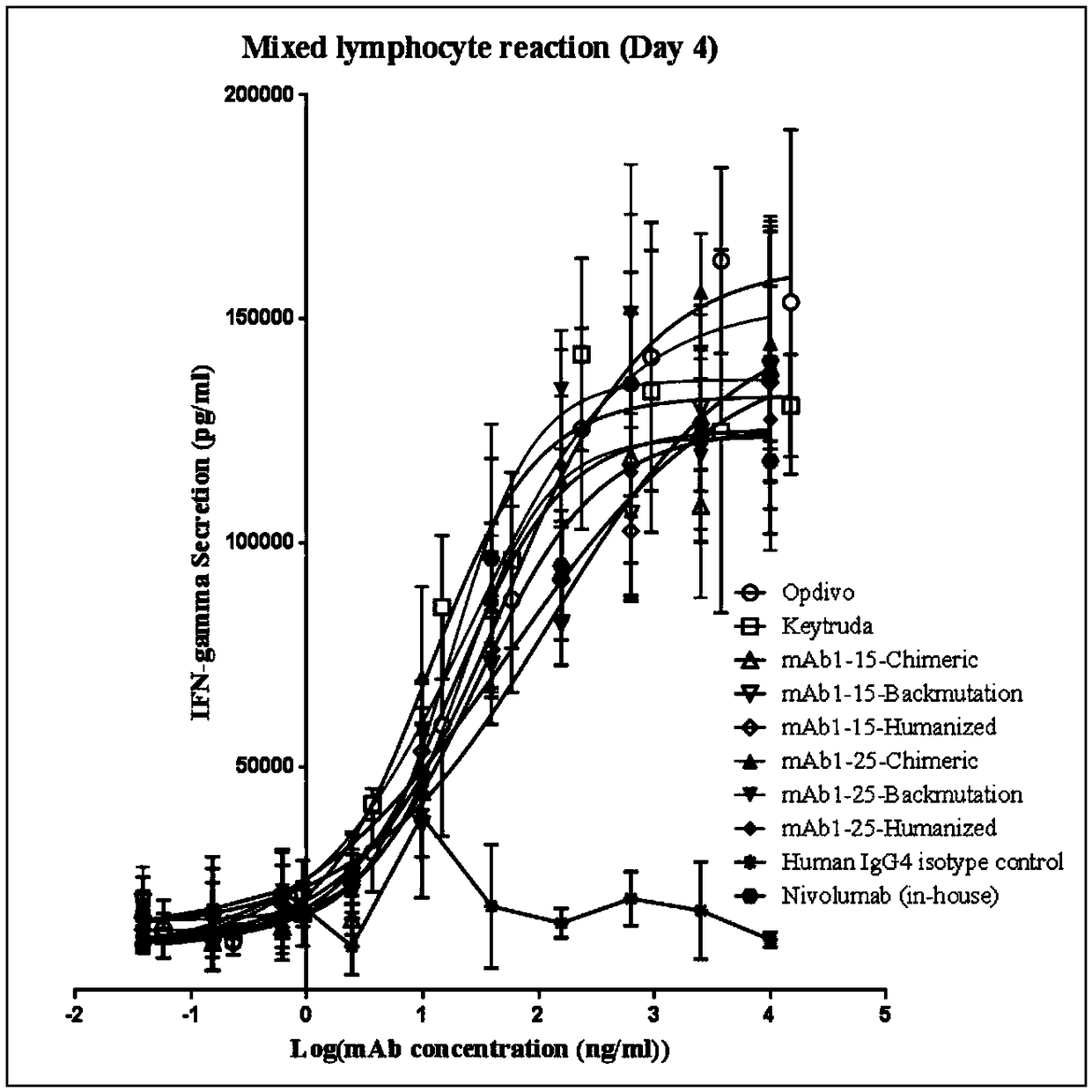
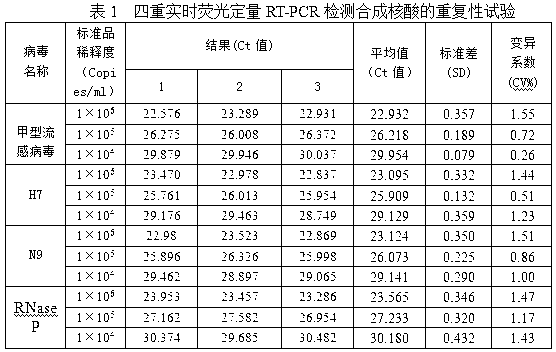
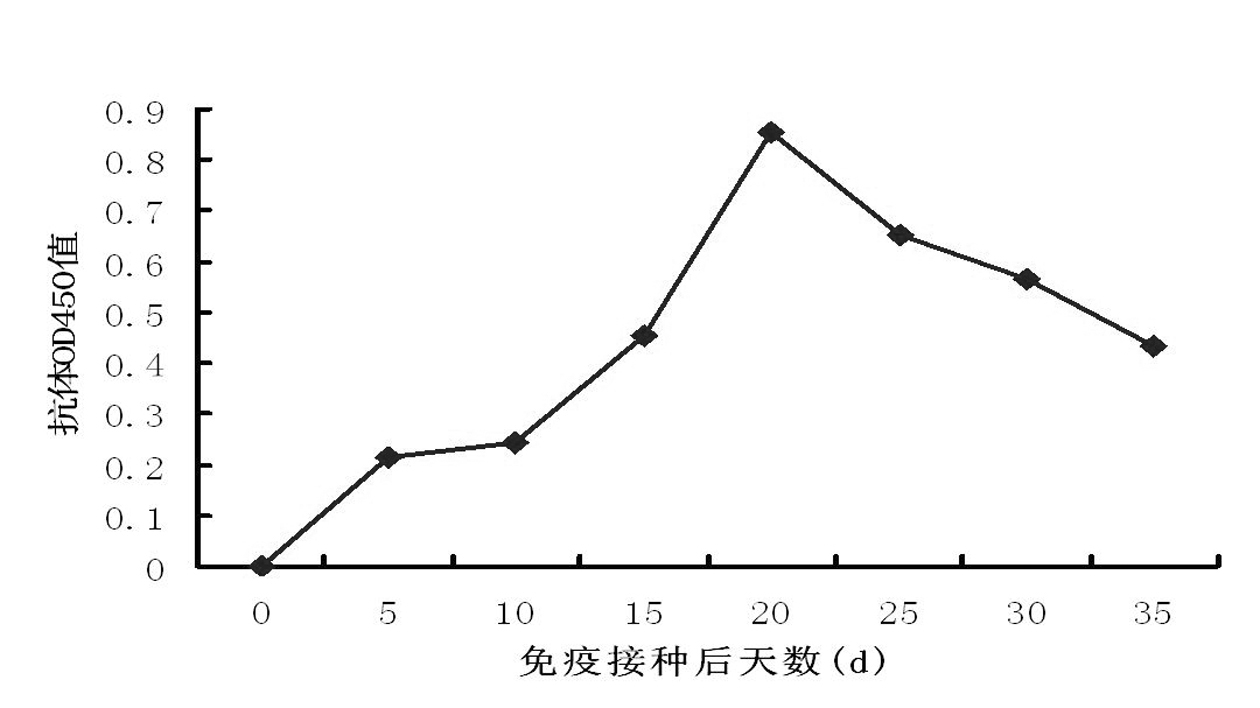
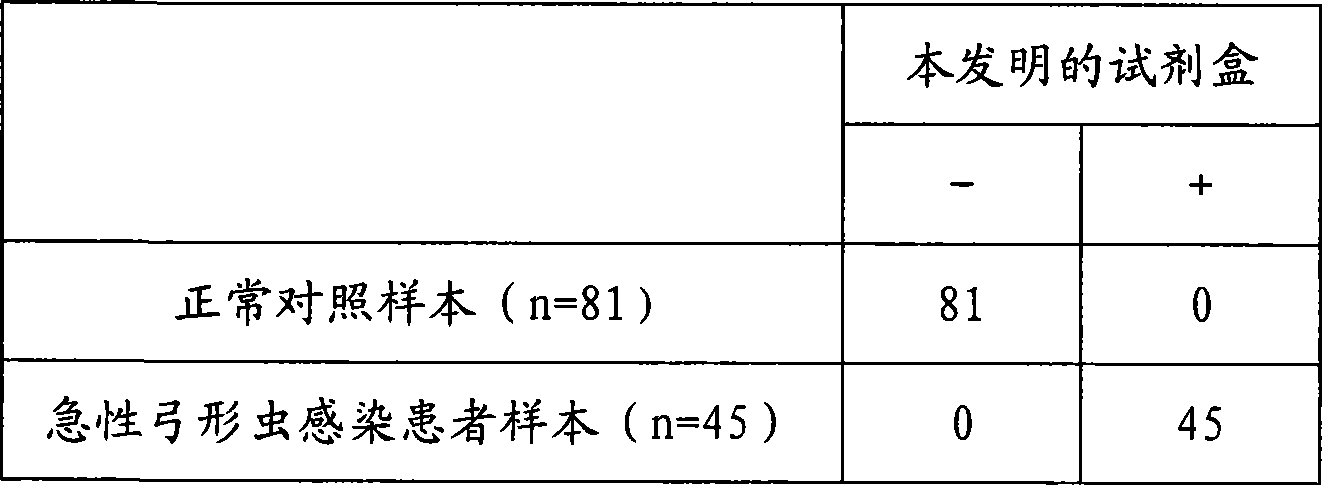
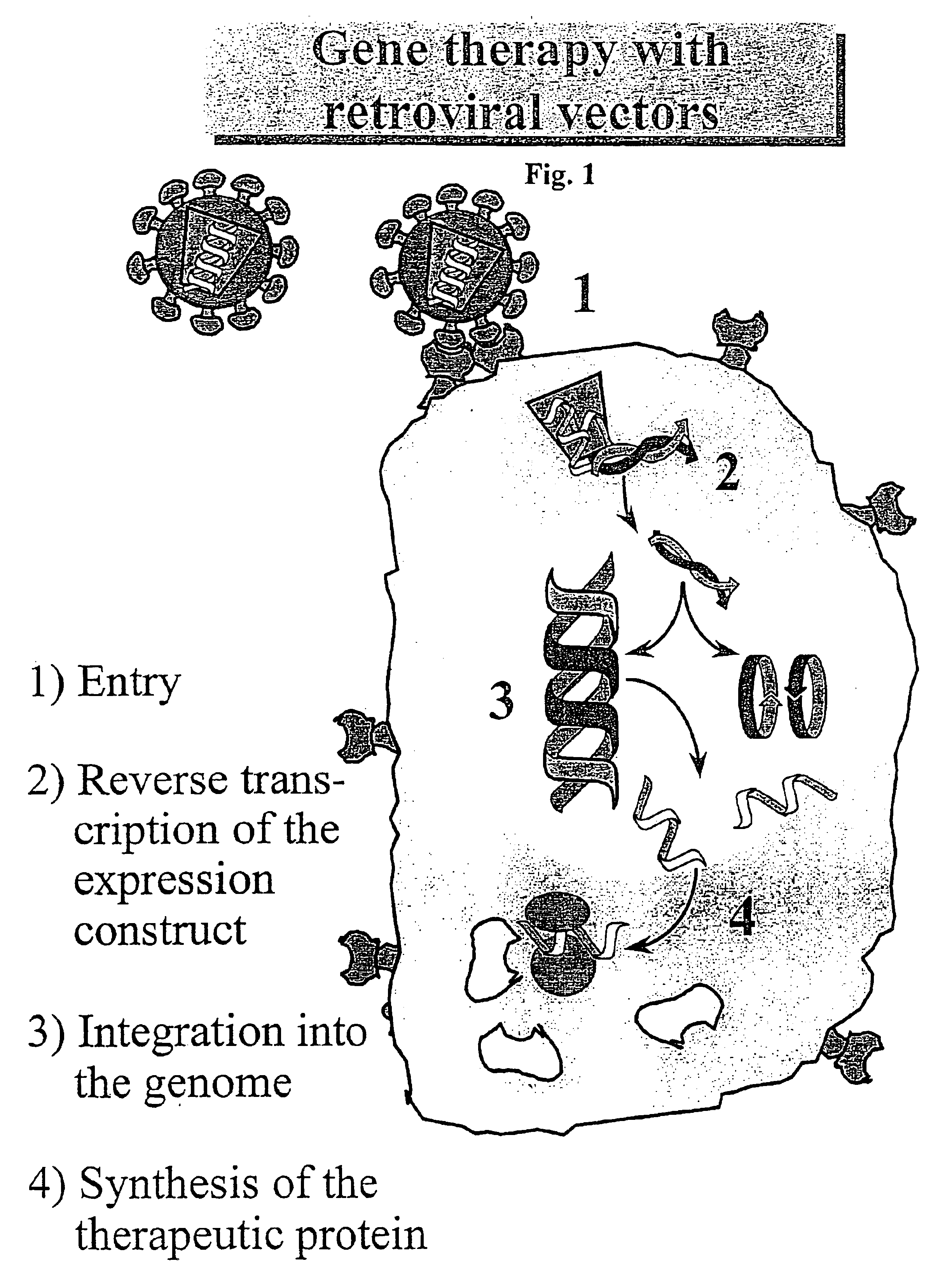
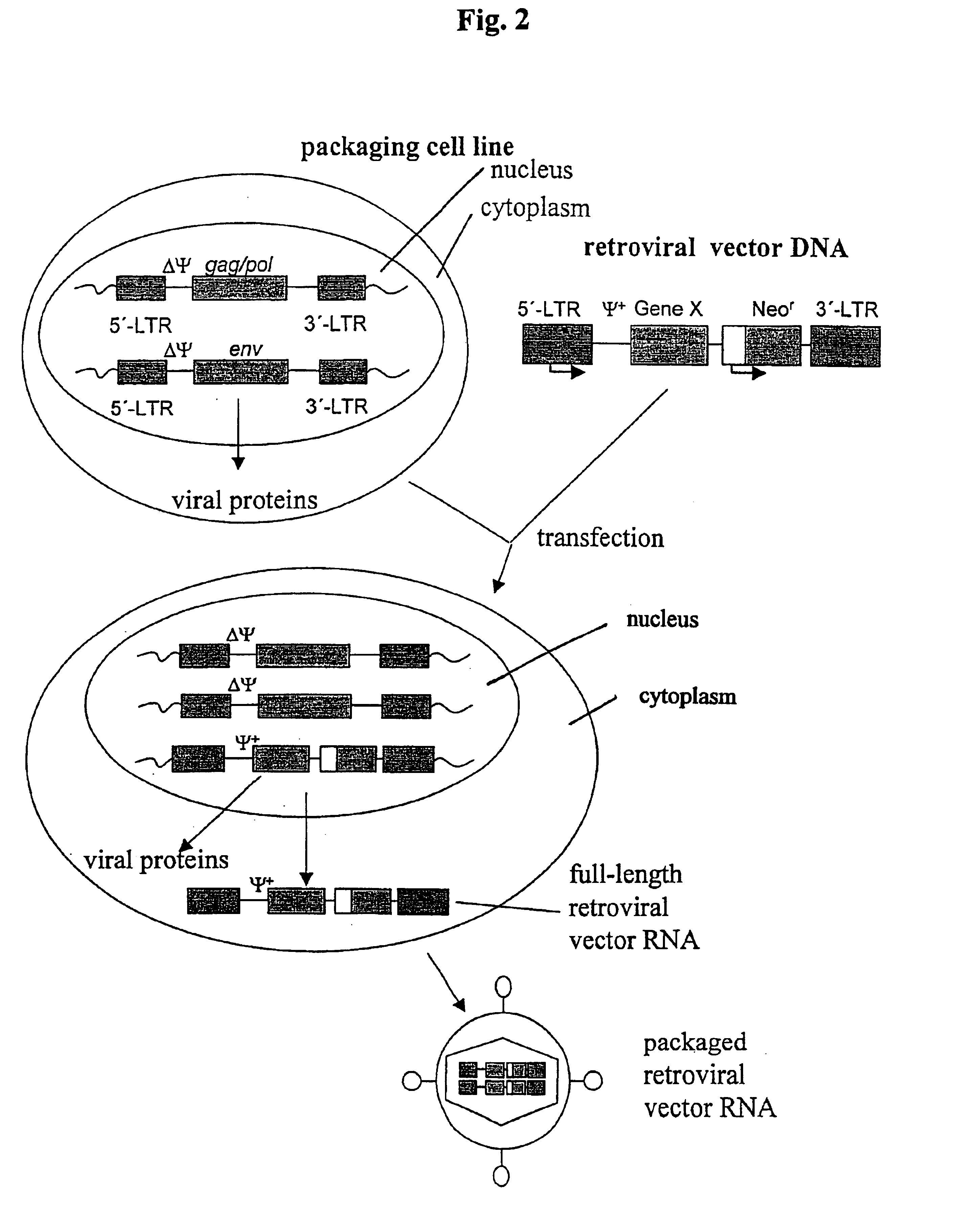
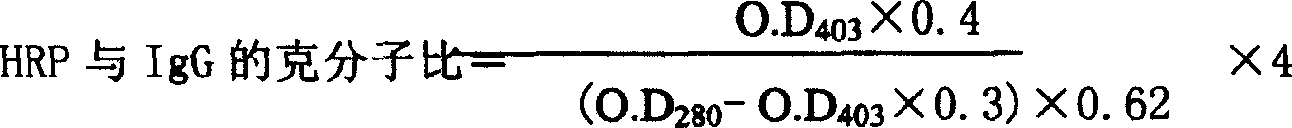Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
836 results about "HIV Positivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infected with the human immunodeficiency virus (HIV), the cause of acquired immunodeficiency syndrome (AIDS).
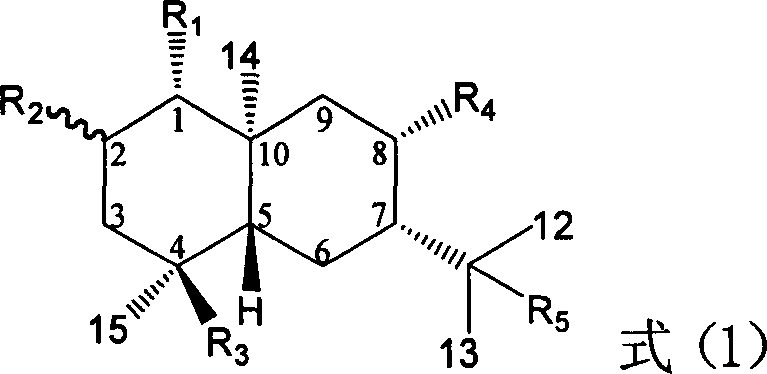
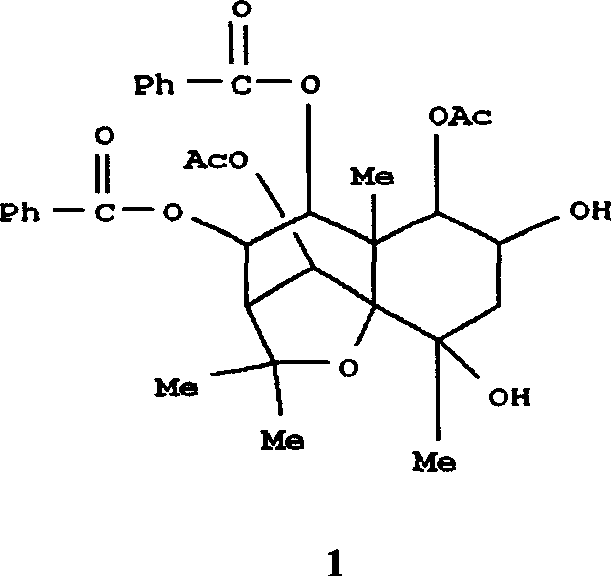
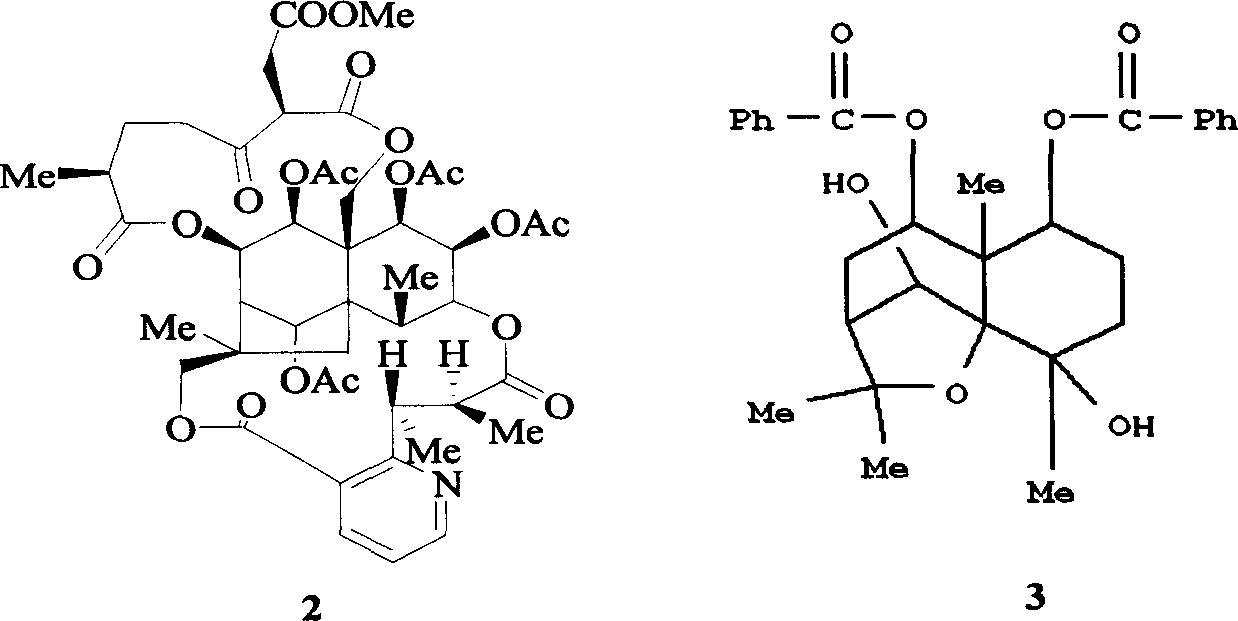
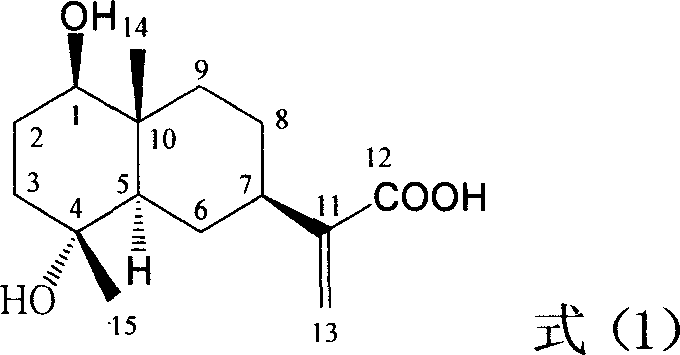
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
HTLV-I/HTLV-II assay and method
InactiveUS6110662AMicrobiological testing/measurementBiological material analysisPeptide antigenSerum samples
Method and assay kit for positively identifying HTLV-I and HTLV-II infection from human serum samples. The kit includes peptide antigens from the C-terminal regions of HLTV-I p19 and HTLV-II p21 gag proteins, and peptide antigens from the HLTV-I and HTLV-II env proteins immobilized on a solid support. After reaction of the serum sample with the solid support, an antibody-detection reagent in the kit is added to the support, to detect binding of human serum antibodies to each of the peptide antigens separately. The test allows positive identification of HTLV-I or HTLV-II when antibody binding to each HTLV-I or HTLV-II gag and env peptide antigen, respectively, is observed. Also disclosed is a kit for screening human sera for evidence of HTLV-I or HTLV-II infection.
Owner:GENELABS TECH INC +1
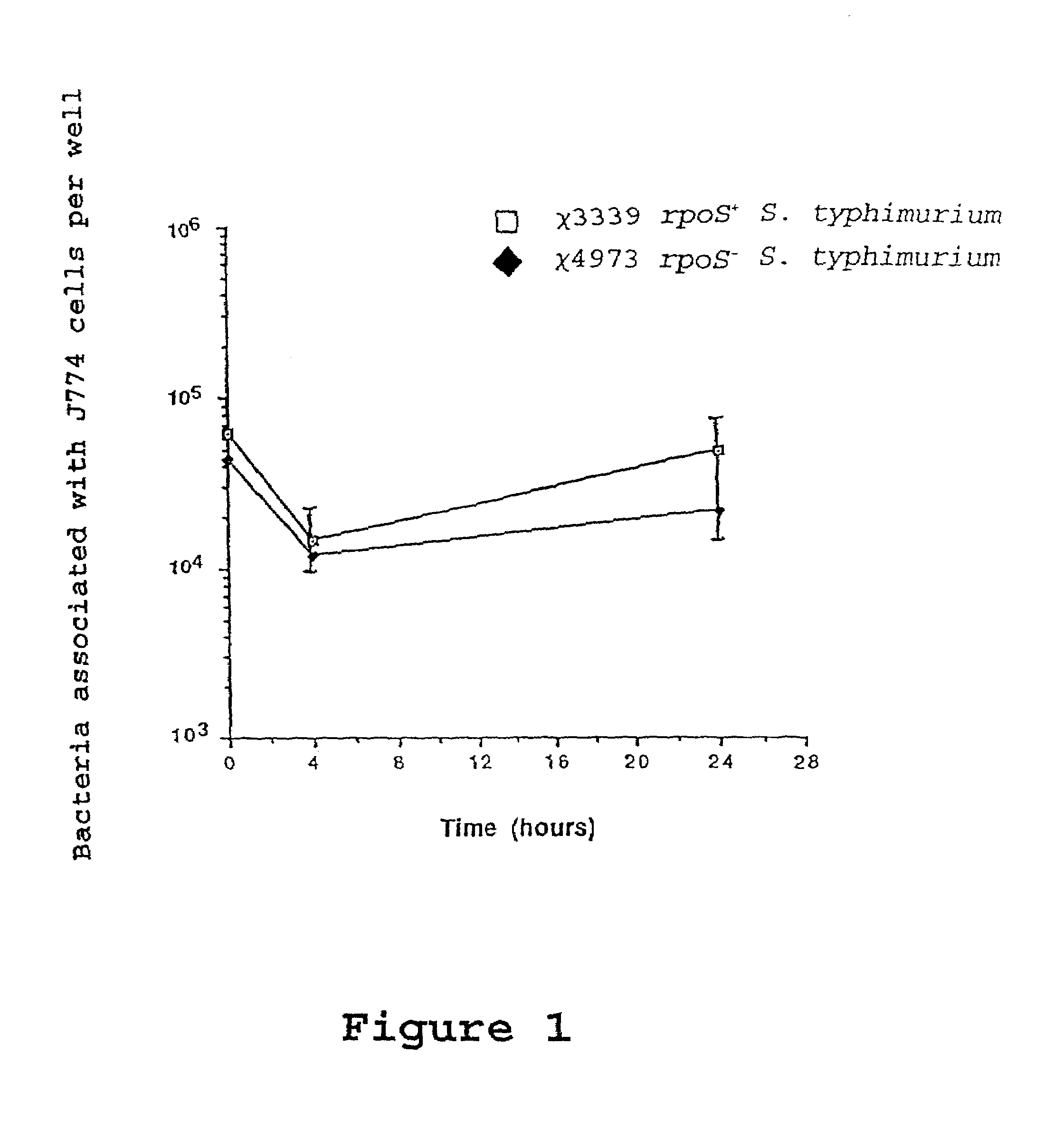
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
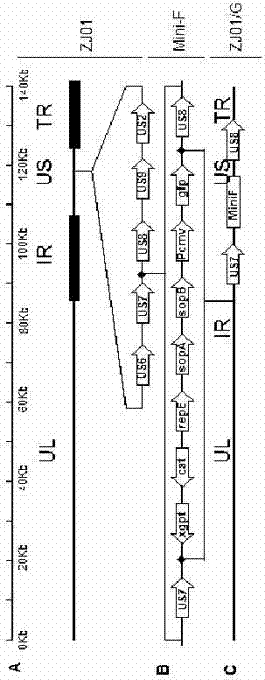
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
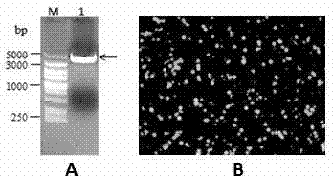
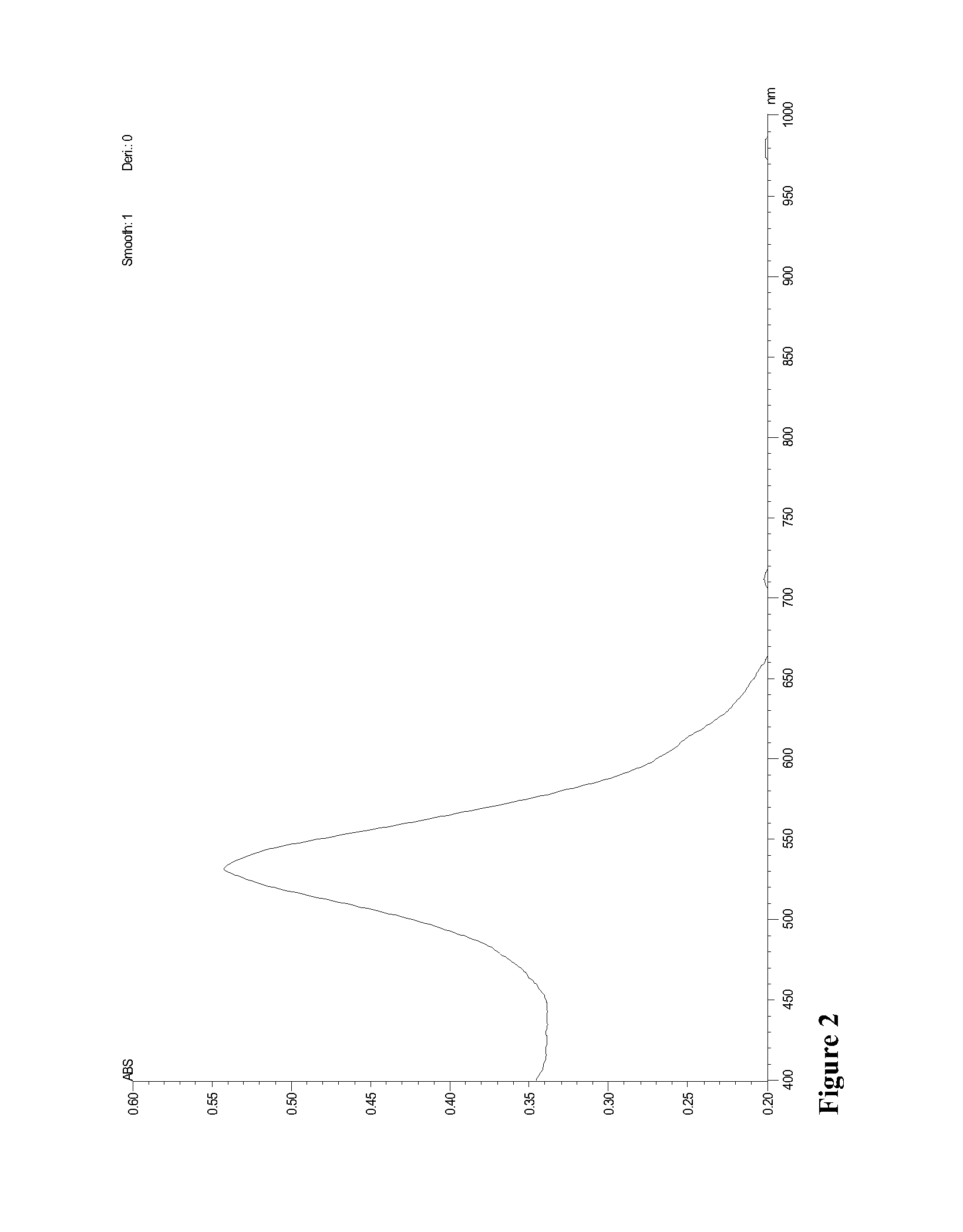
Direct detection of disease biomarkers in clinical specimens using cationic nanoparticle-based assays & versatile and green methods for synthesis of anisotropic silver nanostructures
InactiveUS20150017258A1Enhances thermalImprove electricityBiocideInorganic active ingredientsSilica nanoparticlesPurification methods
A gold nanoparticle-based assay for the detection of a target molecule, such as Hepatitis C Virus (HCV) RNA in serum samples, that uses positively charged gold nanoparticles (AuNPs) in solution based format. The assay has been tested on 74 serum clinical samples suspected of containing HCV RNA, with 48 and 38 positive and negative samples respectively. The developed assay has a specificity and sensitivity of 96.5% and 92.6% respectively. The results obtained were confirmed by Real-Time PCR, and a concordance of 100% for the negative samples and 89% for the positive samples has been obtained between the Real-Time PCR and the developed AuNPs based assay. Also, a purification method for the HCV RNA has been developed using HCV RNA specific probe conjugated to homemade silica nanoparticles. These silica nanoparticles have been synthesized by modified Stober method. This purification method enhanced the specificity of the developed AuNPs assay. The method can detect a target molecule, such as HCV RNA in serum, by employing modified silica nanoparticles to capture the target from a biological sample followed by detection of the captured target molecule using positively charged AuNPs. The assay is simple, cheap, sensitive and specific. Another aspect of the invention is anisotropic silver nanoparticles and methods of their use.
Owner:AMERICAN UNIV OF CAIRO AUC
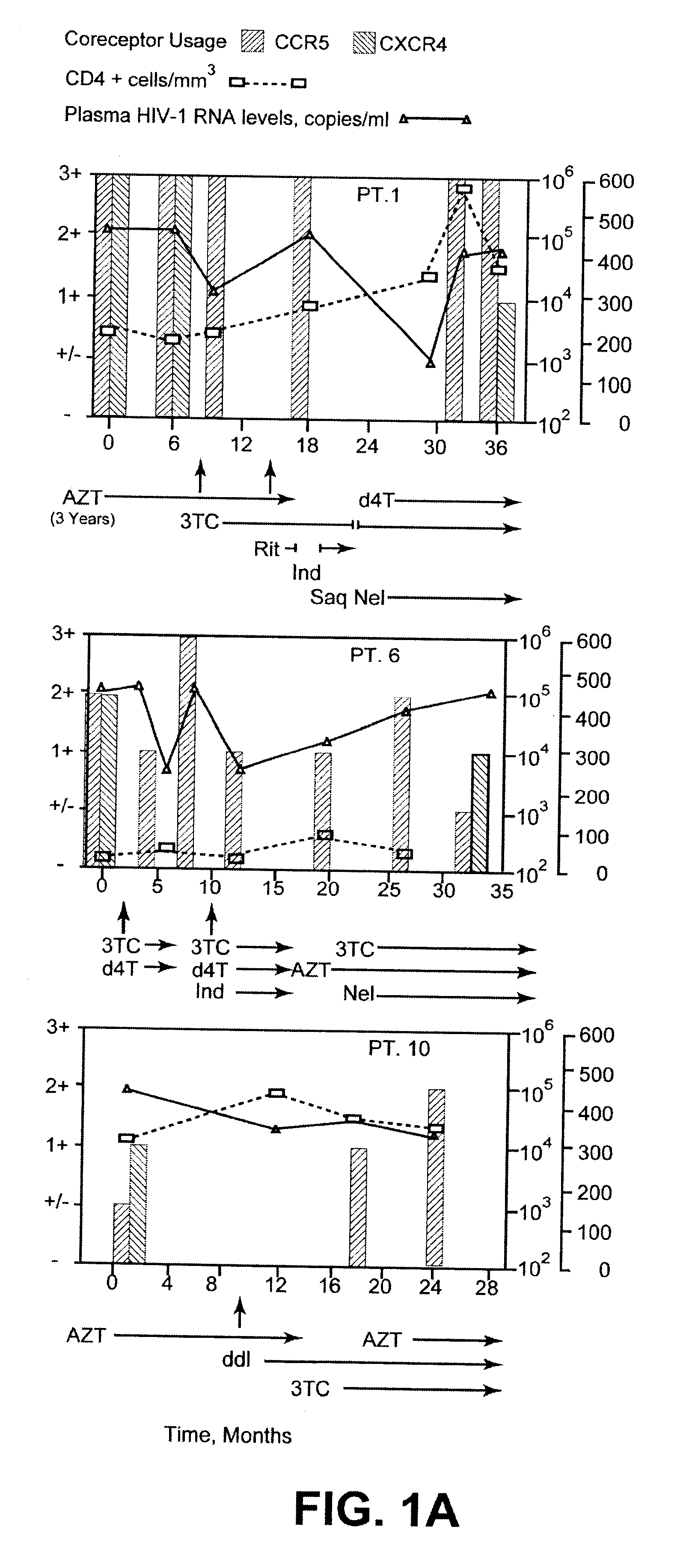
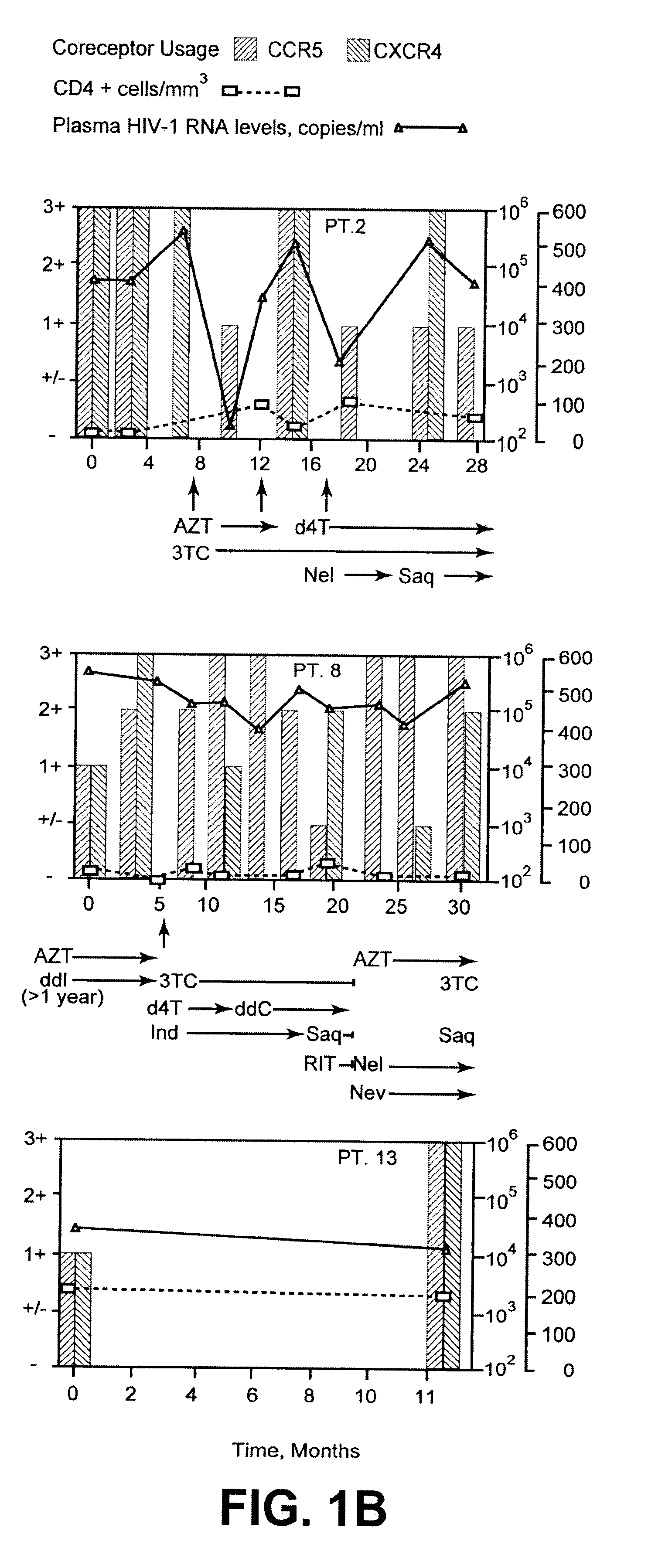
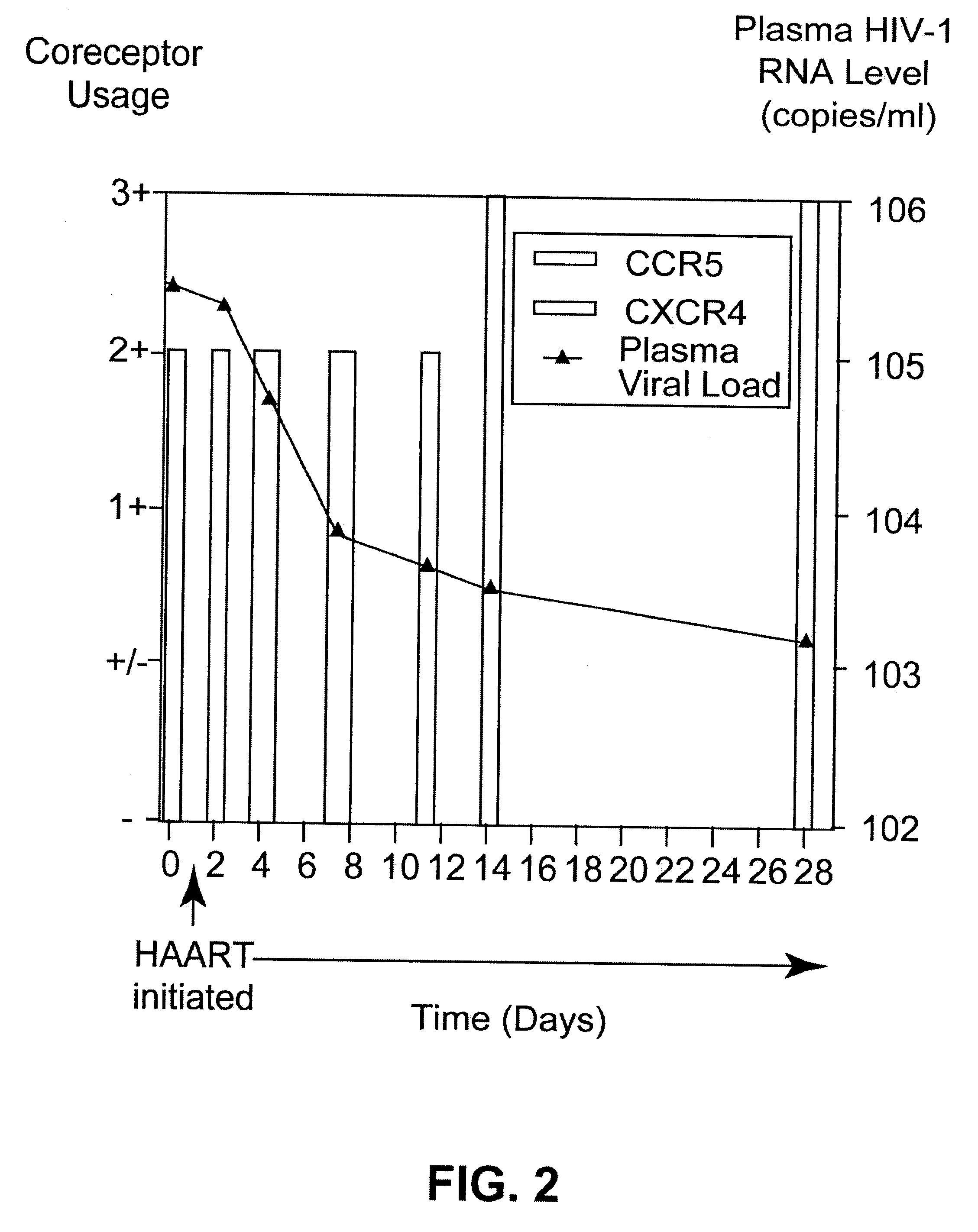
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS6727060B2Accurate predictionMore effectivenessMicrobiological testing/measurementArtificial cell constructsImmunodeficiency virusHIV positives
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
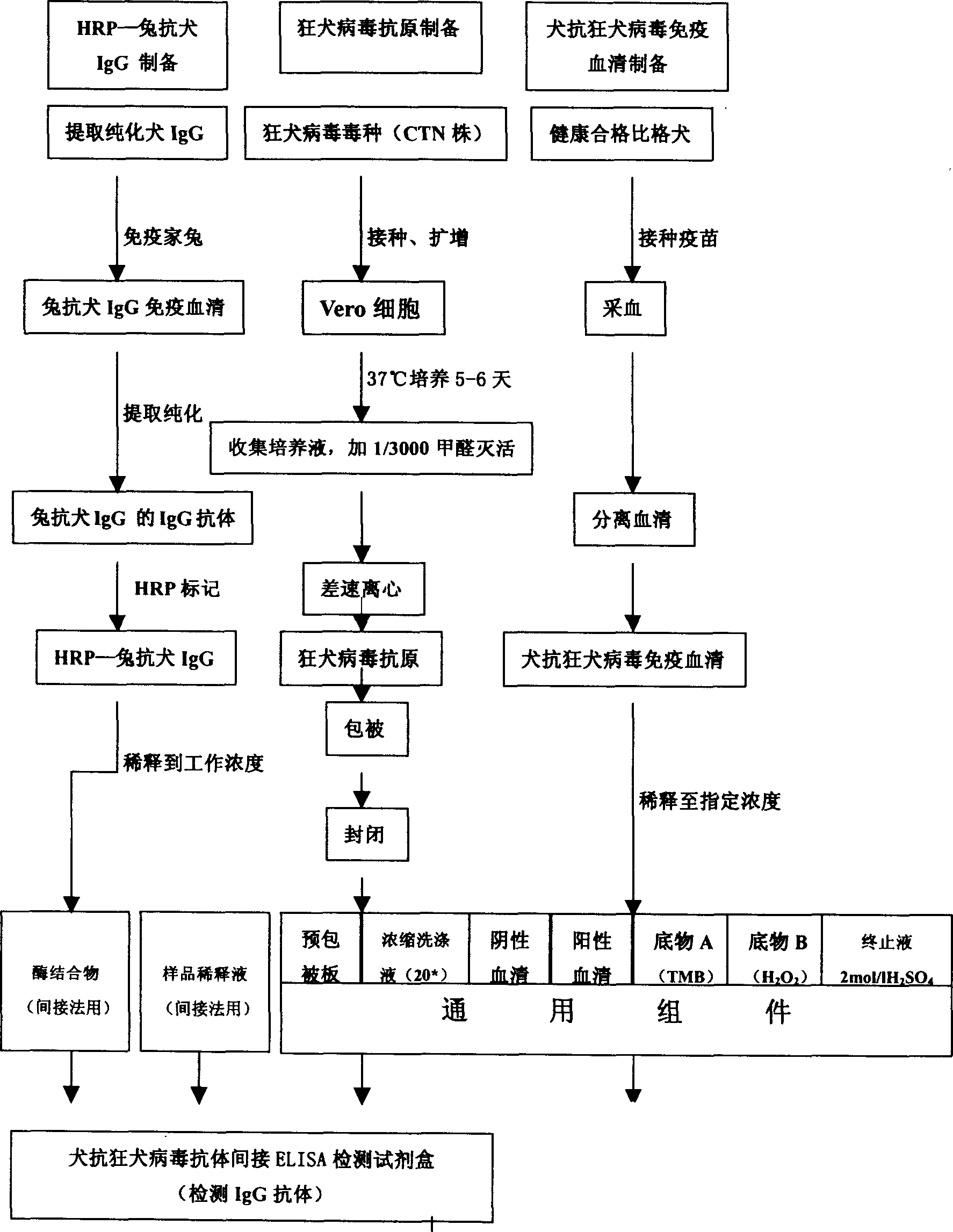
IgG kit for detecting streetvirus of dogs using indirect enzyme immunosorbent assay and preparation method thereof
The invention refers to a kind of detecting reagent box and the manufacturing method, concretely refers to the reagent which is indirect enzyme immune sorption experiment for detecting rabies virus IgG and the manufacturing method. The reagent box compositions are: beforehand enclosed rabies virus antigen enzyme label board, sample diluting solution, HRP-rabies resisting IgG enzyme compound, condensed washer solvent, substrate and stopping liquid. The specificity of the reagent can reach 100%; the sensitivity is 1:640; the accuracy (the variation coefficient) is 6.98%. The reagent uses indirect ELISA to detect the rabies virus IgG antibody.
Owner:湖北省预防医学科学院
Combined therapy for treatment of HIV infection
InactiveUS7094413B2Good curative effectReduce resistanceBiocideSugar derivativesImmunodeficiency virusGastrointestinal complications
The present invention relates to pharmaceutical preparations and methods for treating individuals infected with the human immunodeficiency virus (HIV). The pharmaceutical preparations comprise an immunomodulating agent and a anti-retroviral compound. The pharmaceutical preparations are used to treat HIV infected patients, particularly for gastrointestinal complications arising from viral infection. In addition, the pharmaceutical preparations of the present invention have the effect of raising the levels of CD4+ single positive and CD4+ and CD8+ double positive T cells, thus promoting restoration and normalization of the immune system following HIV infection.
Owner:SANGSTAT MEDICAL +1
Peptides which elicit a high neutralizing antibody titer, cytotoxic T lymphocyte response and T helper cell response in a broad range of MHC type recipients
InactiveUS7094405B1High titerHigh titer of neutralizing antibodyPeptide/protein ingredientsAntibody mimetics/scaffoldsV3 loopT helper cell
Peptide constructs comprised of multideterminant T helper peptides from the envelope glycoprotein of HIV previously identified to induce proliferative responses in four different haplotypes of mice and IL-2 responses in 52-73% of HIV positive, flu positive patients (cluster peptides), were co-linearly synthesized with the peptide 18 of the V3 loop of HIV-1 gp 160, corresponding to the principal neutralizing determinant of HIV-IIIB and also shown to contain a dominant CTL epitope. Cognate help for peptide 18 antibody was elicited following a single immunization in all strains of mice which had previously responded to a T cell epitope encompassed by the peptides. In two strains of mice, the level of neutralizing antibody achieved was comparable to levels adequate for protection from homologous viral challenge in chimpanzees. After a single boost, much higher antibody titers for 90% neutralization in the range of 1:1000 to 1:16,000 were achieved. Spleen cells from mice of three distinct MHC haplotypes sharing the Dd class I MHC molecule but with different class II molecules, immunized with the compound peptides, exhibited enhanced gp160-specific CTL activity.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
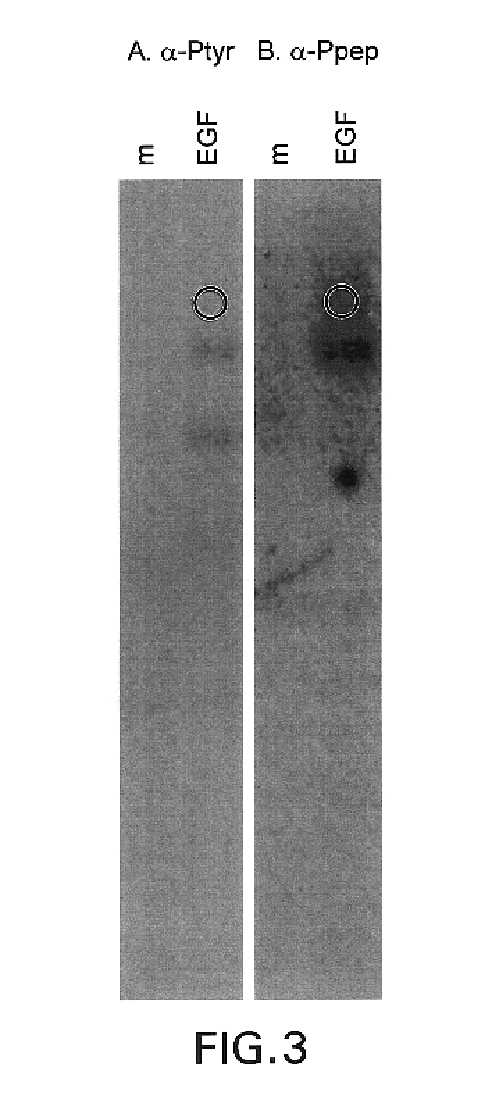
Phosphopeptide-specific antibodies that are activity specific; methods of production and antibody uses
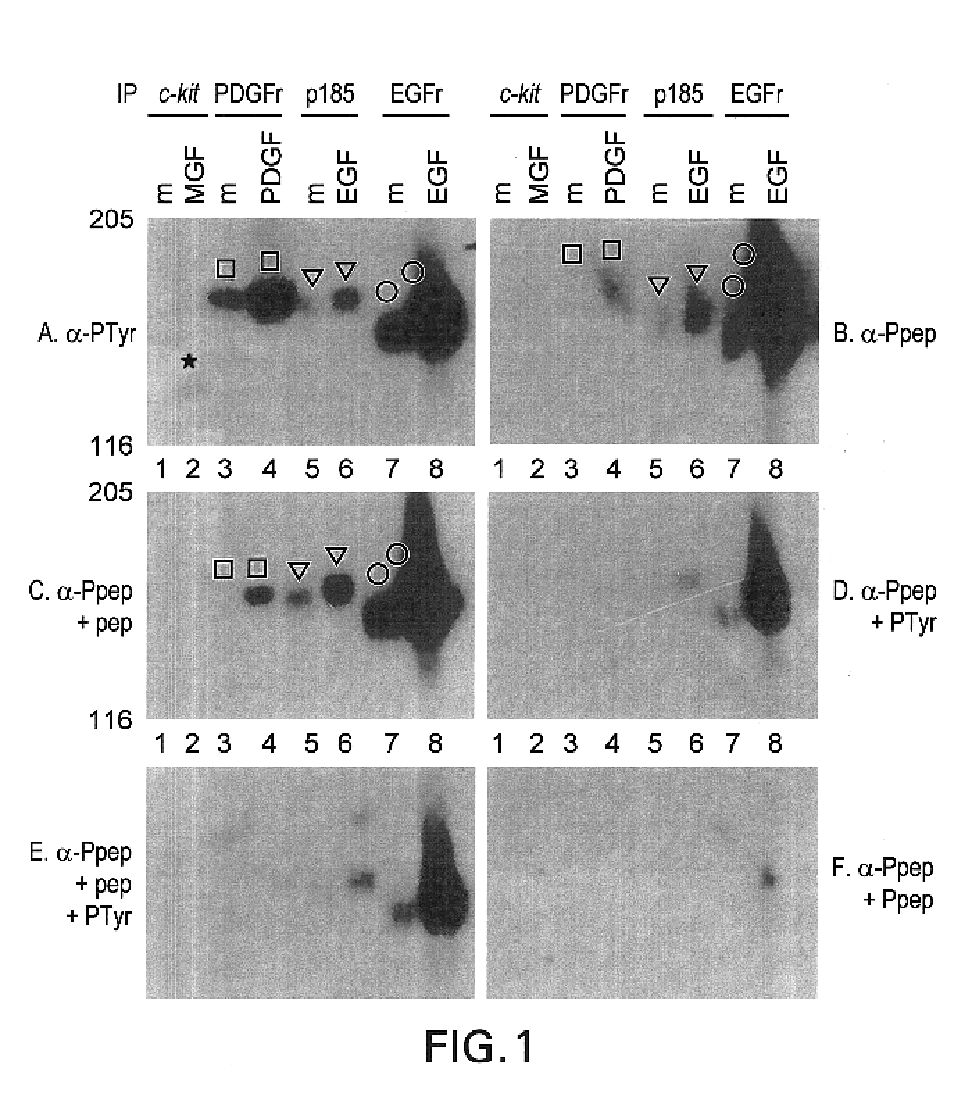
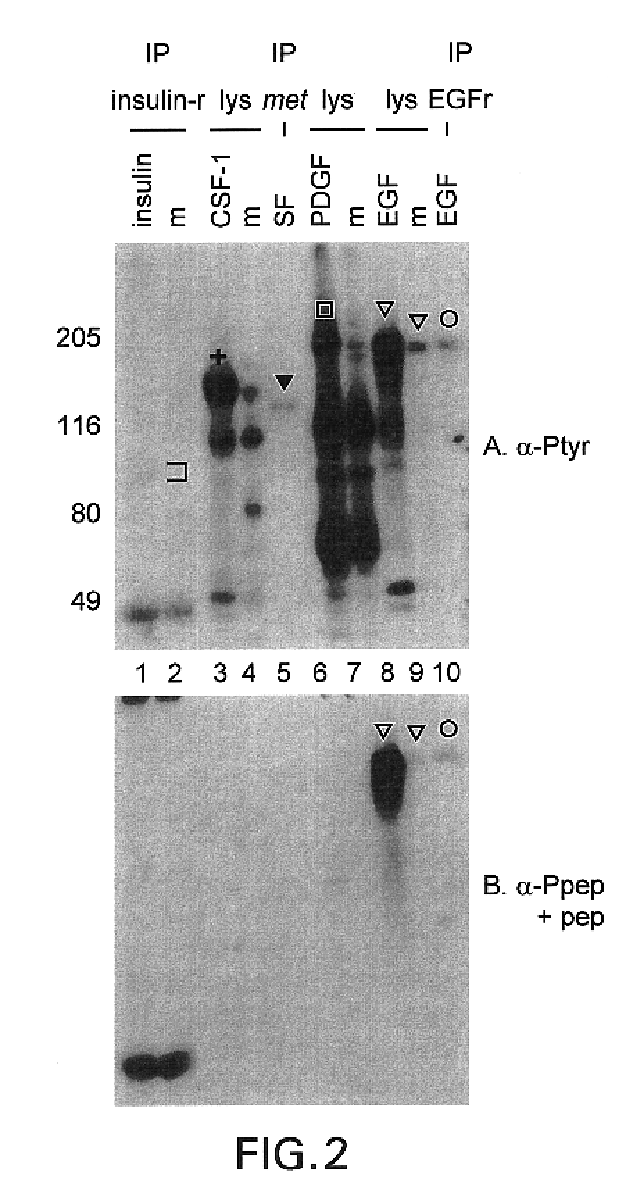
This invention relates to growth regulatory proteins expressed in various disease states, especially receptor tyrosine kinases and similar growth factor receptors. Prior to the Applicants invention, it was not clear that specific antibodies could be generated that recognized peptide epitopes comprising phosphotyrosine in the context of its surrounding amino acid sequence. Applicants generated antibodies to such phosphotyrosine specific peptides, distinct from antibodies that recognize only phosphotyrosine itself. The invention includes methods for producing phosphopeptide specific antibodies by removing contaminating antibody specificities by negative and / or positive selections. Phosphospecific antibodies and their uses in immunodetection, diagnostic or therapeutic applications are also disclosed.
Owner:PHOSPHOPROTEOMICS
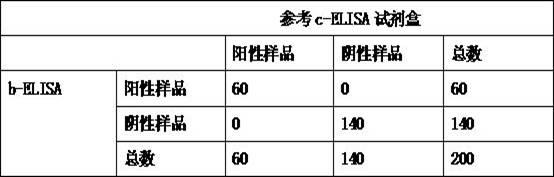
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
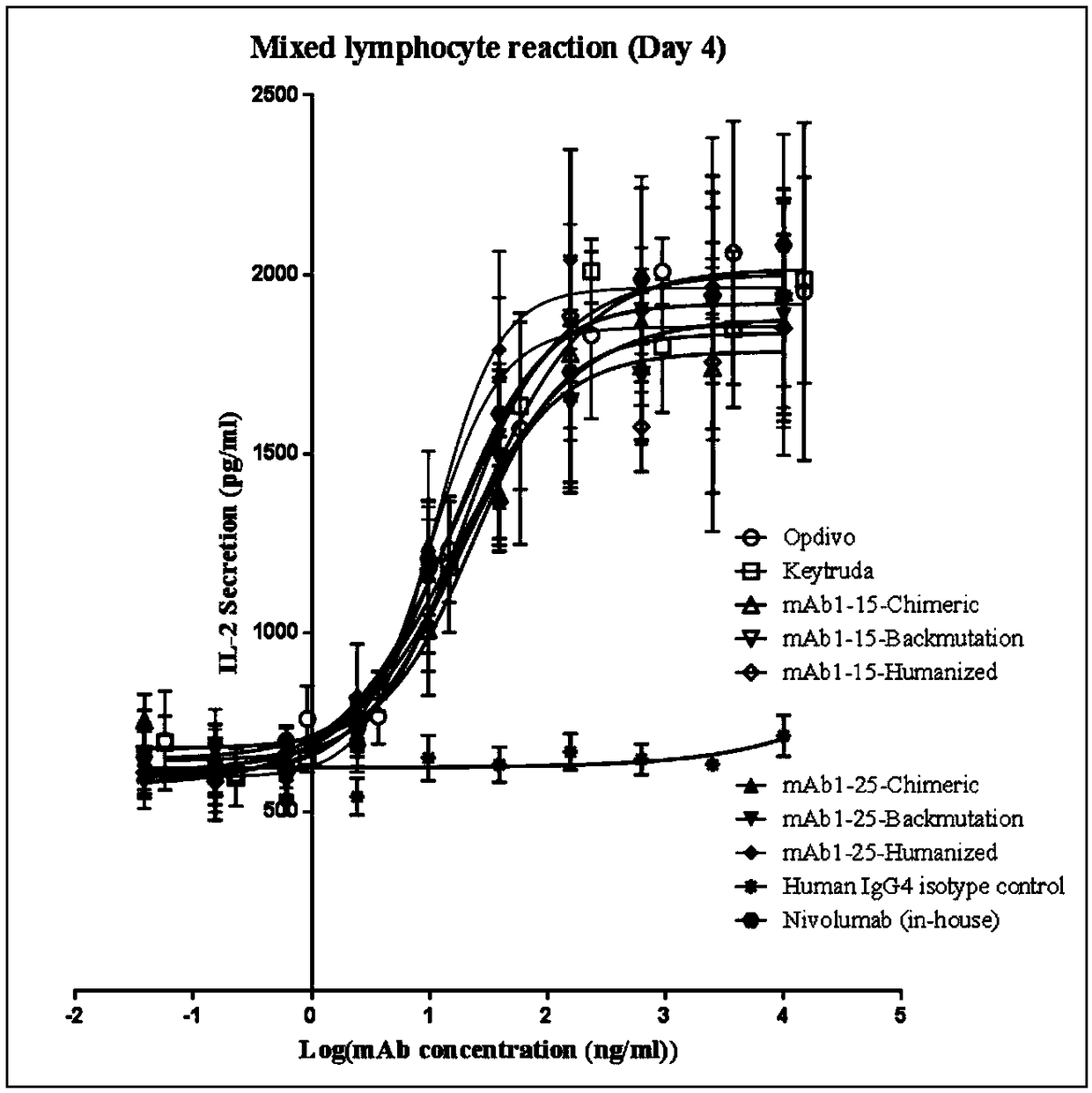
Monoclonal antibody against PD-1, and preparation method and application thereof
InactiveCN108341871AImprove biological activityEffective combinationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseasePD-L1 Positive
The invention specifically discloses a monoclonal antibody against human PD-1, and a preparation method and application thereof, belonging to the field of antibodies. The monoclonal antibody against human PD-1 in the invention has good biological activity, and can effectively bind to the extracellular region of a human PD-1 protein receptor, block PD-1 protein at a protein level and a cellular level, prevent binding of the PD- 1 protein to ligand PD-L1 and enhances immunity. The monoclonal antibody can be individually used or used in combination with other anti-tumor drugs for tumor immunotherapy and the diagnosis and screening of patients with PD-L1 positive tumors, and has good prospects in preparation of drugs for treating tumors, anti-autoimmune diseases and the like.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
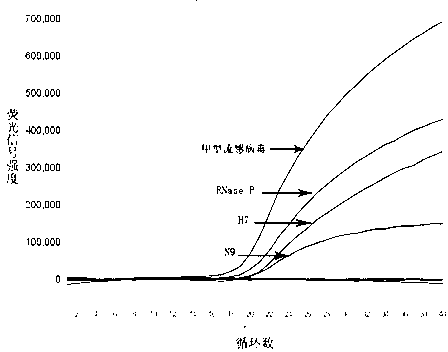
Fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting influenza A virus subtype H7N9
ActiveCN103275862AQuantitatively accurateLow costBioreactor/fermenter combinationsBiological substance pretreatmentsConserved sequenceInfluenza Viruses Type A
The invention provides a fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) kit for detecting an influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit can be used for detection of influenza A viruses and the influenza A virus subtype H7N9. The fluorescent quantitative RT-PCR kit comprises a quantitative RT-PCR reaction solution, an enzyme mixed liquor, a primer and probe mixed liquor, standard substances of influenza A viruses, H7, N9 and RNaseP, positive reference substances of influenza A viruses, H7, N9 and RNaseP), and negative reference substances. Specific primers and probes are designed according to conserved sequences of influenza A viruses, H7 and N9. The RNaseP primers and probes are used as internal references. Through the one-step quadruple real-time fluorescent RT-PCR technology, the influenza A virus and the influenza A virus subtype H7N9 in the sample can be fast and accurately detected. The fluorescent quantitative RT-PCR kit has a reasonable design, very high singularity, sensitivity and repeatability, can be used for laboratory emergency diagnosis and fast screening of an epidemic disease caused by the influenza A virus subtype H7N9, and for an epidemiology study on the influenza A virus and the influenza A virus subtype H7N9 causing fever and respiratory tract syndrome.
Owner:ZHEJIANG UNIV
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Kit for nucleic acid combined detection of influenza virus A, influenza virus B and respiratory syncytial virus
InactiveCN105400907AEasy to prepareAvoid pollutionMicrobiological testing/measurementMicroorganism based processesRespiratory virusRespiratory syncytial virus (RSV)
The invention provides a kit for nucleic acid combined detection of the influenza virus A, the influenza virus B and the respiratory syncytial virus. The kit comprises RT-PCR reaction liquid, an RT-PCR enzyme mixture, a respiratory virus primer probe, internal reference, negative contrast, clinical positive contrast and strong positive contrast. The one-step RT-PCR reaction can be directly conducted on the well-extracted respiratory virus nucleic acid, the influenza virus A, the influenza virus B and the respiratory syncytial virus in a sample can be detected in a classified and qualitative mode, the gene of the internal reference serves as the internal contrast, and contamination is prevented through UNG enzymes. The kit is simple in one-step amplification method, short in procedure, easy and convenient to operate, capable of preventing contamination, high in detection result specificity, high in sensitivity, clear in result, high in credibility, and capable of being used for qualitatively authenticating and detecting the influenza virus A, the influenza virus B and the respiratory syncytial virus in a human nose pharynx-mop sample.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD
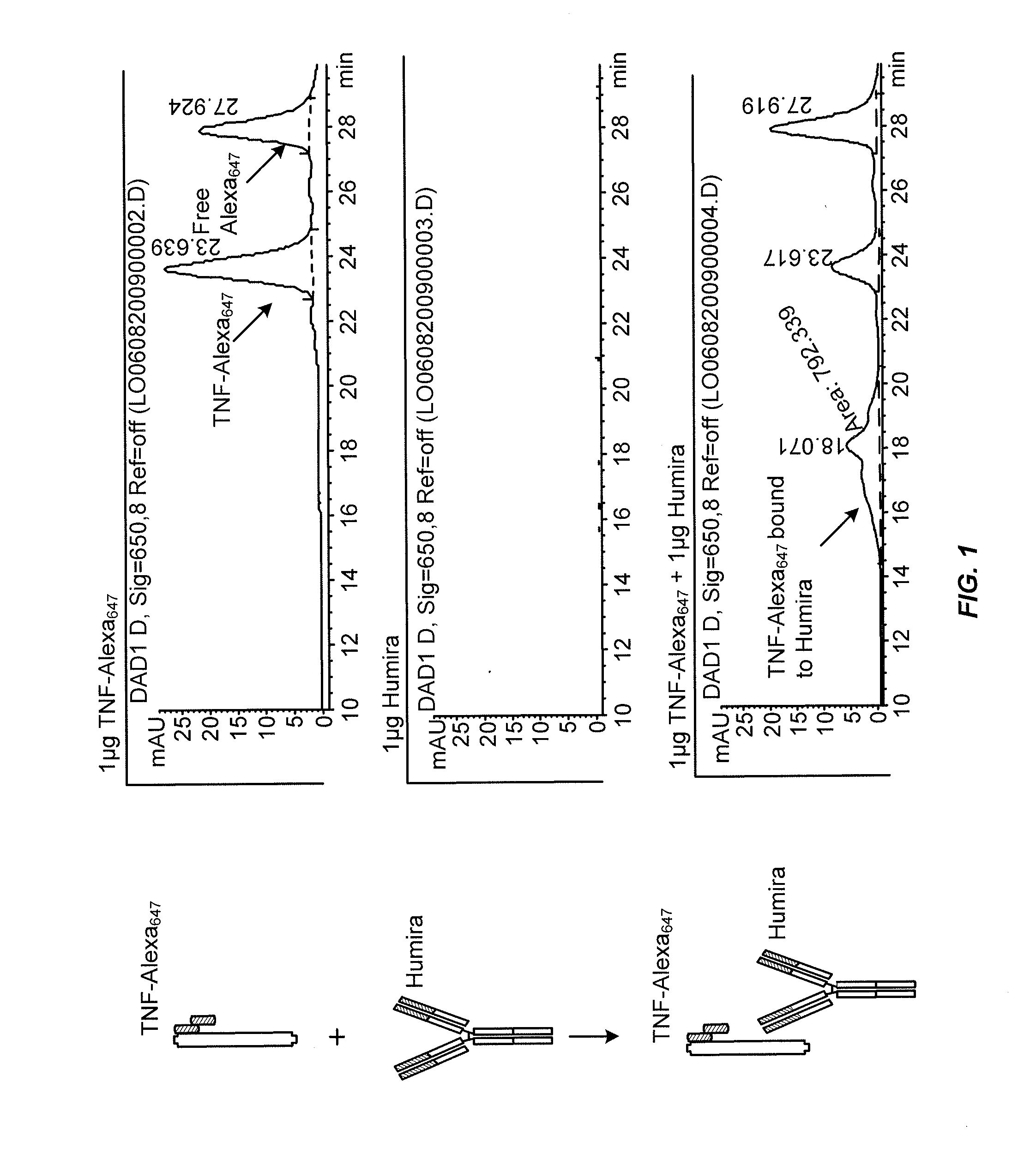
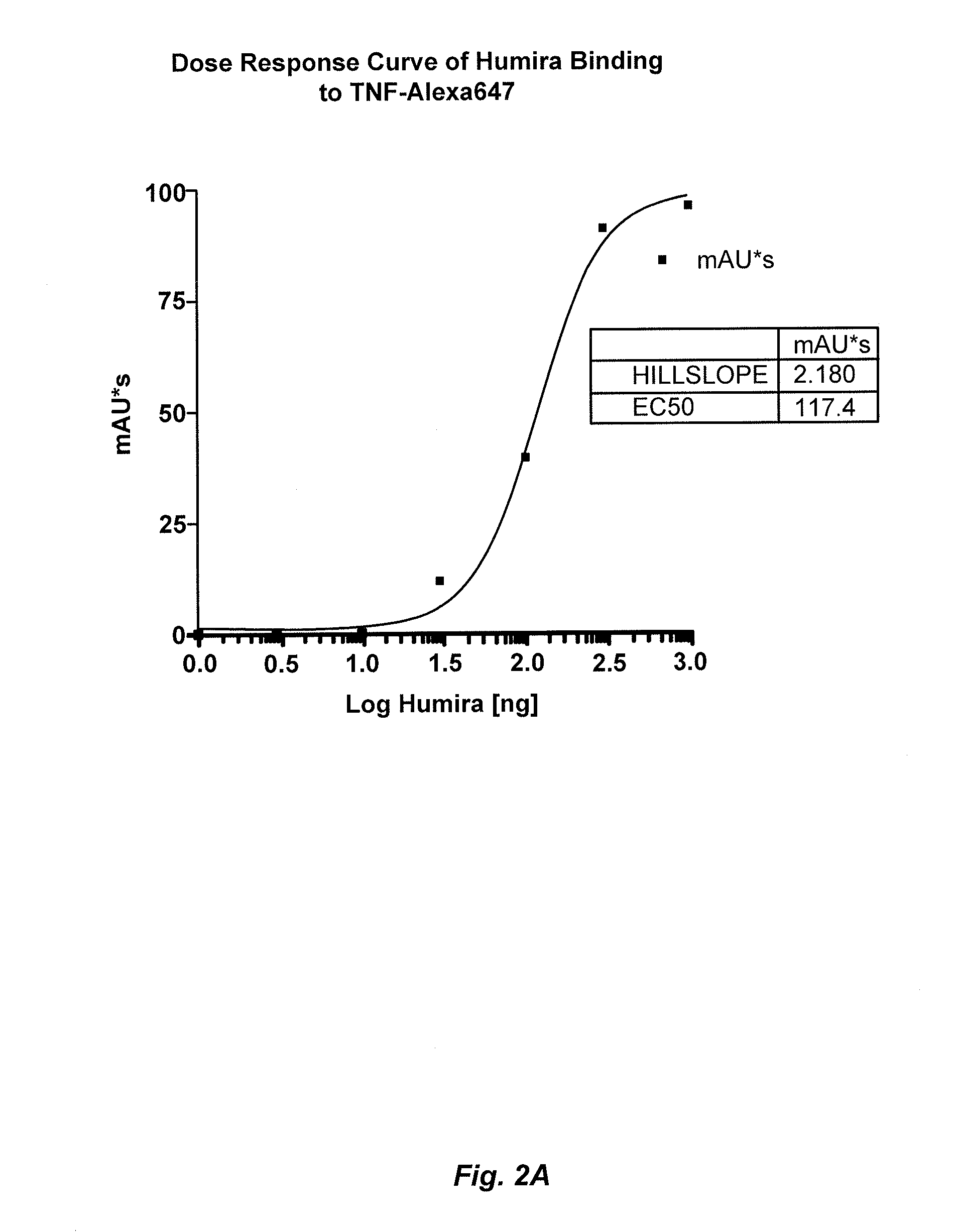
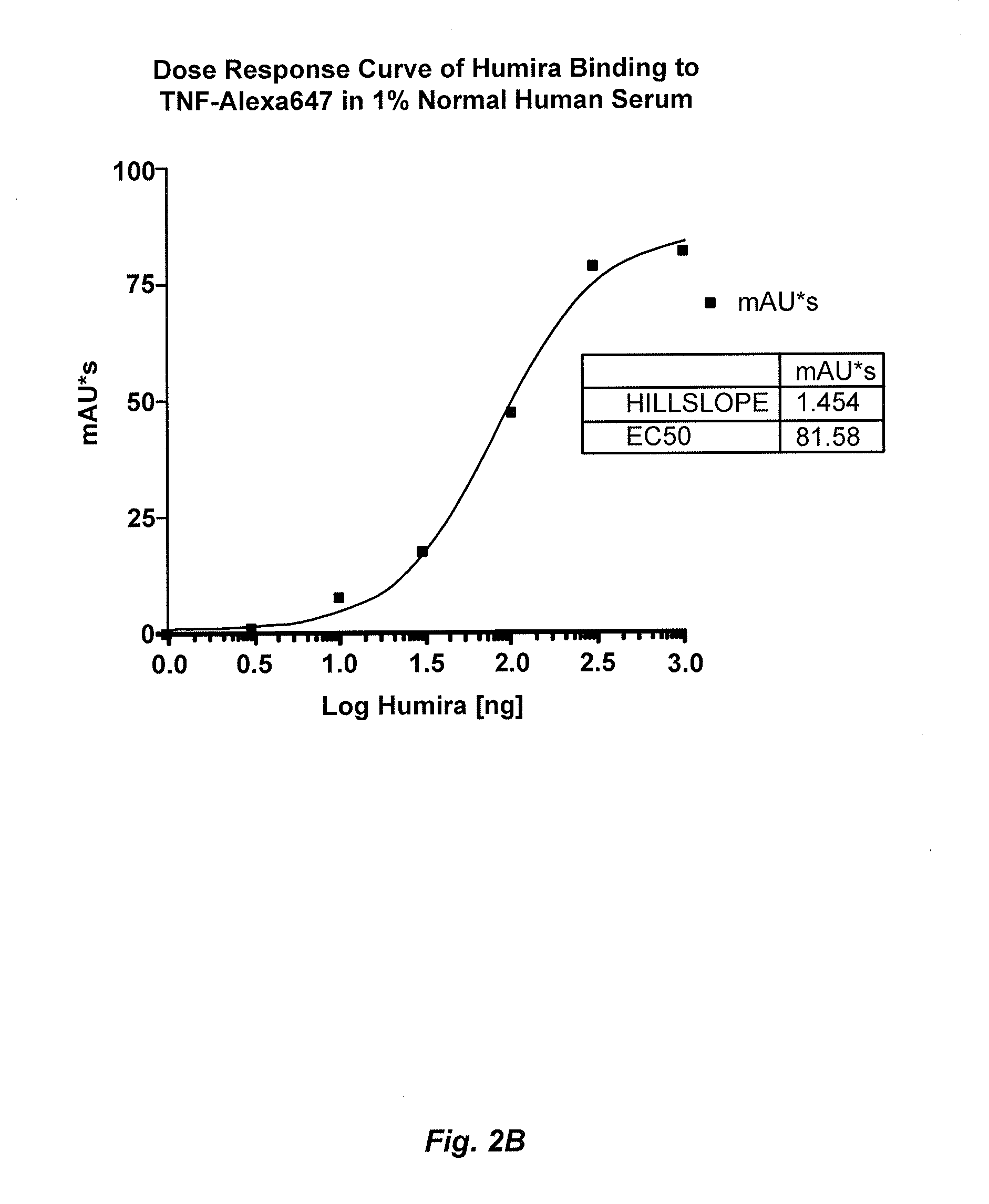
Methods for determining Anti-drug antibody isotypes
ActiveUS20130344621A1Convenient treatmentLow toxicityComponent separationBiological testingAntiendomysial antibodiesAssay
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFα drug such as REMICADE™ (infliximab) or HUMIRA™ (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFα inhibitors for the treatment of TNFα-mediated disease or disorders.
Owner:PROMETHEUS LAB
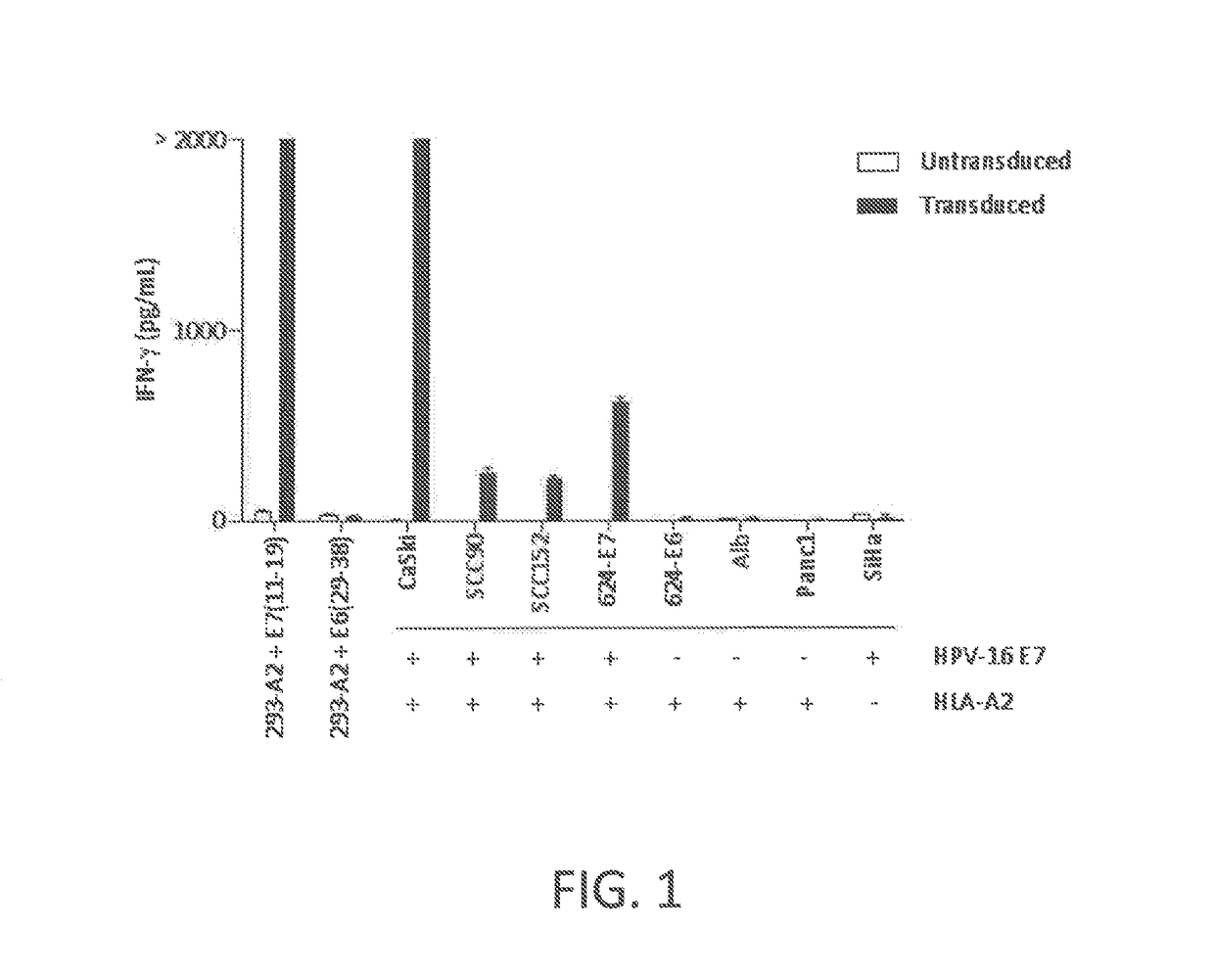
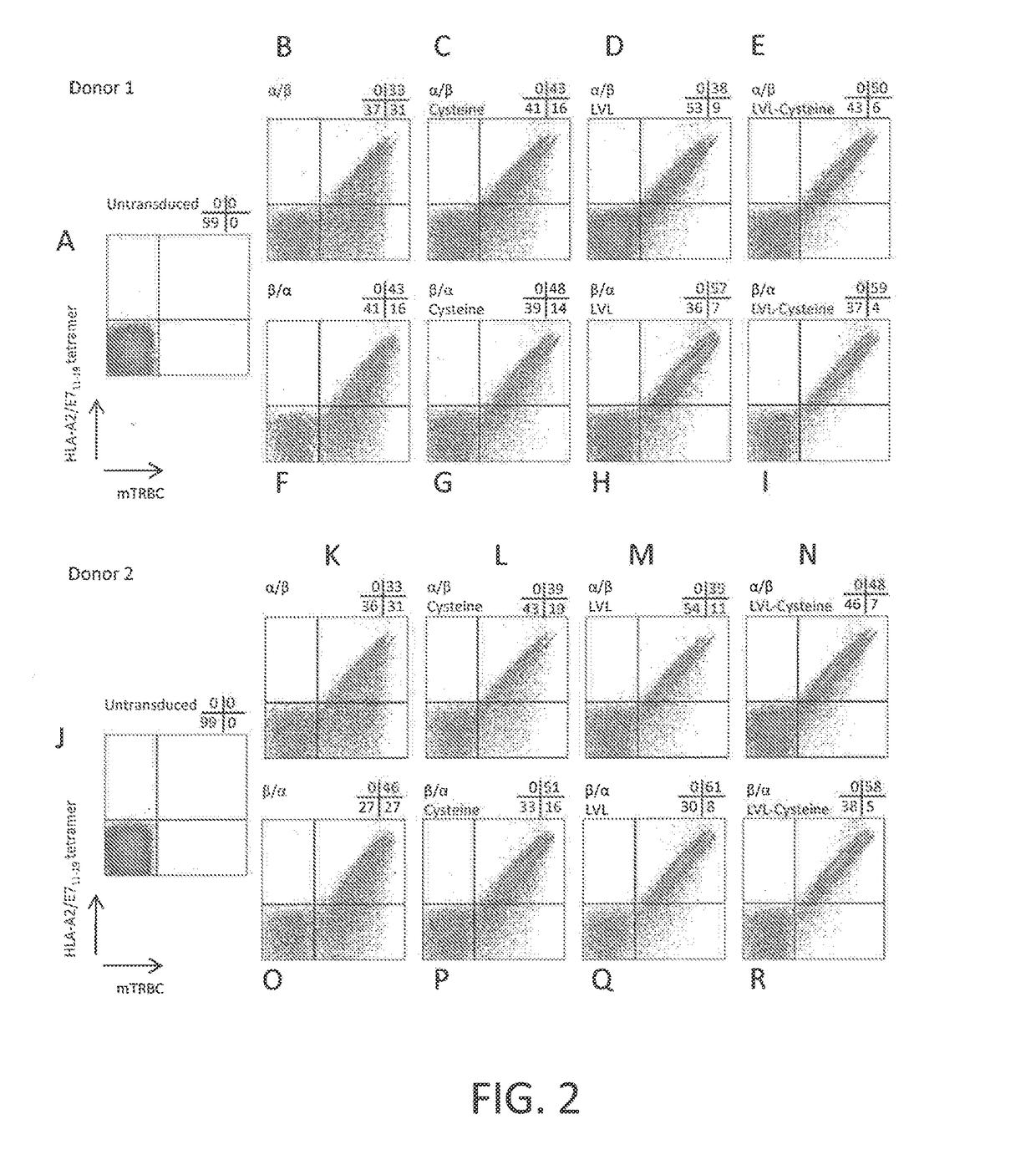
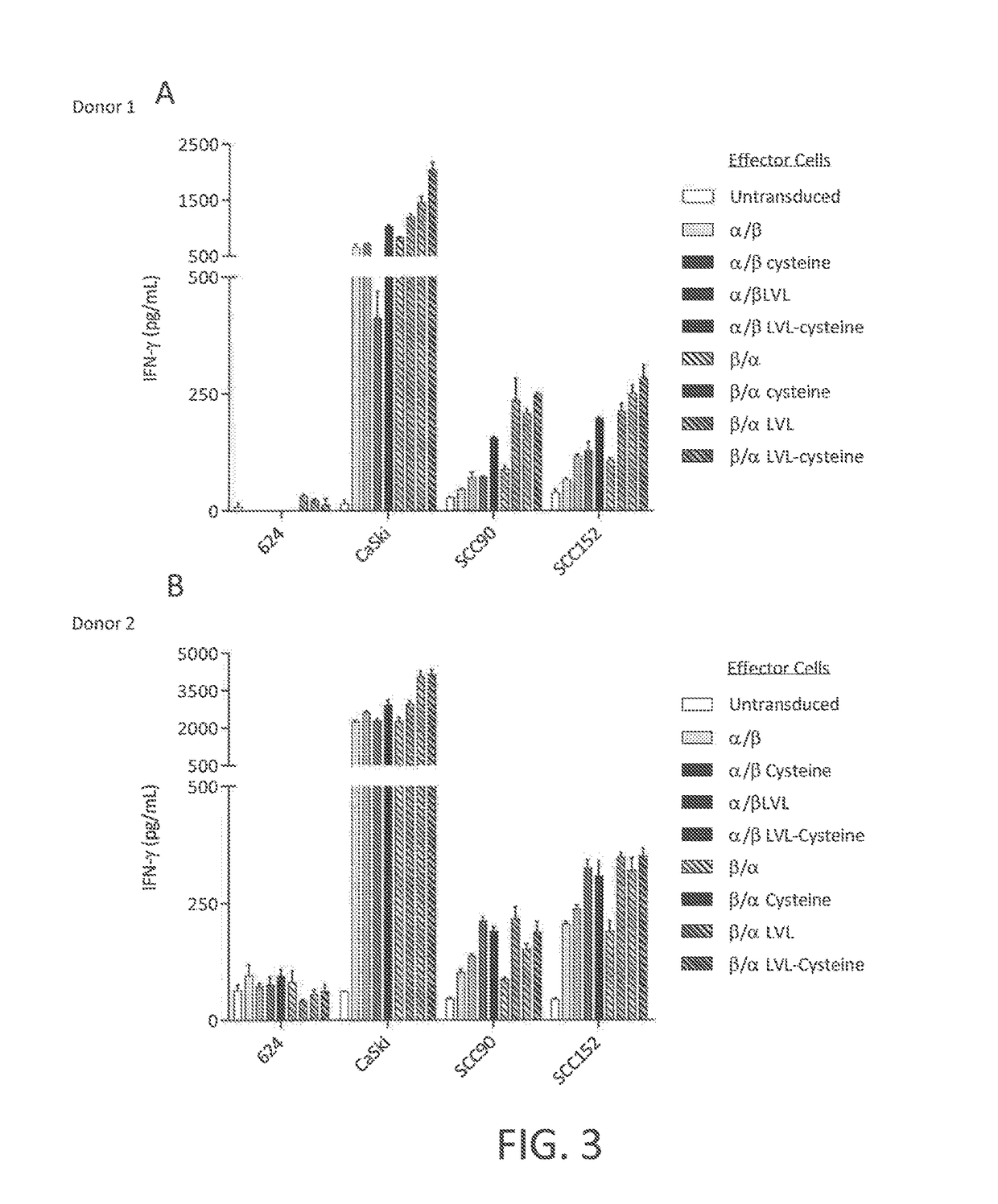
Anti-human papillomavirus 16 e7 t cell receptors
ActiveUS20170145070A1Minimize ToxicityImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a synthetic T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E7, E711-19. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
Chemiluminescence immune analysis determination reagent kit for detecting Toxoplasma Gondi IgM antibody
The invention discloses a toxoplasma gondii IgM antibody detection kit combined with the FITC-anti-FITC indirect coating technology and the chemiluminescent immunoassay technology, and a preparation method thereof. The kit of the invention is composed of a negative control, a positive control, solid-phase vectors for anti-FITC antibodies, anti-human Mu-chain monoclonal antibodies of FITC markers, toxoplasma gondii antigens which are marked by horse radish peroxidase, chemiluminescent substrates and concentrated washing solutions. The kit of the invention can be used as the aided detection index for prenatal prepotency diagnosis, and has vital significances for improving the birth population quality and doing the family planning and the prepotency well.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
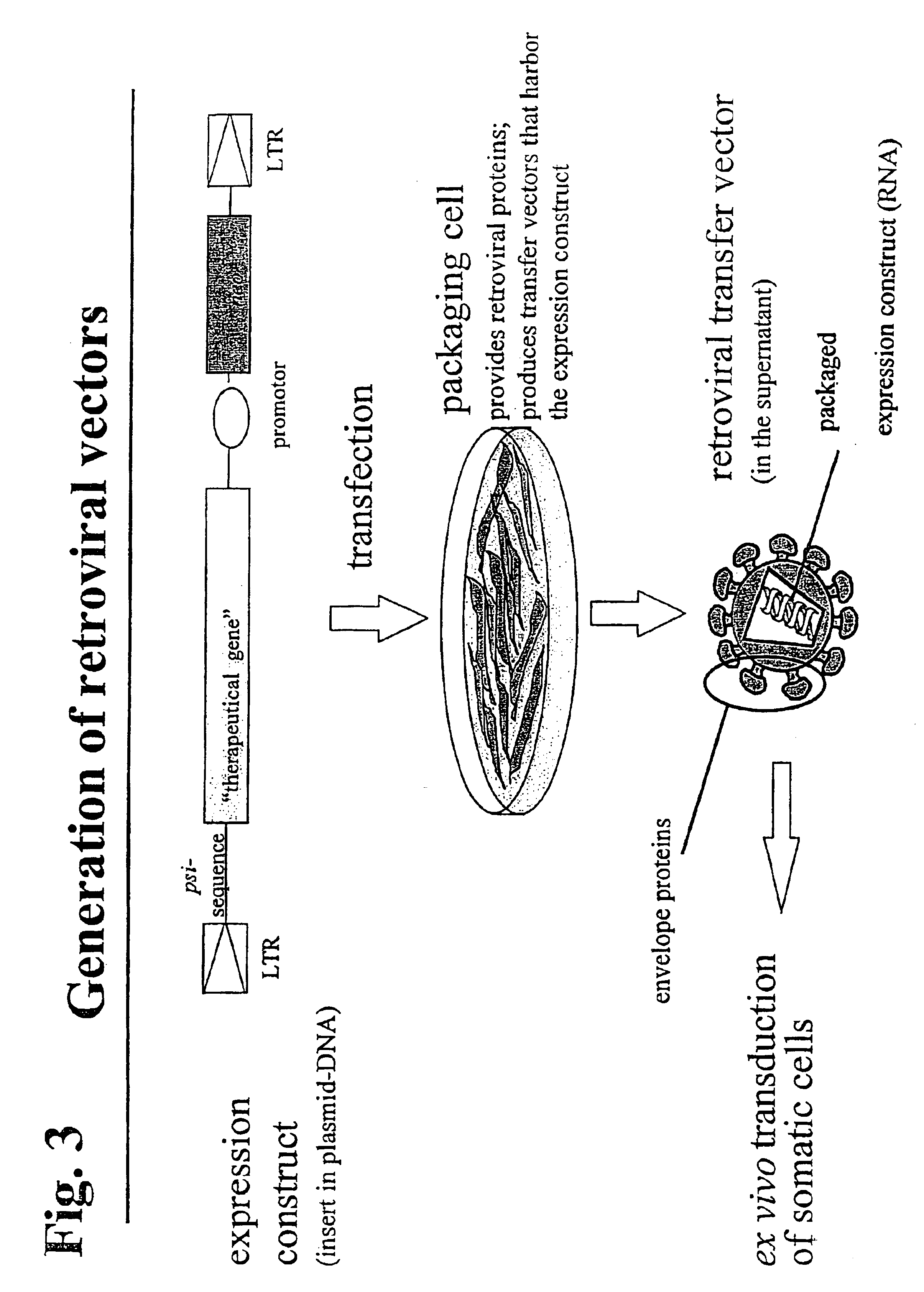
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
ActiveCN101196518AWide applicabilityLow costChemiluminescene/bioluminescenceBiological testingAntigenChemiluminescence
The invention relates to a diagnostic reagent kit for testing the hepatitis c virus (HCV) and the preparation and test method, which is to add the HCV recombinant antigen used for peridium into the buffer solution, blend it, move into the luminous microplate, make incubation for 18 hours under 4DEG.C, wash the luminous microplate, add into the confining liquid, leave the liquid after incubation and fully dry the luminous microplate to complete the preparation of the pre-peridium luminous microplate; combine the anti-human IgG used for marking and the horse radish peroxidase by improving the sodium periodate to complete the preparation of the enzyme marker; prepare the chemical luminous substrate solution A with luminal, Tween20 and luminous intensifier and prepare the chemical luminous substrate solution B with the hydrogen peroxide. The reagent kit also comprises the sample diluent and concentrated scrub solution. The negative corresponds to the normal human serum while the positive corresponds to the people with serum of pooled serum with HCV antibody. The reagent kit provided in the invention has much higher detection sensitivity than the ELISA, which is safe and reliable, easy to operate with low cost, and without any expensive full-automatic chemical luminous measuring apparatus required.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Composition, kit and method for detecting and typing viruses causing respiratory tract infection and application of composition, kit and method
ActiveCN111074011AReduce wasteReduce psychological burdenMicrobiological testing/measurementMicroorganism based processesBioinformaticsInfluenza B viruses
The invention relates to the field of molecular biology detection, in particular to detection of novel coronavirus, influenza A virus and influenza B virus. The invention provides a composition for detecting the viruses, and meanwhile, the invention further provides a kit containing the composition, application of the composition and a method for detecting and typing the viruses causing respiratory tract infection. The composition is combined with a fluorescent probe method, three viruses causing respiratory tract infection can be detected and typed at the same time in one tube, and the composition, the kit and the method have the advantages of being low in cost, high in flux, short in consumed time, easy and convenient to operate and capable of avoiding false positive caused by cross among samples and environmental pollution.
Owner:SANSURE BIOTECH INC
On-site detection immuno-chip and preparation method thereof and application
The invention discloses an on-site detection immuno-chip, comprising a chip carrier and an agarose gel layer which is paved on the chip carrier, wherein, the agarose gel layer is fixed with a plurality of antibody microarrays of 4*4, and chip-dedicated fences or Super PAP Pen scribings are used for separating the microarrays from each other; the double antibody sandwich principle is adopted to detect the antigen to carry out the dot matrix of corresponding capture antibody, positive control and negative control on the solid phase carrier simultaneously; the antibody protein is connected with the solid phase carrier through the covalent bond and physical adsorption; the sample liquid to be detected and the chip are directly incubated; the antigen to be detected in the samples combine with the corresponding antibody which is fixed on the chip; a specific monoclonal antibody probe marked by horse radish peroxidase is added and the macroscopic detection results can be obtained after the coloration of substrates. The invention can detect viruses in multiple aquatic animal samples and the results are macroscopic, thus being applicable to rapid and accurate detection of viruses of aquatic animals in breeding production.
Owner:OCEAN UNIV OF CHINA
Methods and devices to enhance sensitivity and evaluate sample adequacy and reagent reactivity in rapid lateral flow immunoassays
ActiveUS20110143365A1Reduce chanceBioreactor/fermenter combinationsBiological substance pretreatmentsIntravenous gammaglobulinLateral flow immunoassay
Methods and devices for rapid lateral flow immunoassays to detect specific antibodies within a liquid sample while also validating the adequacy of the liquid sample for the presence of immunoglobulin and the integrity and immunoreactivity of the test reagents that detect the antibodies of interest, without requiring instrumentation. The methods and devices provide for delivery of a diluted liquid sample to a single location that simultaneously directs the liquid flow along two or more separate flow paths, one that serves as a positive control to confirm that all critical reagents of the test are immunoreactive, and that the sample being tested is adequate, and the other to detect specific antibodies if present.
Owner:BUCHANAN
Multiplex PCR (polymerase chain reaction) primer, probe and gene chip for detecting bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus
InactiveCN103695566AImprove throughputShorten diagnostic timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerMultiplex
The invention relates to a multiplex PCR (polymerase chain reaction) primer, a probe and a gene chip for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus. The multiplex PCR primer and probe have the nucleotide sequences shown by SEQ ID No.1 to SEQ ID and No.9. The gene chip comprises a solid-phase carrier, a sample application quality control probe, a positive hybrid quality control probe and a multiplex PCR primer for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus and the corresponding probe. In the invention, the forward primers of three viruses are marked with fluorescence, a gene chip detection technology carrying three viruses in animal fur is established based on multiplex RT-PCR (reverse transcription-polymerase chain reaction), and the RNA virus in the fur can be sensitively and specifically detected with high flux; the three viruses are screened at the same time in detection once, and the situation that a specific method is required for each virus before is changed, thereby saving the diagnosis time, meeting the needs for quick detection of mass imported / exported fur samples of the exit-entry inspection and quarantine departments and the fur import and export enterprises, and realizing relatively high application values.
Owner:徐超
Anti-human papillomavirus 16 e6 t cell receptors
ActiveUS20160152681A1Highly avid recognitionMinimize destructionPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E6, E629-38. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Heteroduplex tracking assay
InactiveUS20060194227A1Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
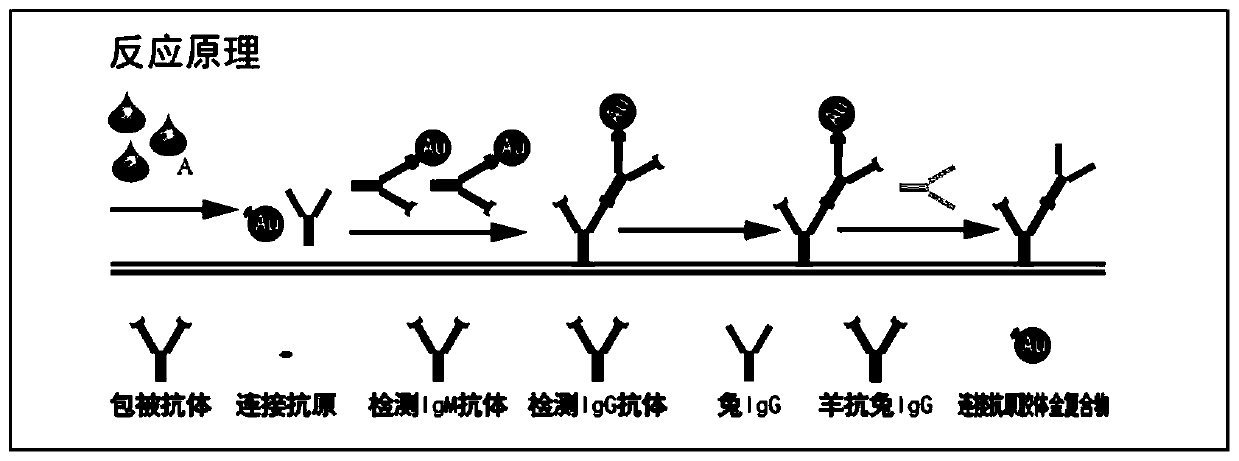
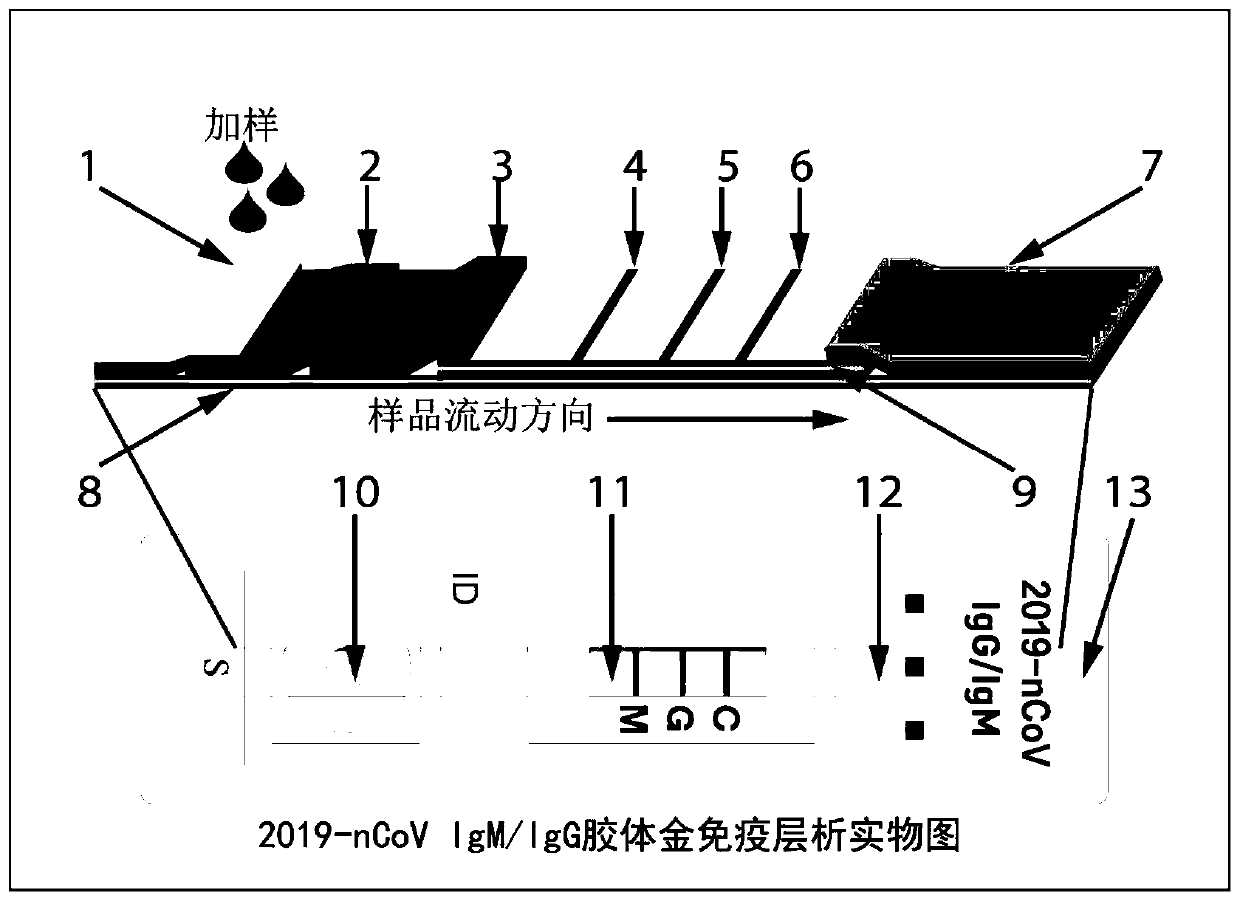
System for rapidly detecting new coronavirus 2019-nCoV in blood sample and preparation method of system
PendingCN111273001ASimple and fast operationLow costMaterial analysisWhole blood unitsBlood specimen
The invention provides a system for rapidly detecting new coronavirus 2019-nCoV in a blood sample. The system is used for detecting IgM and IgG antibodies of the new coronavirus 2019-nCoV, and comprises a buckle, a colloidal gold immunochromatography test strip and a sample buffer solution. The colloidal gold immunochromatography-free test strip comprises a sample pad, a whole blood separation membrane, a marking pad, a chromatography membrane, a water absorption pad and a bottom plate which are connected in sequence, a colloidal gold-new coronavirus N protein connecting antigen compound and acolloidal gold-quality control molecular compound are fixed on the marking pad at the same time, and a mouse anti-human IgM-mu chain antibody and a mouse anti-human IgG-mu chain antibody are respectively fixed on a detection line. During detection, after the blood sample to be detected is added to the sample pad, the sample buffer solution is added; if the sample buffer solution is not used, butthe sample is directly detected, the new coronavirus IgM and IgG in the sample cannot be detected or false positive easily occurs, so that the existence of the sample buffer solution is crucial for realizing the detection of the sample.
Owner:北京大弘生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com