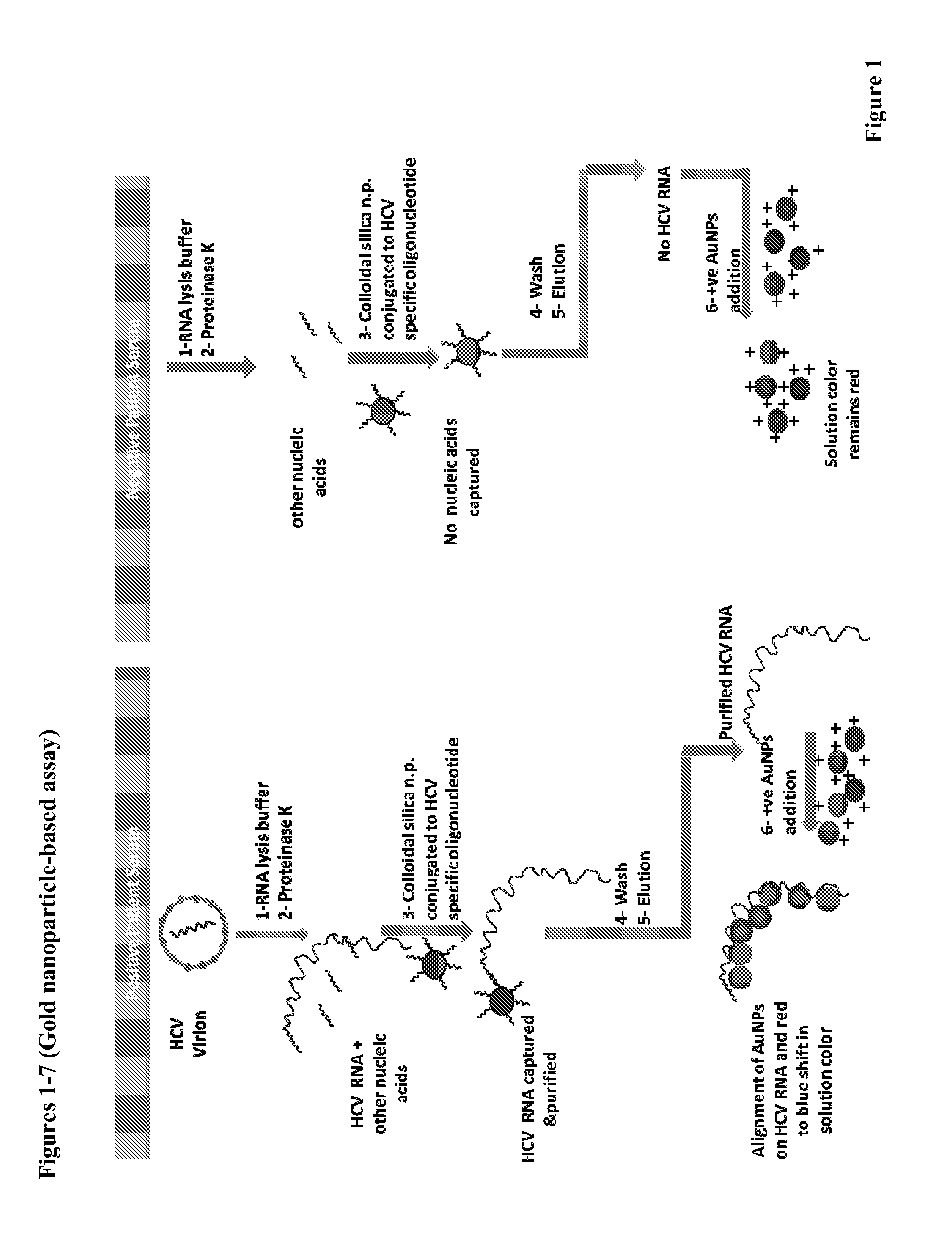
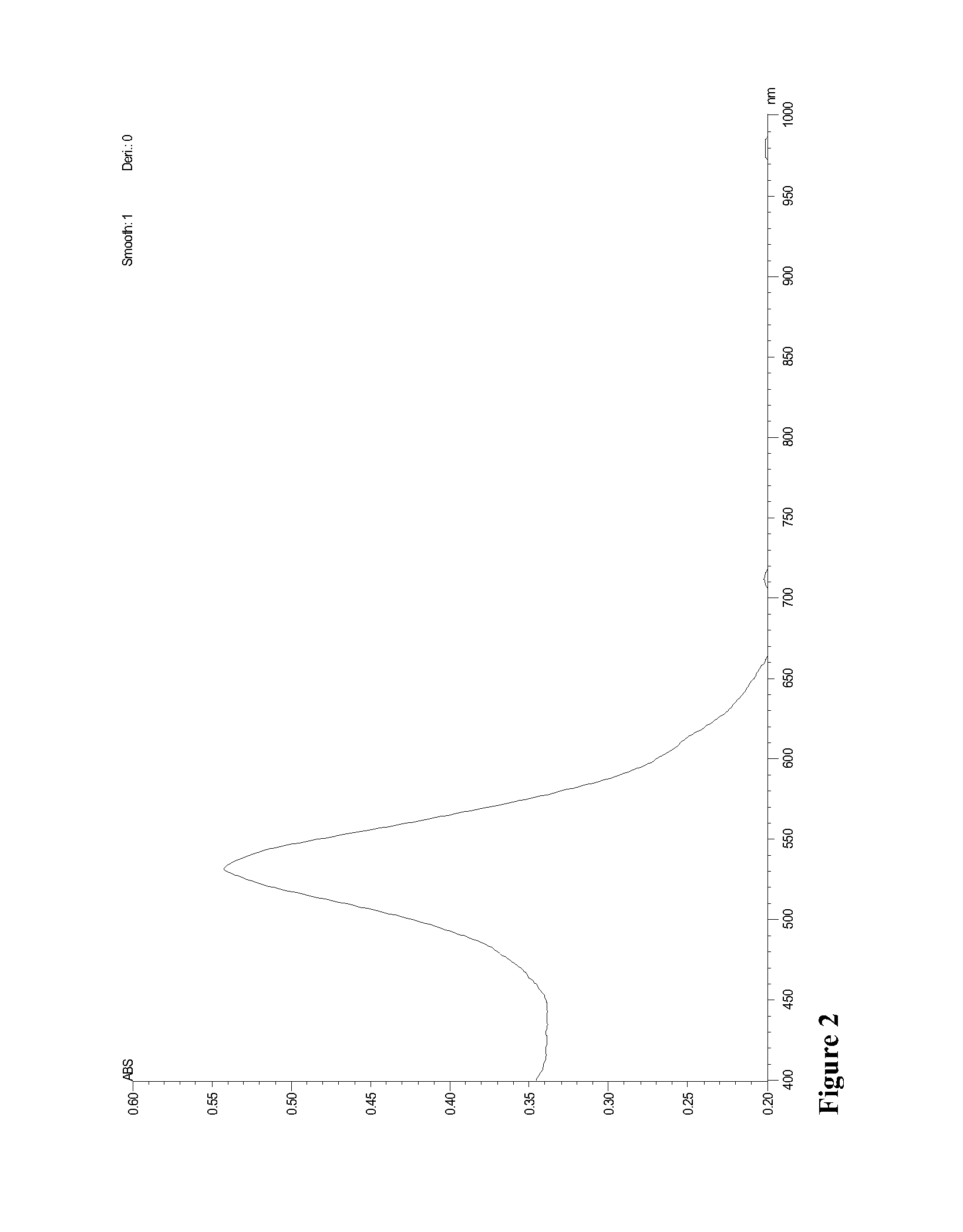
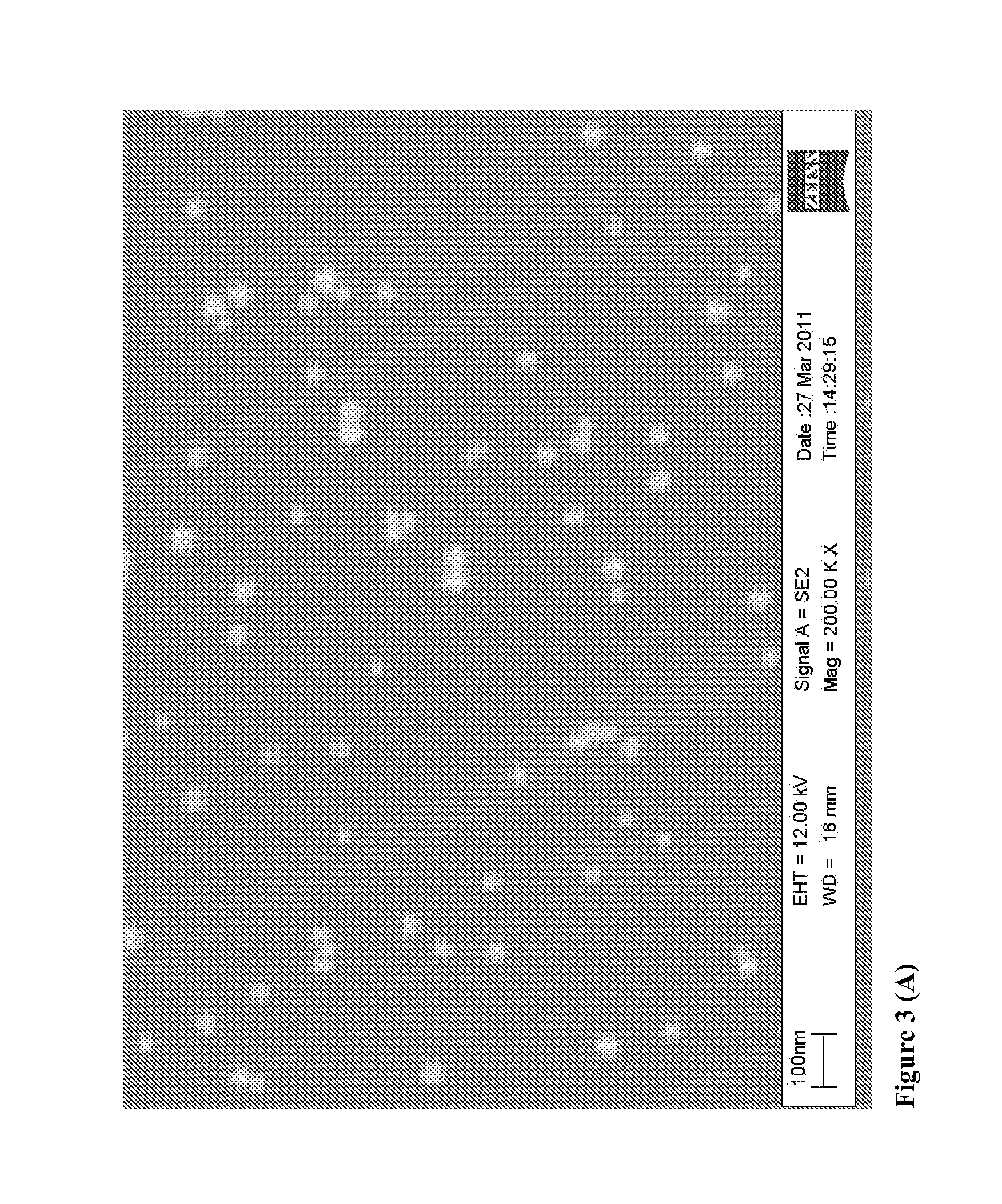
Direct detection of disease biomarkers in clinical specimens using cationic nanoparticle-based assays & versatile and green methods for synthesis of anisotropic silver nanostructures
a technology assays, which is applied in the field of direct detection of disease biomarkers in clinical specimens using cationic nanoparticle-based assays and versatile and green methods for synthesis of anisotropic silver nanoparticles, can solve the problems of aggregation of gold nanoparticles, time-consuming, labor-intensive, etc., and achieves the effect of evaluating the safety of nanoparticles and improving thermal, electrical or magneti
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0211]The 3d flower-like silver structures with multi layer of hollow, rough surface, external channels surrounded particles were synthesized according to the above condition but using 0.2 mL of AgNO3, 0.4 mL of TSC, 0.4 mL of dextrose added to 15 mL of DDI water; stirring at room temperature the color turned to deep yellow immediately after addition of 100 μL of NaOH then the solution is stirred for an additional 5 min, stirrer turned off and samples collected by centrifugation as mentioned above (FIG. 8).
example 2
[0212]The 3d flower-like silver structures with more multilayer of hollow, more rough surface, with larger hollows, highly external channels surrounded particles were synthesized according above condition but using 0.2 mL of AgNO3, 0.4 mL of TSC, 0.4 mL of dextrose added to 15 mL of DDI water, stirring at room temperatures the color turn to gray immediately after addition of 250 μL of NaOH then the solution is stirred for an additional 5 minutes, stirrer turned off and samples collected by centrifugation as mentioned above (FIG. 9).
example 3
[0213]The 3D scaffold fibers like, flakes, and cluster silver structures little hollow pores, more rough surface, highly external arms surrounded particles were synthesized according above condition but using 1 mL of AgNO3, 1 mL of TSC, 1 mL of dextrose added to 15 mL of DDI water, stirring at room temperatures the color turn to green immediately after addition of 50 μL of NaOH then the solution is stirred for an additional 5 min, stirrer turned off and samples collected by centrifugation 3 times at 8,000, 12,000, and 14,000 rpm, respectively.
Claim 80; Example 3 at page 16 the word (3D shell-like) should be substituted by (3D scaffold fibers like, flakes, and cluster). Also, the following statement should be added centrifugation 3 times at 8,000, 12,000, and 14,000 rpm respectively (calim80)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Microstructure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com