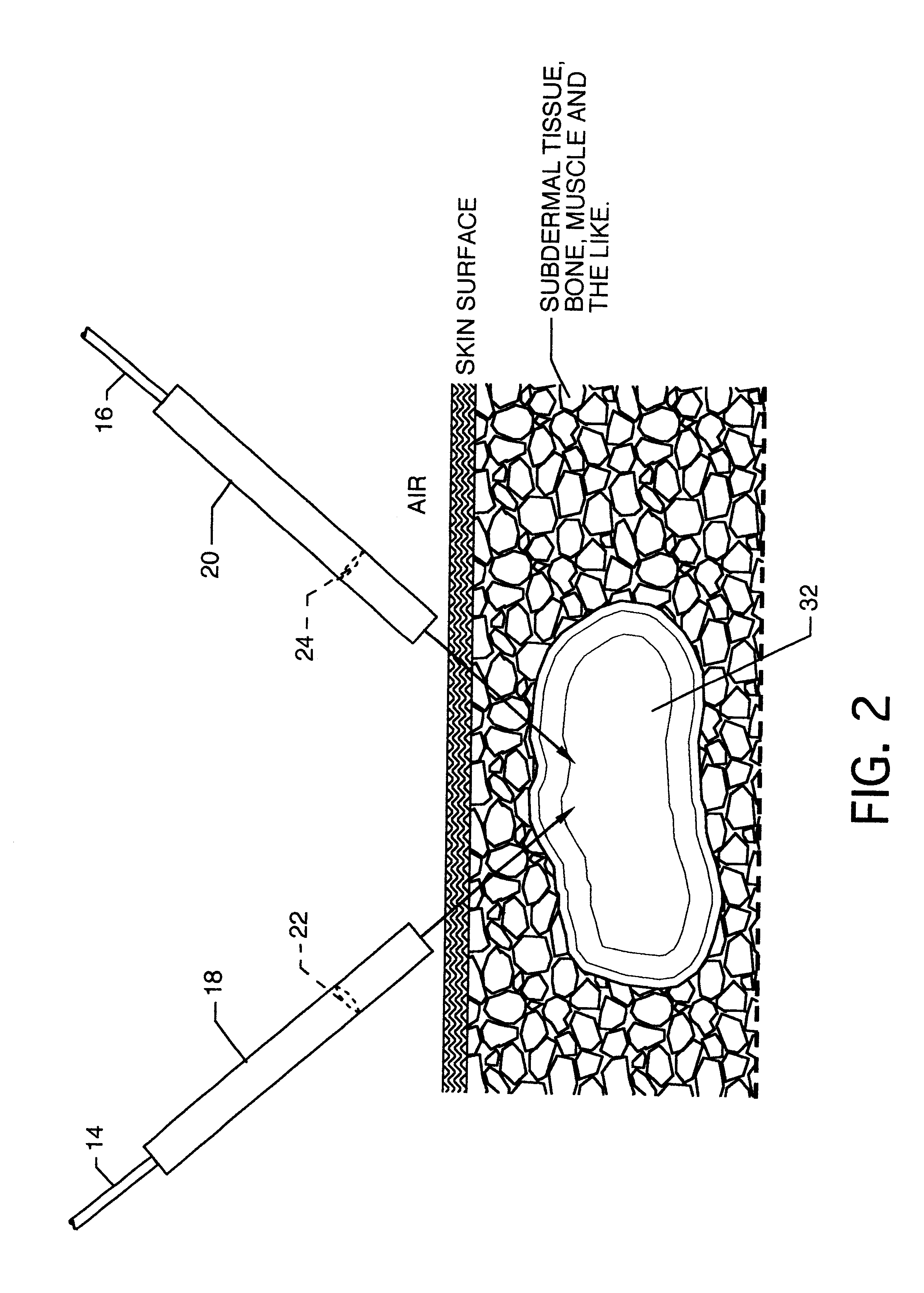
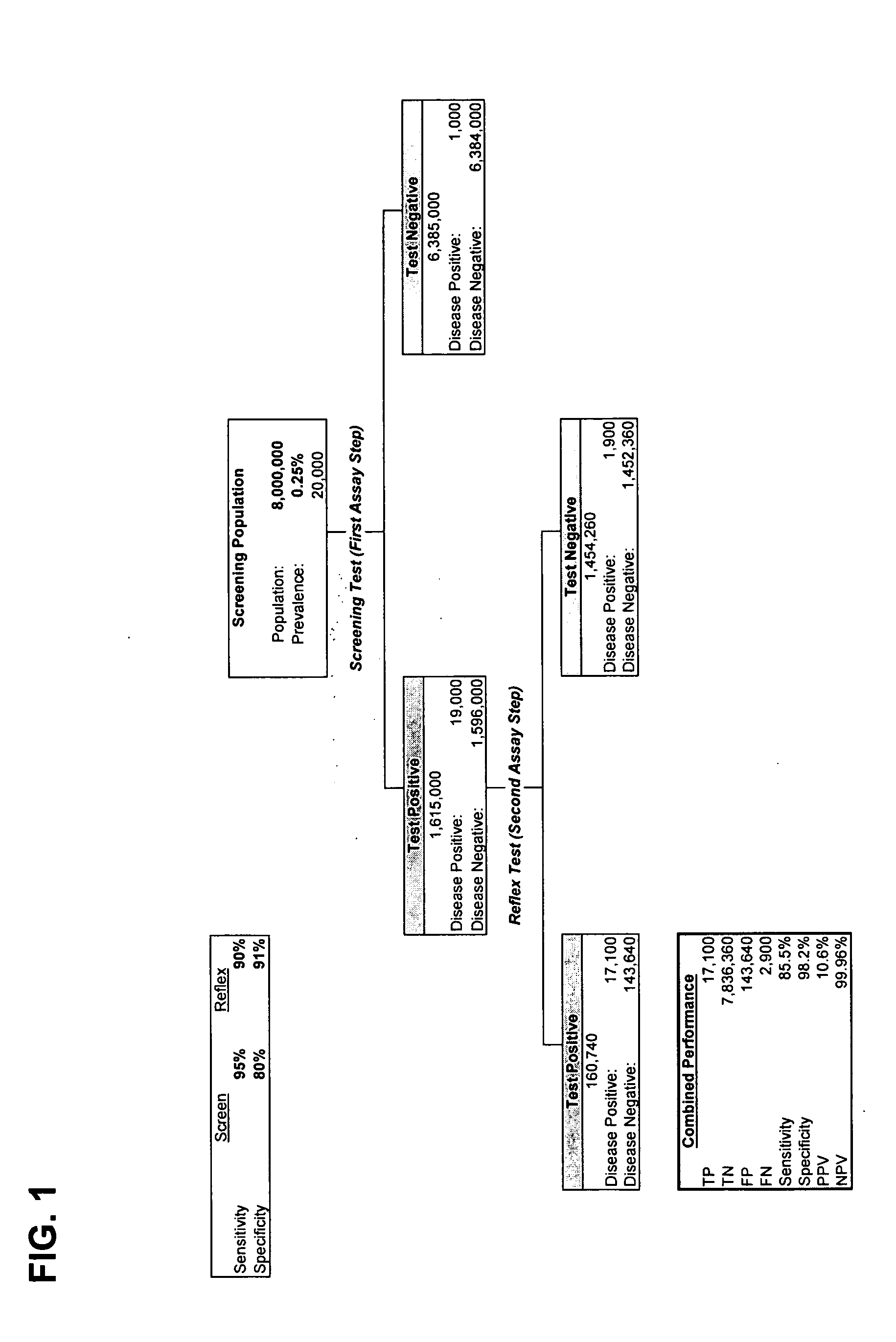
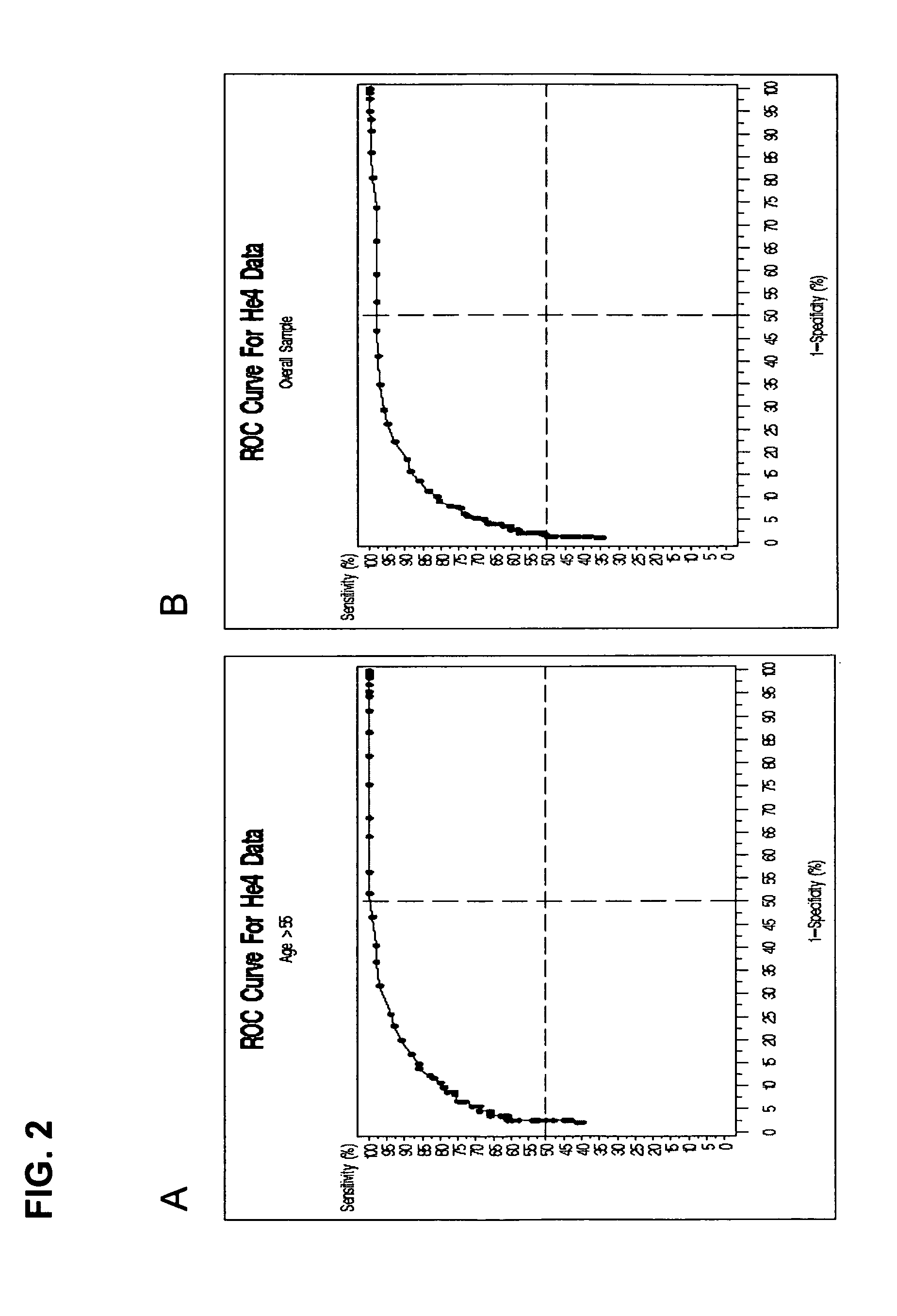
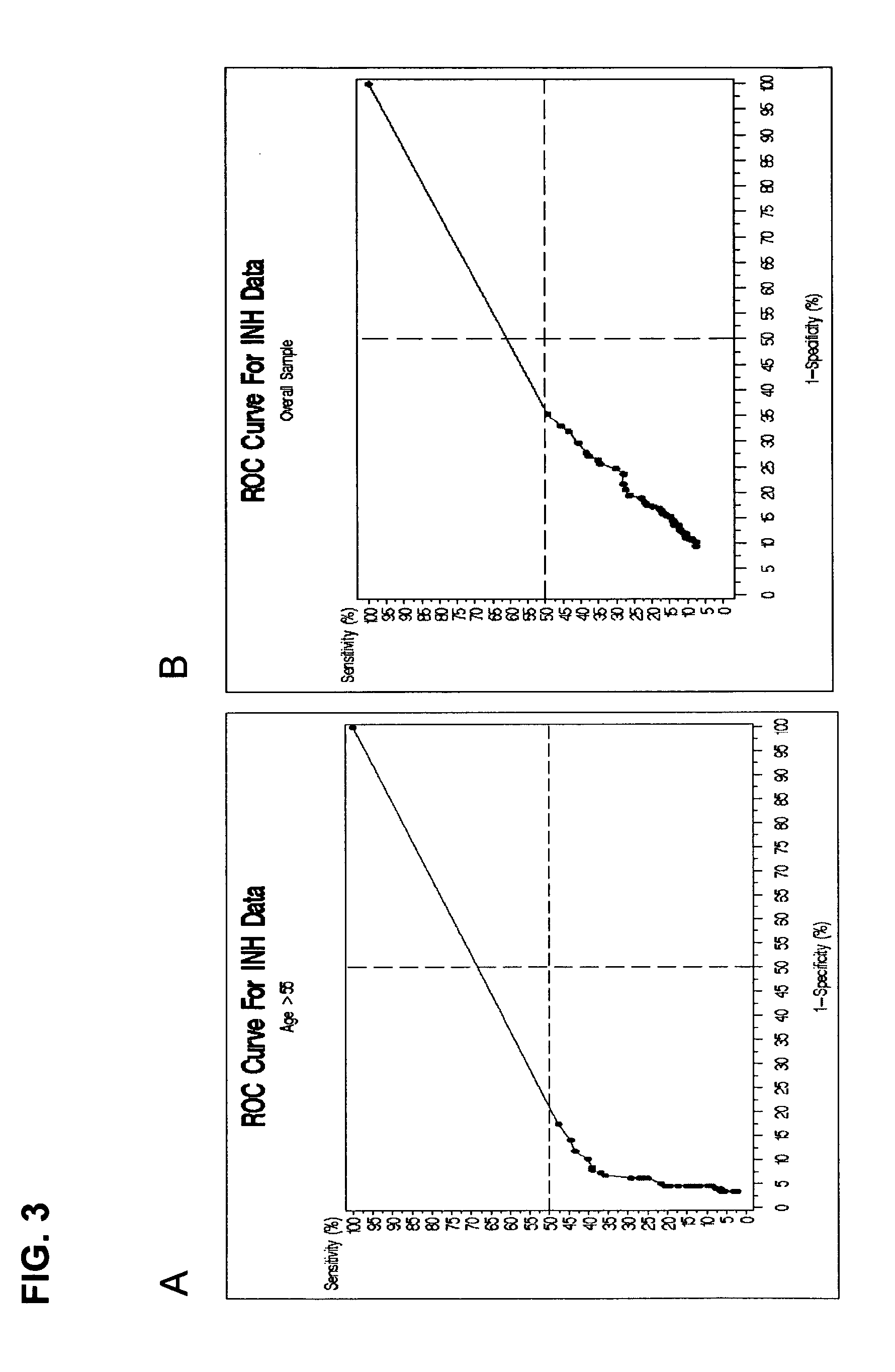
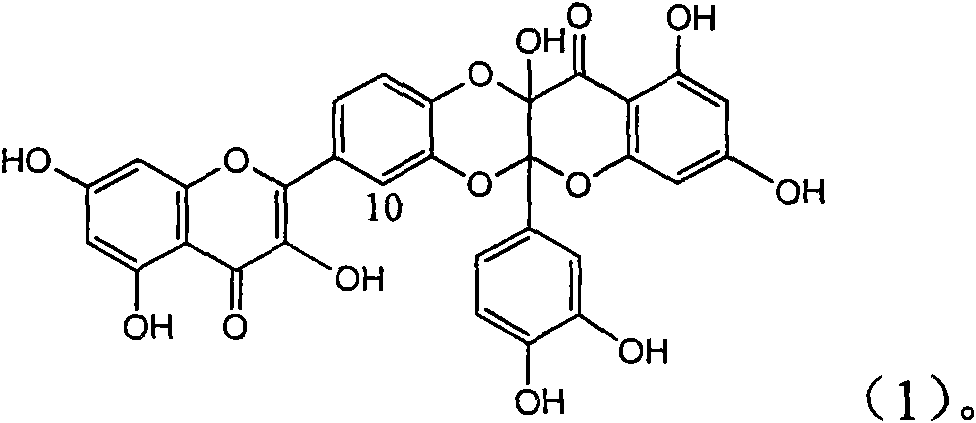
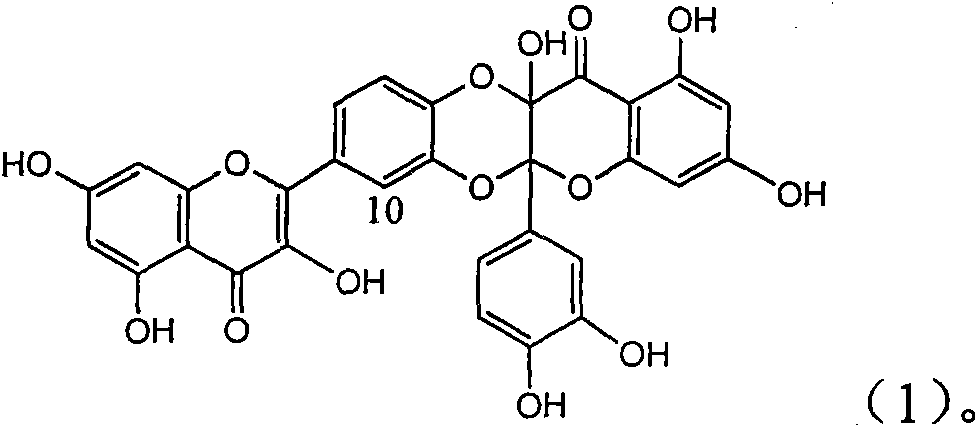
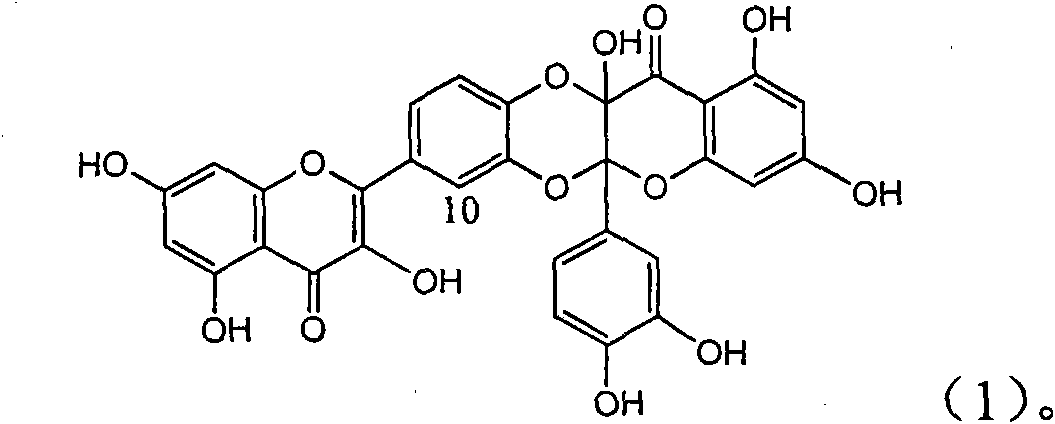
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
240 results about "DNA replication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
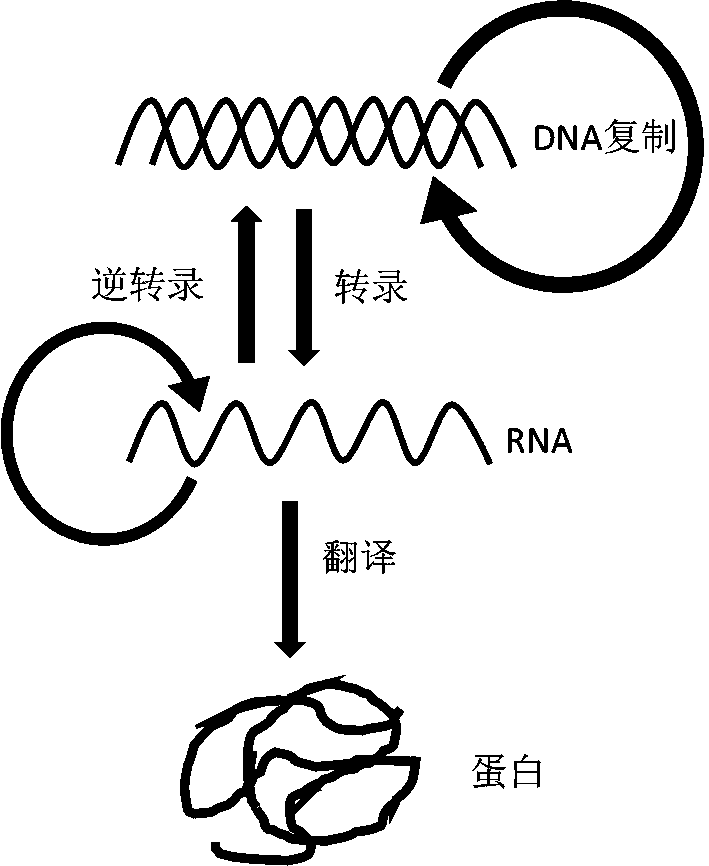
In molecular biology, DNA replication is the biological process of producing two identical replicas of DNA from one original DNA molecule. DNA replication occurs in all living organisms acting as the basis for biological inheritance. The cell possesses the distinctive property of division, which makes replication of DNA essential.
Method and devices for extremely fast DNA replication by polymerase chain reactions (PCR)
InactiveUS6180372B1Shorten cycle timeHigh sensitivityBioreactor/fermenter combinationsNanotechPolymerase LBiological materials
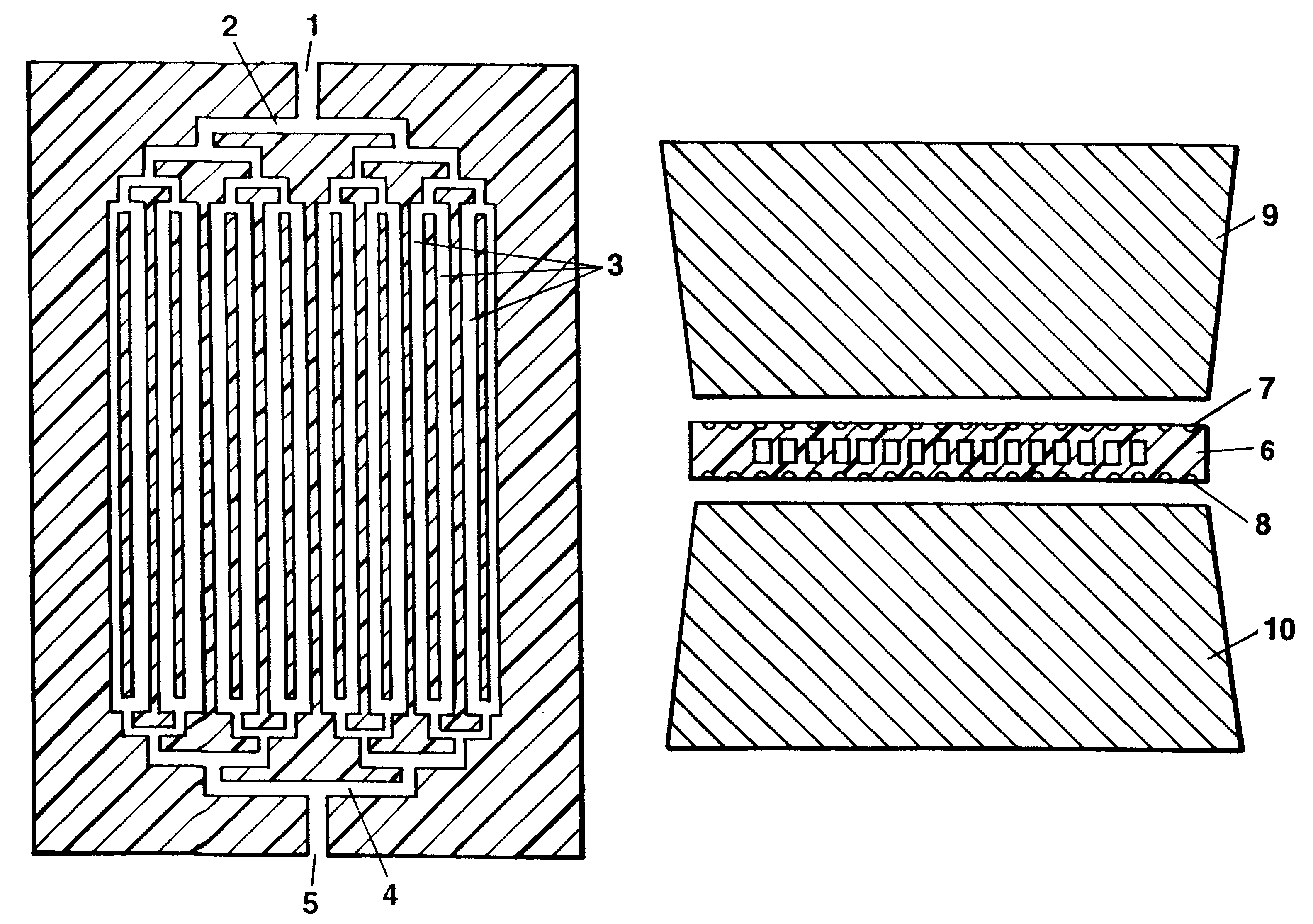
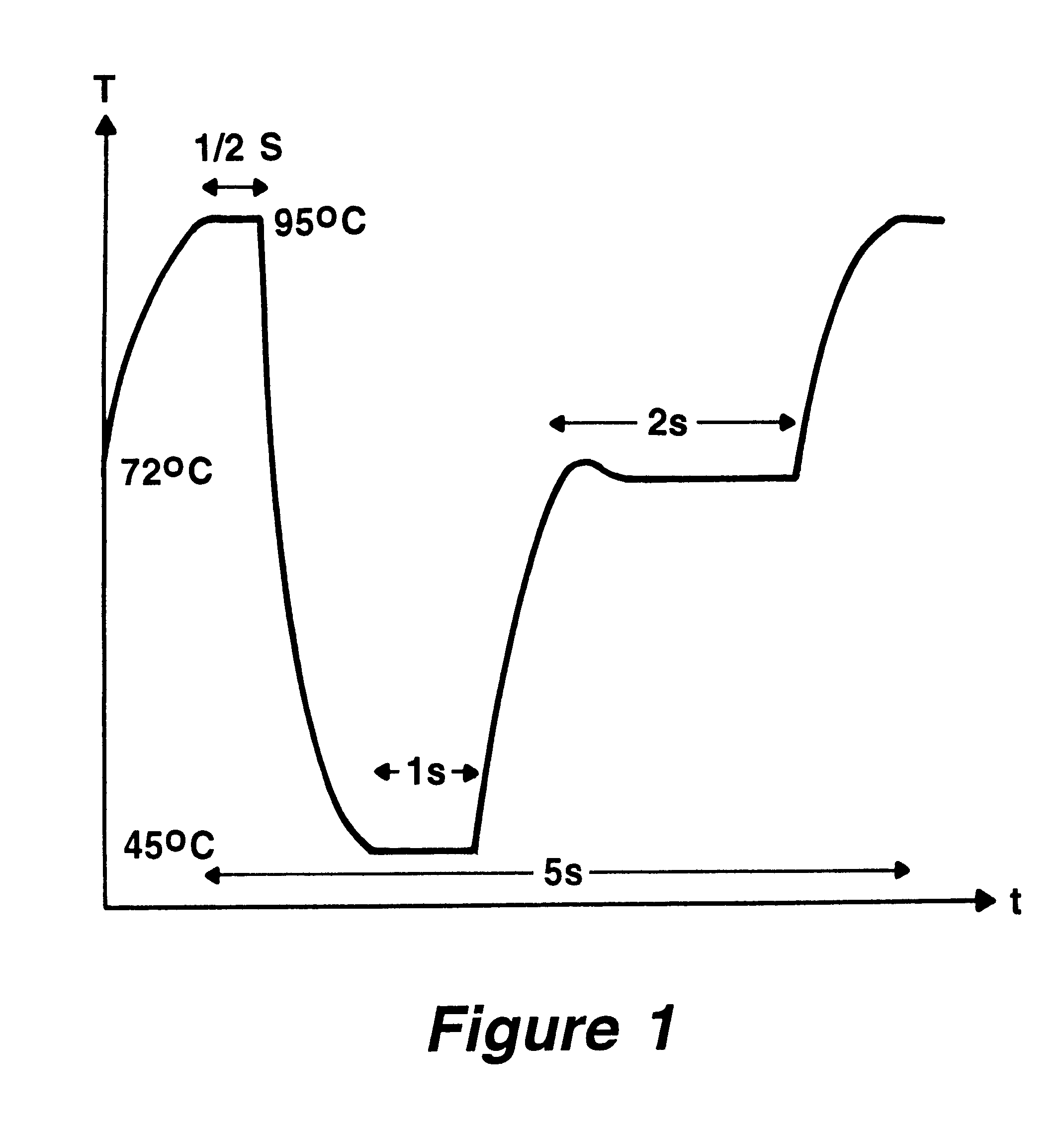
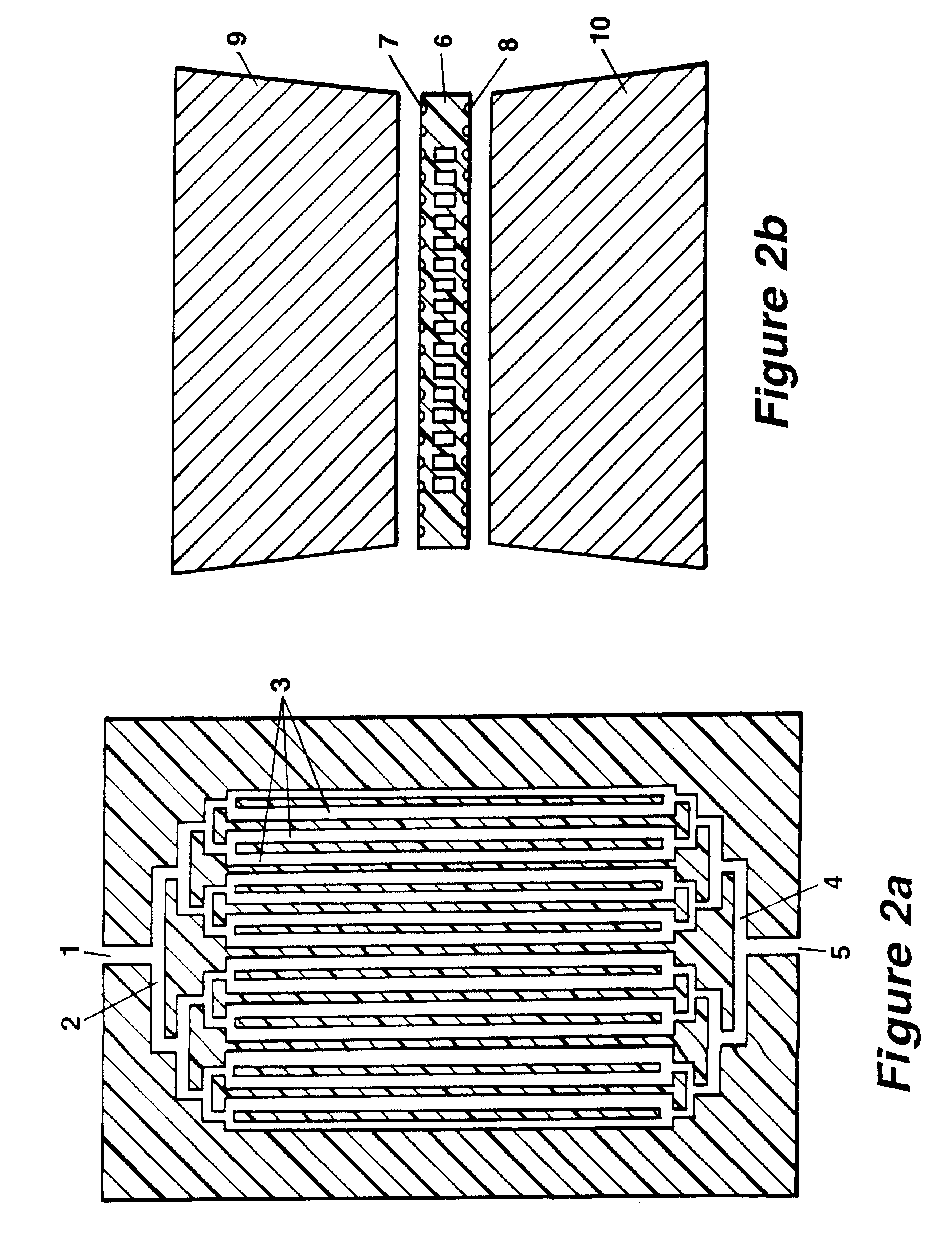
The invention concerns methods and instruments for fast, selective replication of deoxyribonucleic acid (DNA) from biomaterial through the known polymerase chain reaction (PCR), working in individual duplication thermocycles. The invention consists of extremely brief cycle times of only a few seconds for the PCR reactions, generated, on the one hand, by reaction chambers for the reception of the reaction solution constructed of a pattern of fine capillaries in close proximity to heating and cooling elements in order to optimally accelerate the temperature setting in the reaction solution for the three temperature phases of the PCR duplication cycles and, on the other hand, by keeping the flow rates in the capillaries to a minimum during the amplification phase so that the polymerase reaction is not disturbed. The capillary pattern can be simply produced by means of microsystern technology.
Owner:BRUKER FRANZEN ANALYTIK
Methods and compositions for the detection of cervical disease
ActiveUS20050260566A1Improve the level ofIncreased proliferationAnimal cellsMicrobiological testing/measurementDiseaseCervical cells
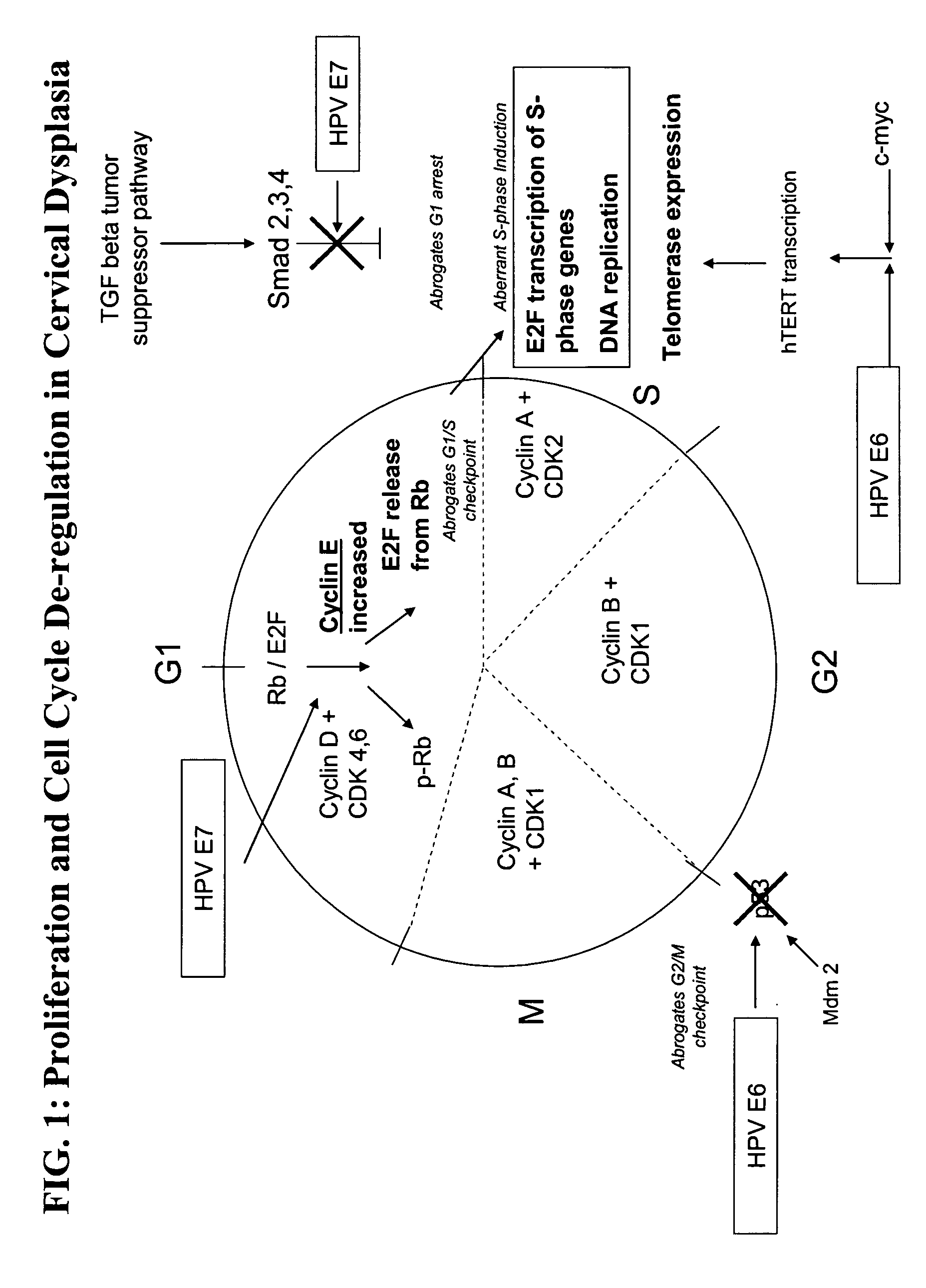
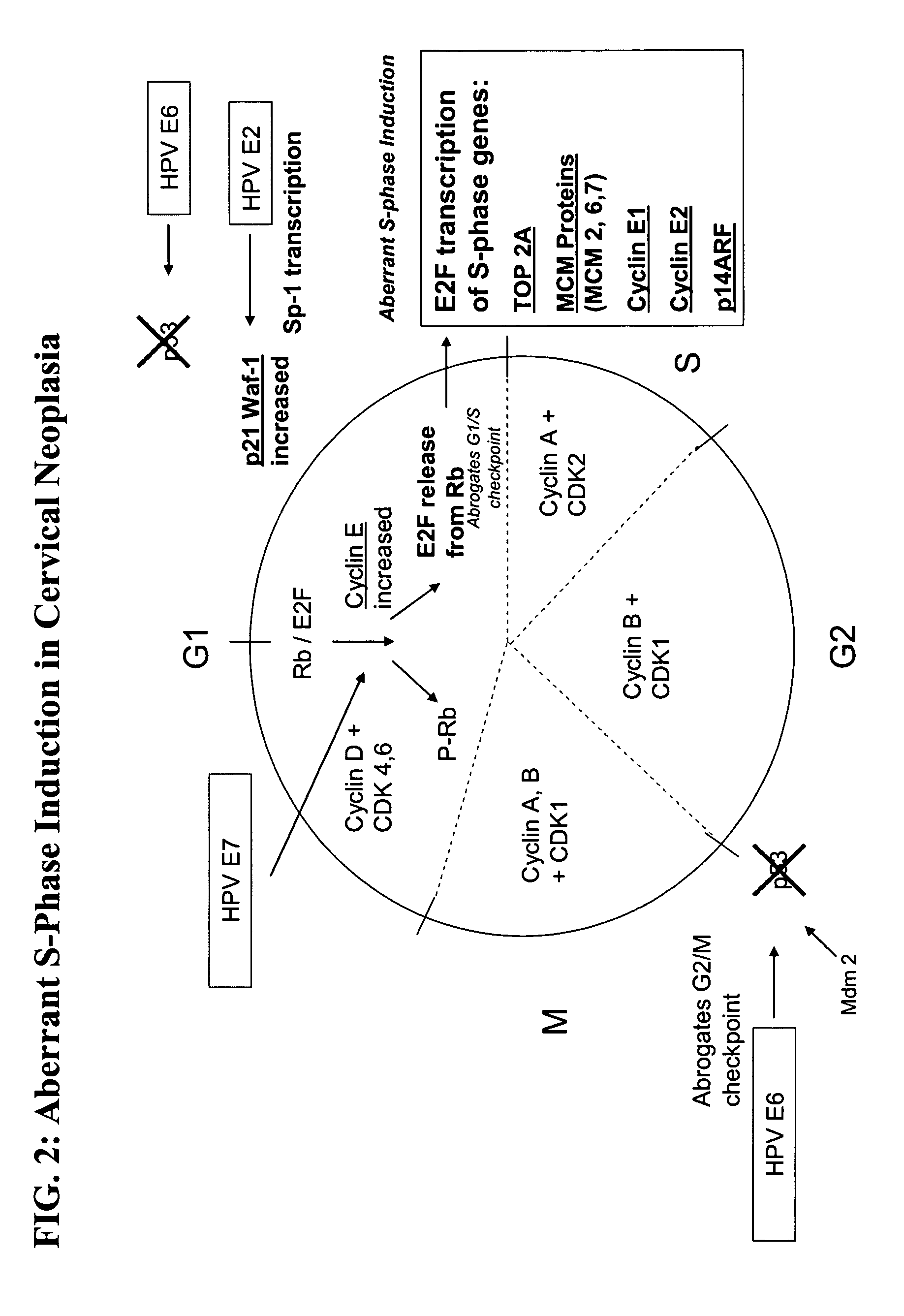
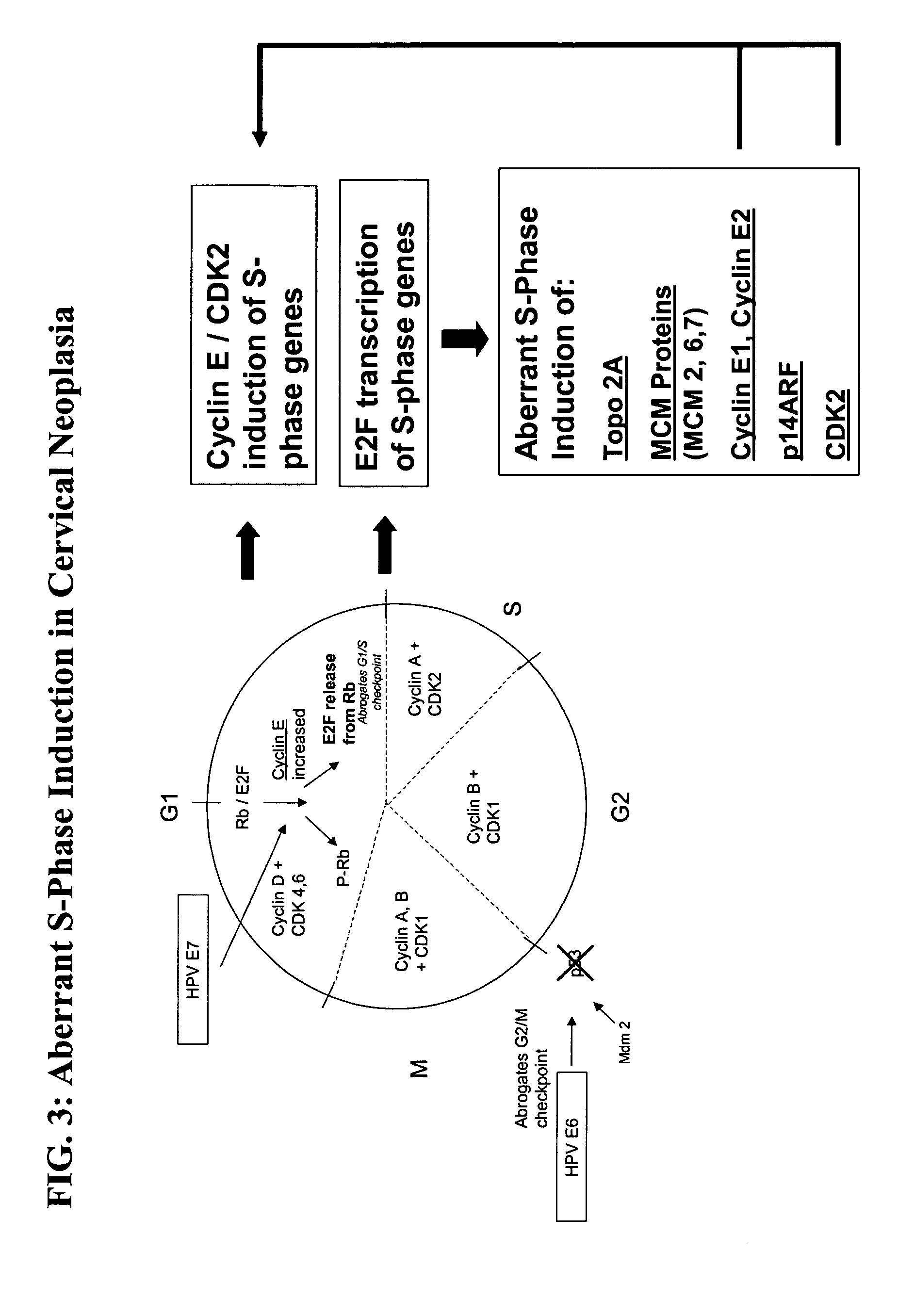
Methods and compositions for identifying high-grade cervical disease in a patient sample are provided. The methods of the invention comprise detecting overexpression of at least one biomarker in a body sample, wherein the biomarker is selectively overexpressed in high-grade cervical disease. In particular claims, the body sample is a cervical smear or monolayer of cervical cells. The biomarkers of the invention include genes and proteins that are involved in cell cycle regulation, signal transduction, and DNA replication and transcription. In particular claims, the biomarker is an S-phase gene. In some aspects of the invention, overexpression of a biomarker of interest is detected at the protein level using biomarker-specific antibodies or at the nucleic acid level using nucleic acid hybridization techniques. Kits for practicing the methods of the invention are further provided.
Owner:TRIPATH IMAGING INC
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Methods and compositions for evaluating breast cancer prognosis
InactiveUS20060063190A1Accurate assessmentImproved prognosisMicrobiological testing/measurementProteomicsLymphatic SpreadNucleic acid hybridisation
Methods and compositions for evaluating the prognosis of a breast cancer patient, particularly an early-stage breast cancer patient, are provided. The methods of the invention comprise detecting expression of at least one, more particularly at least two, biomarker(s) in a body sample, wherein overexpression of the biomarker or a combination of biomarkers is indicative of breast cancer prognosis. In some embodiments, the body sample is a breast tissue sample, particularly a primary breast tumor sample. The biomarkers of the invention are proteins and / or genes whose overexpression is indicative of either a good or bad cancer prognosis. Biomarkers of interest include proteins and genes involved in cell cycle regulation, DNA replication, transcription, signal transduction, cell proliferation, invasion, proteolysis, or metastasis. In some aspects of the invention, overexpression of a biomarker of interest is detected at the protein level using biomarker-specific antibodies or at the nucleic acid level using nucleic acid hybridization techniques.
Owner:TRIPATH IMAGING INC
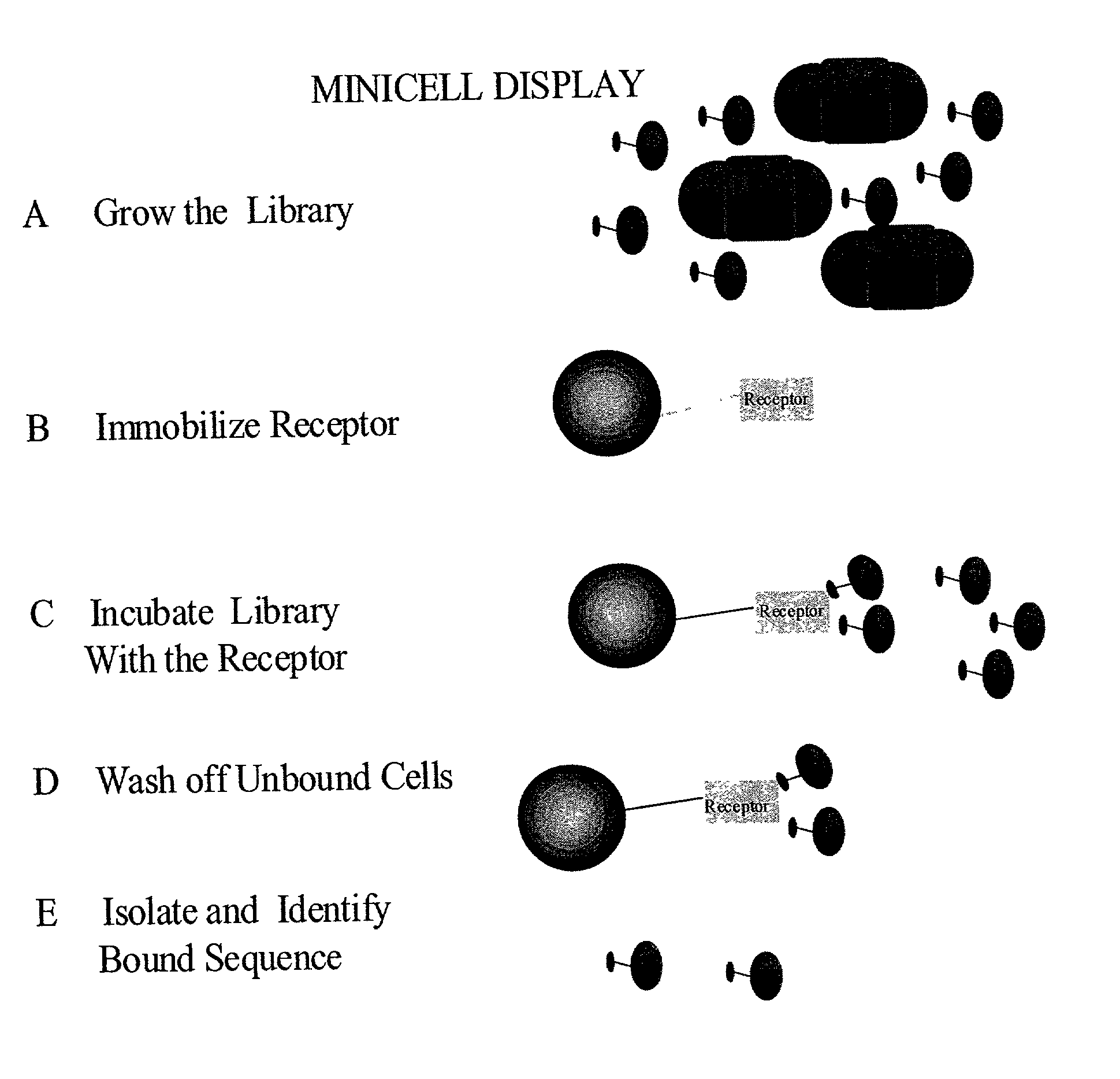
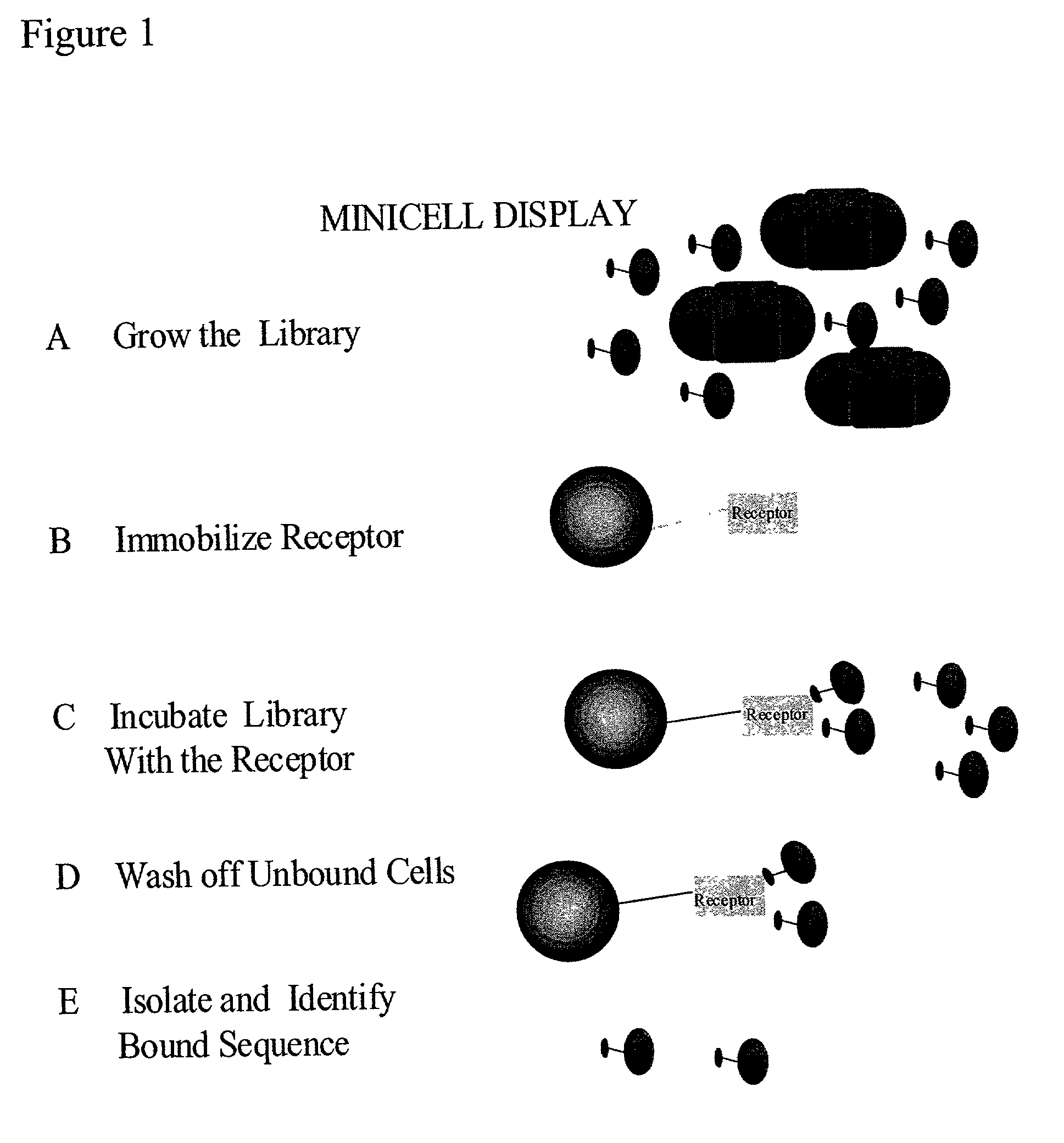
Methods to screen peptide libraries using minicell display
InactiveUS7125679B2Easy to eluteIncrease mutation frequencyPolypeptide with localisation/targeting motifSugar derivativesIn vivoMutagenesis
A minicell display method has been developed which has significant advantages for screening peptide libraries for candidates that can bind and effectively modulate a particular biological process. The method, based on the small, anucleate minicell, has increased versatility in generating unique sequences to screen as well as increasing the size of the peptides to be screened. In vivo mutagenesis, at the level of protein synthesis, as well as DNA replication, increases diversification of the library to be screened and therefore substantially increases the number of potential peptides that can modulate a particular biological response or mechanism.
Owner:CHILDRENS MEDICAL CENT CORP
Violation of time reversal invariance in living tissue
InactiveUS6592611B1Eliminate needSynergistic effectivenessSurgical instrument detailsLight therapyDiseaseWavenumber
The present invention provides for a device, a method, and a treatment system for chronic disease conditions. The present invention was designed using the theoretical concepts of Quantum Biology. The principles of operation are based on the device's ability to stimulate a Bose-Einstein condensate and excitation of Frolich resonance in living tissue The wavenumbers necessary for this excitation are derived from the solution to the equations for optical phonon scattering in living tissue generated by optical photon excitation. The establishment of this degeneracy condition induces a super conducting state in the tissue. This super conducting state facilitates DNA replication, transcription and translation, thereby allowing the proper formation or regeneration of healthy tissue. This superconducting state provides the conditions necessary for establishing the violation of time reversal invariance in living tissue.
Owner:HOBSON MICHAEL A
Methods for identifying patients with an increased likelihood of having ovarian cancer and compositions therefor
InactiveUS20070212721A1Easy to detectRaise the possibilityMicrobiological testing/measurementBiological testingCancer cellMotility
Screening methods for identifying patients with an increased likelihood of having ovarian cancer are provided. The screening methods involve the detection of expression of a plurality of biomarkers in a body sample, wherein overexpression of the biomarkers is indicative of an increased likelihood of having ovarian cancer. The screening methods may further comprise a two-step analysis. Biomarkers of interest include genes and proteins that are, for example, involved in defects in DNA replication / cell cycle control, cell growth and proliferation, escape from apoptosis, angiogenesis or lymphogenesis, or the mechanisms of cancer cell motility and invasion. In some aspects of the invention, expression of a biomarker is detected at the protein level using a biomarker-specific antibody or at the nucleic acid level using nucleic acid hybridization techniques. Methods for detecting ovarian cancer in patients are further disclosed herein. Kits for practicing the methods of the invention are further provided.
Owner:TRIPATH IMAGING INC
Regulation of epigenetic control of gene expression
Methods are provided for the identification of compounds that selectively modulate epigenetic changes in gene expression. Compounds, compositions, kits or assays devices, and methods are provided for modulating the expression, endogenous levels or the function of small non-coding RNAs cognate to or transcribed by heterochromatic regions subject to epigenetic regulation (i.e., promoters, enhancers, centromeres, telomeres, origins of DNA replication, imprinted loci, or loci marked by dosage-compensation), and for modulating the formation or function of heterochromatin in cells, tissues or animals.
Owner:IONIS PHARMA INC
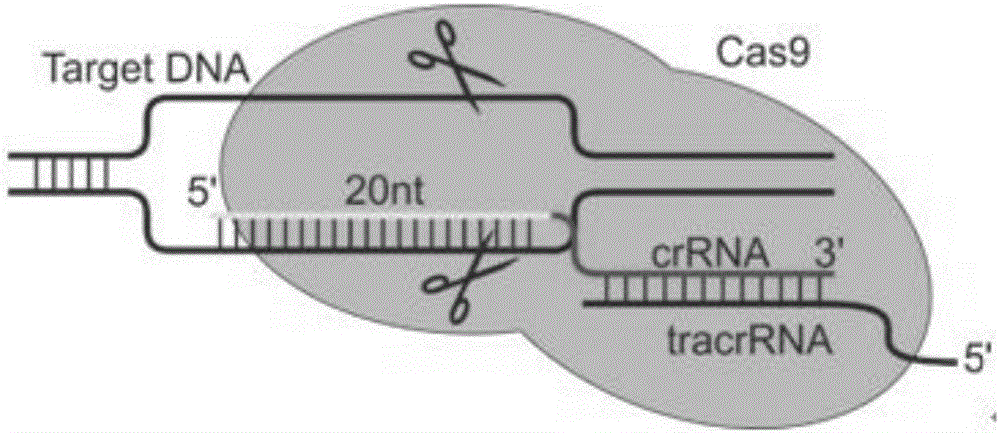
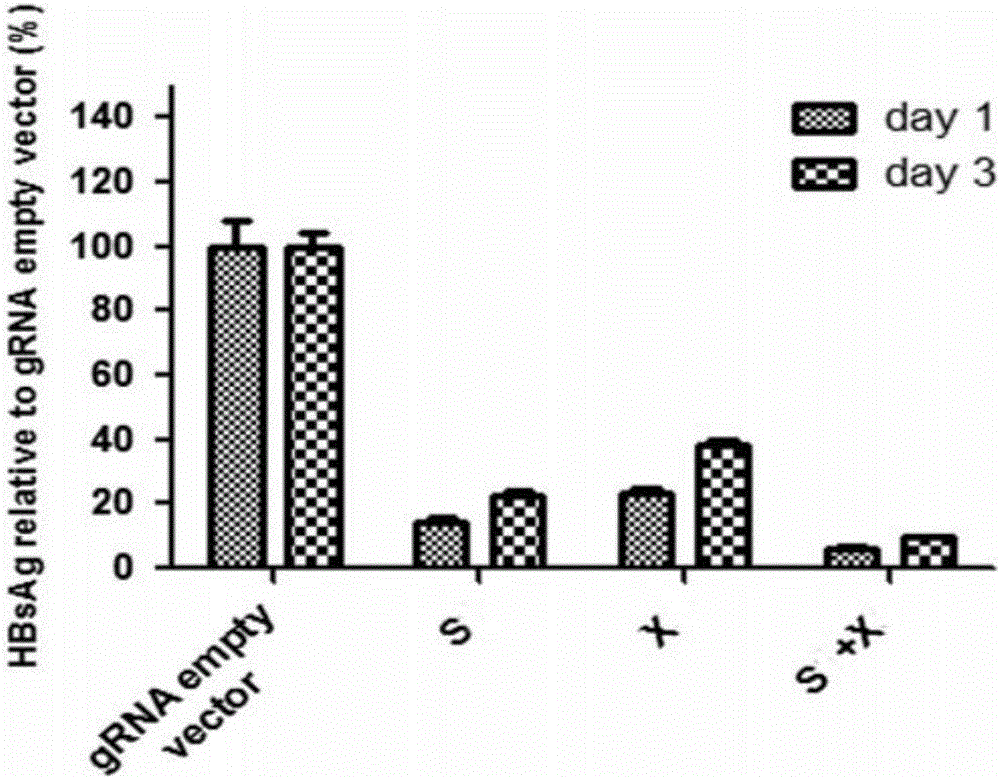
Specific sgRNA combined with immunogene to inhibit HBV replication, expression vector thereof, and application of specific sgRNA and expression vector
ActiveCN105821039AImprove targetingHigh knockout efficiencyOrganic active ingredientsGenetic material ingredientsHepatitis B Virus AntigenIn vivo
The invention provides a specific sgRNA combined with an immunogene to inhibit HBV replication, an expression vector thereof, and an application of the specific sgRNA and the expression vector. The sgRNA sequences of a human hepatitis B virogene suitable for CRISPR-Cas9 targeting editing and a PD-1 gene are designed, the plasmid vector of the sgRNA inhibiting HBV and PD-1 genes is constructed, and the plasmid vector and a nuclease gene expression vector are transferred to HBV transgenic mice, and can obviously inhibit HBV DNA replication. The gene expression vector prepared in the invention has the advantages of simple method steps, good sgRNA targeting property, and high CRISPR-Cas9 knockout efficiency. The sgRNAs specifically targeting the HBV and PD-1 genes can accurately splice the HBV and PD-1 genes, inhibit in vivo hepatitis B virus replication, and reduce the hepatitis B virus antigen expression.
Owner:李旭
Synthesis system, preparation, kit and preparation method of in-vitro DNA-to-Protein (D2P)
The invention provides a theoretical design and technical design of cell-free protein synthesis for DNA replication, transcription and translation coupling, a preparation, a kit and a preparation method. Specifically, with the application of the in-vitro cell-free synthesis system provided by the invention, complex protein can be synthesized, and moreover, DNA and mRNA can be synthesized; and effective, high-throughput and quite convenient protein synthesis can be completed with the use of a DNA template by a minute quantity (nanogram-microgram).
Owner:KANGMA SHANGHAI BIOTECH LTD
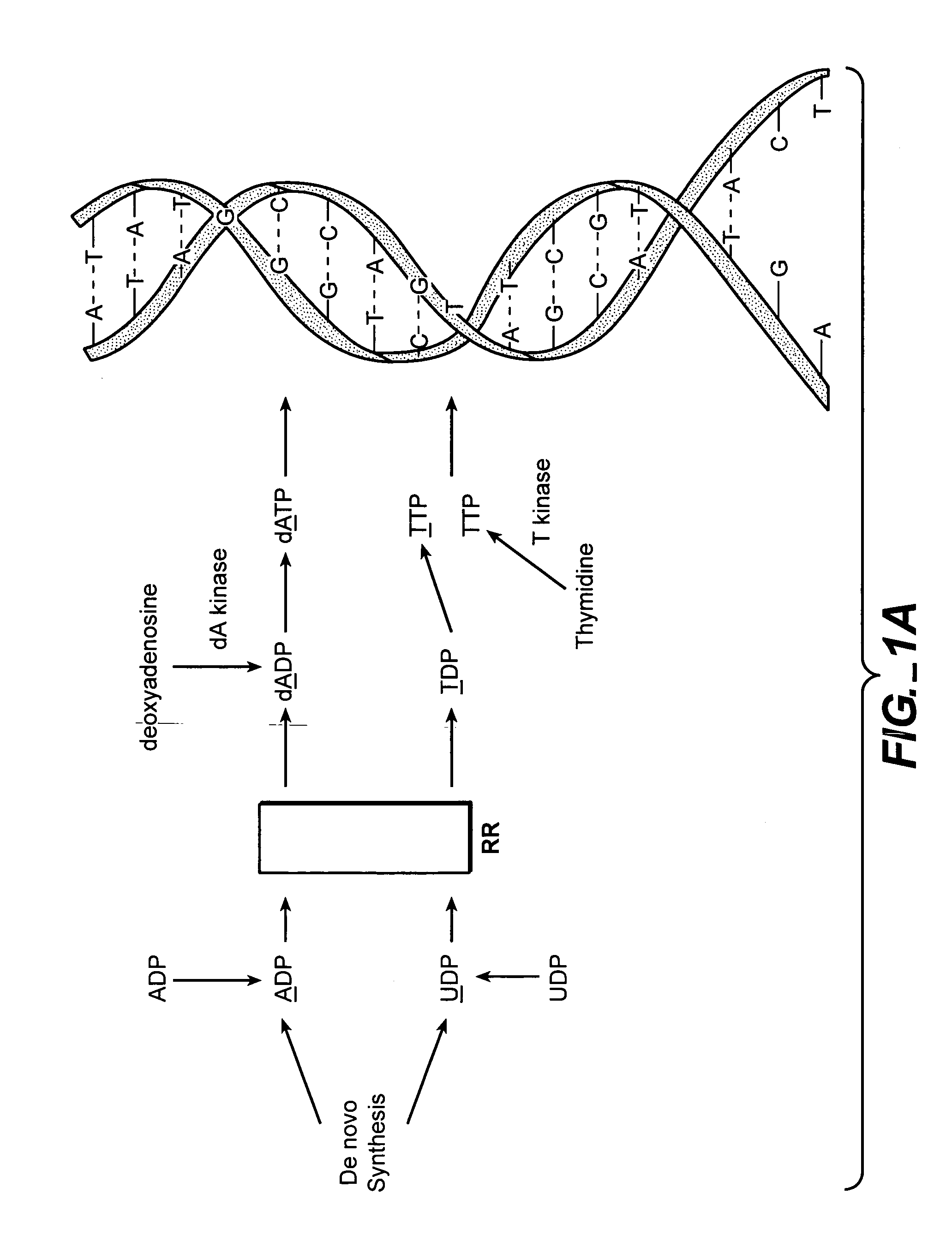
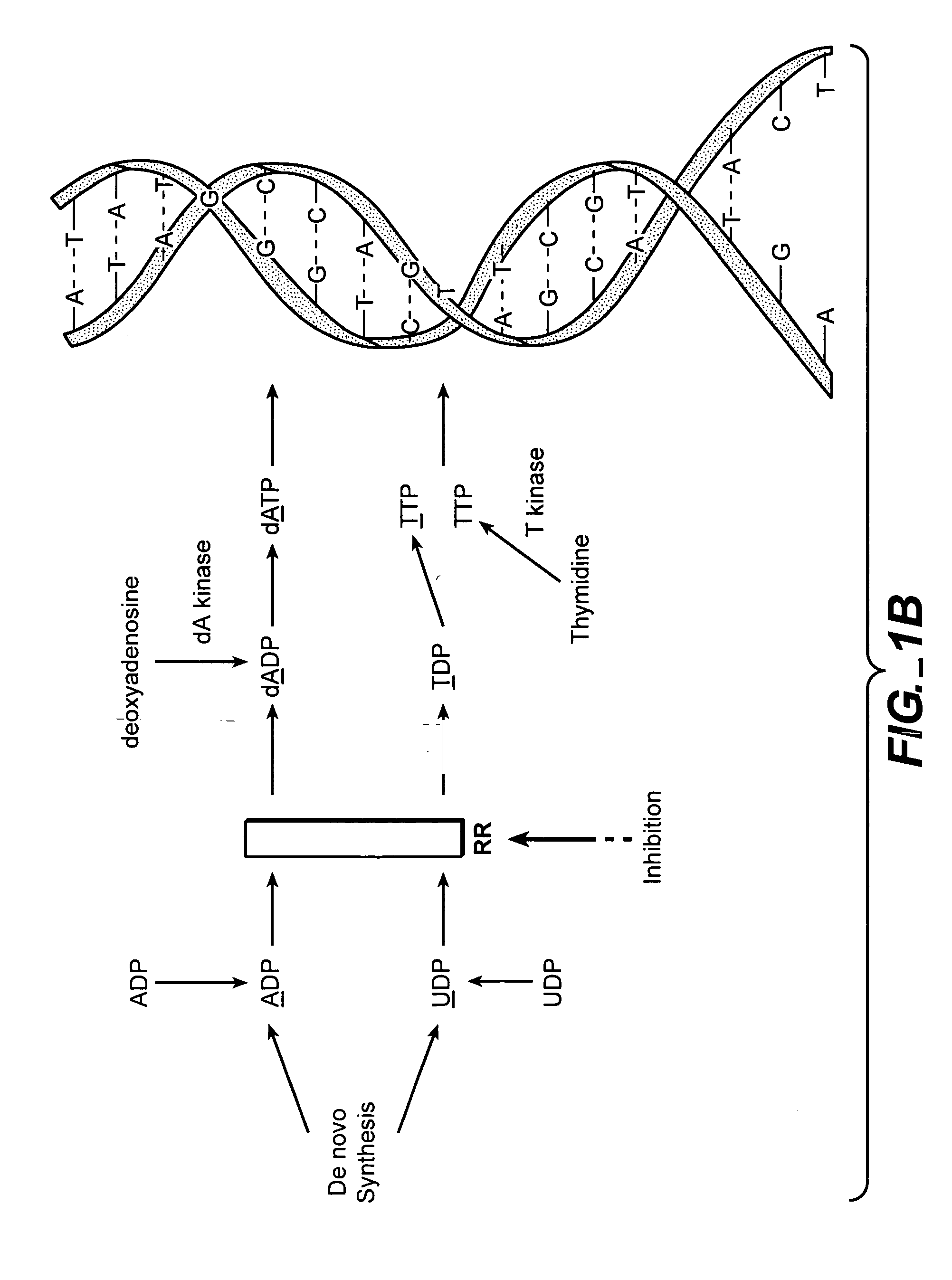
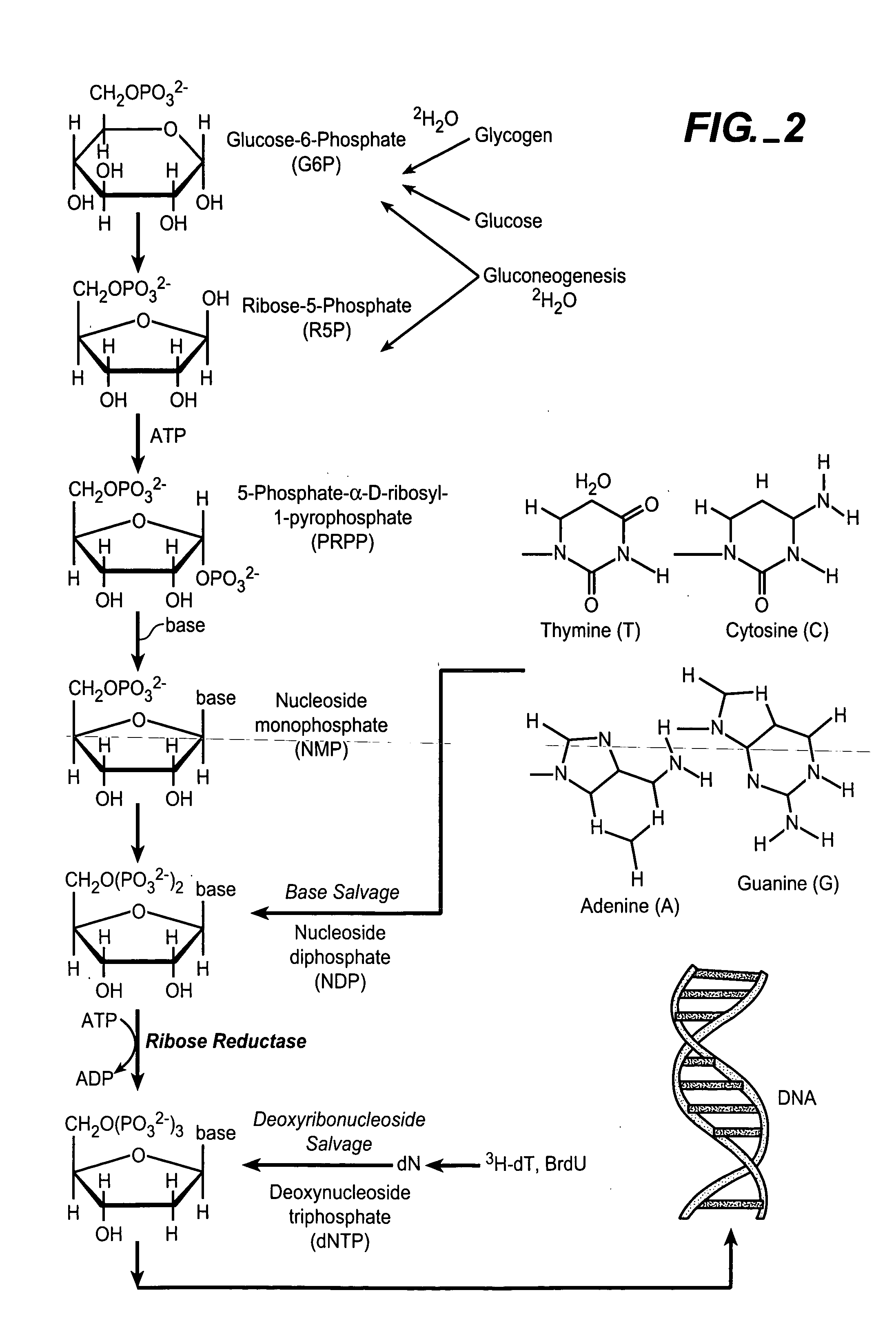
In vivo measurement of the relative fluxes through ribonucleotide reductase vs. deoxyribonucleoside pathways using isotopes
InactiveUS20050255509A1Increase salvageHigh activityCompound screeningApoptosis detectionRate-determining stepDeoxyribonucleotide biosynthesis
The methods of the present invention allow for the measurement of ribonucleotide reductase (RR) activity, an important enzyme in the de novo DNA synthesis pathway. Ribonucleotide reductase converts all four ribonucleotides to their deoxy form and is a rate-controlling step in this pathway. Biosynthetic pathways of deoxyribonucleotides (dN) have received considerable attention in the context of anti-proliferative chemotherapy. Inhibitors of various steps in dN biosynthesis, including inhibitors of RR are among the most useful chemotherapeutic agents in cancer, viral infections, and other therapeutic uses. DNA synthesis from the dN salvage pathway is also an important component to DNA replication. The relative contributions from RR vs. salvage pathways are critical to the actions and effectiveness of chemotherapeutic agents that act on nucleoside metabolic pathways. Until now, however, it has not been possible to study these metabolic processes in vivo. Disclosed within are methods of measuring RR activity in vivo and in vitro which find use, among other things, in drug discovery, development, and approval.
Owner:KINEMED
Compounds useful for inhibiting Chk1
Aryl- and heteroaryl-substituted urea compounds useful in the treatment of diseases and conditions related to DNA damage or lesions in DNA replication are disclosed. Methods of making the compounds, and their use as therapeutic agents, for example, in treating cancer and other diseases characterized by defects in DNA replication, chromosome segregation, or cell division also are disclosed.
Owner:ICOS CORP
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
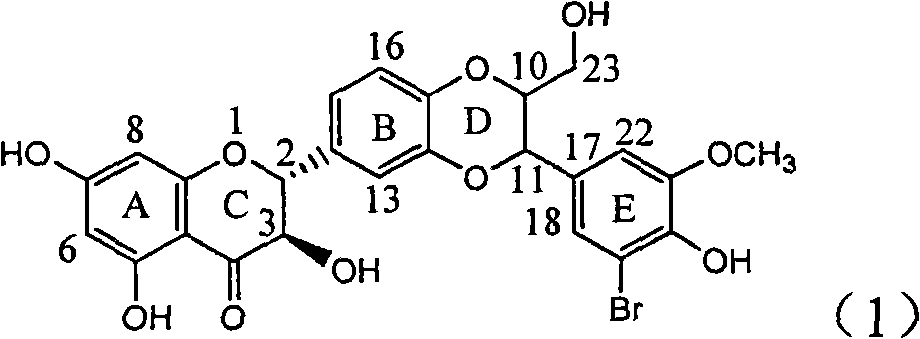
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
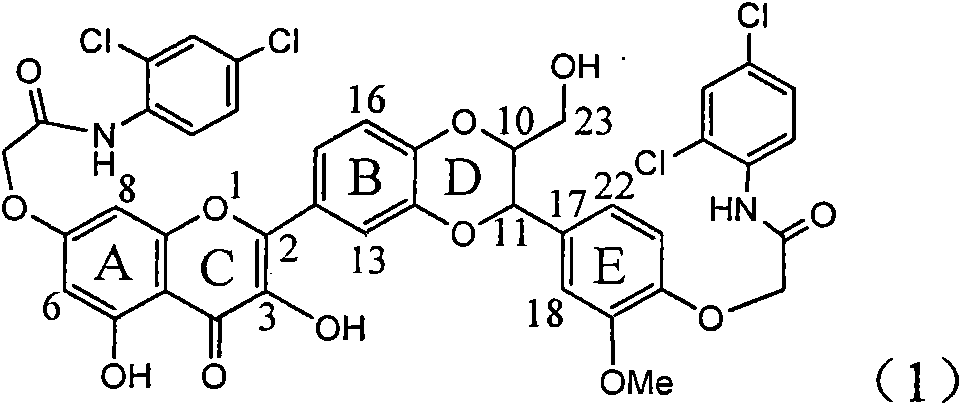
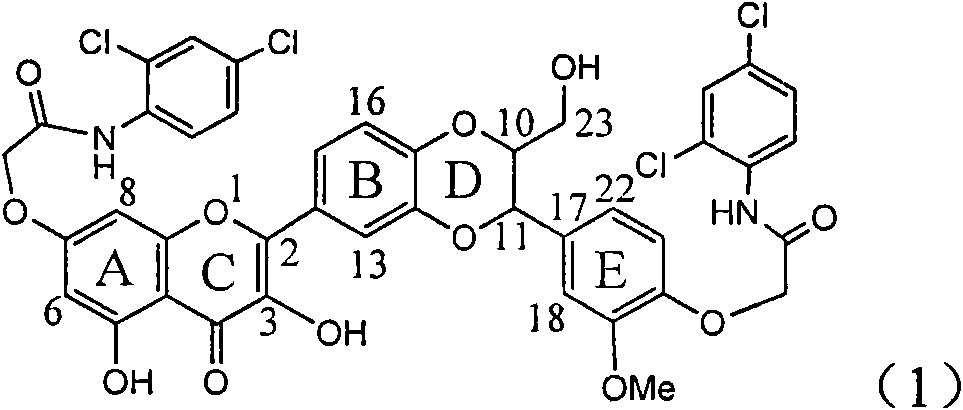
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
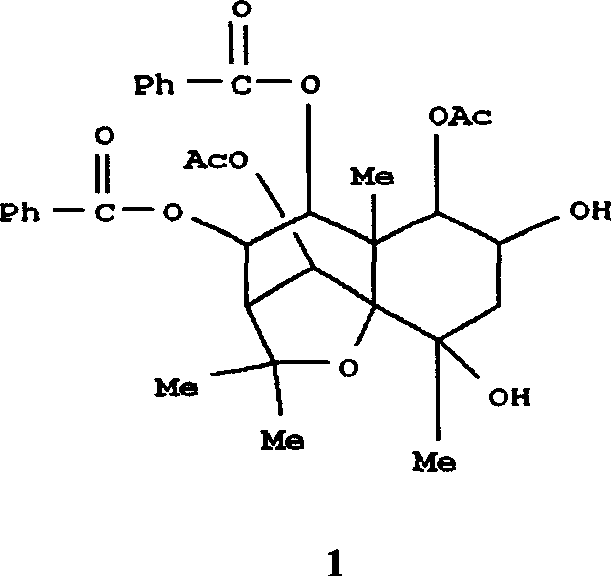
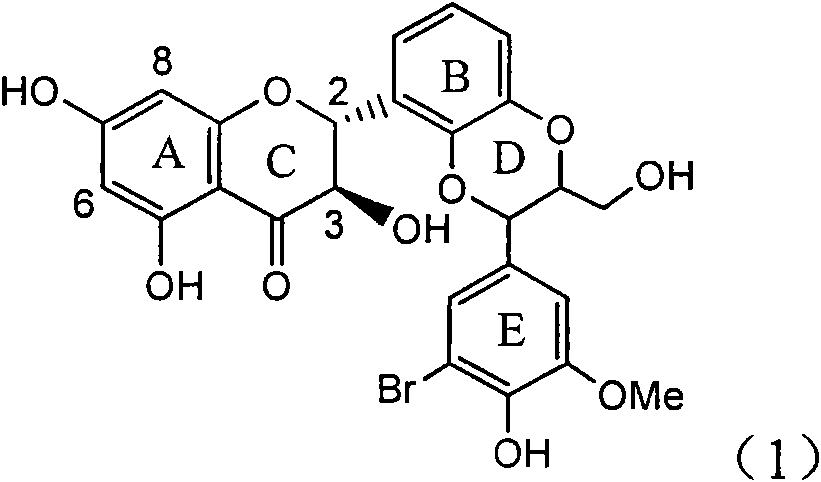
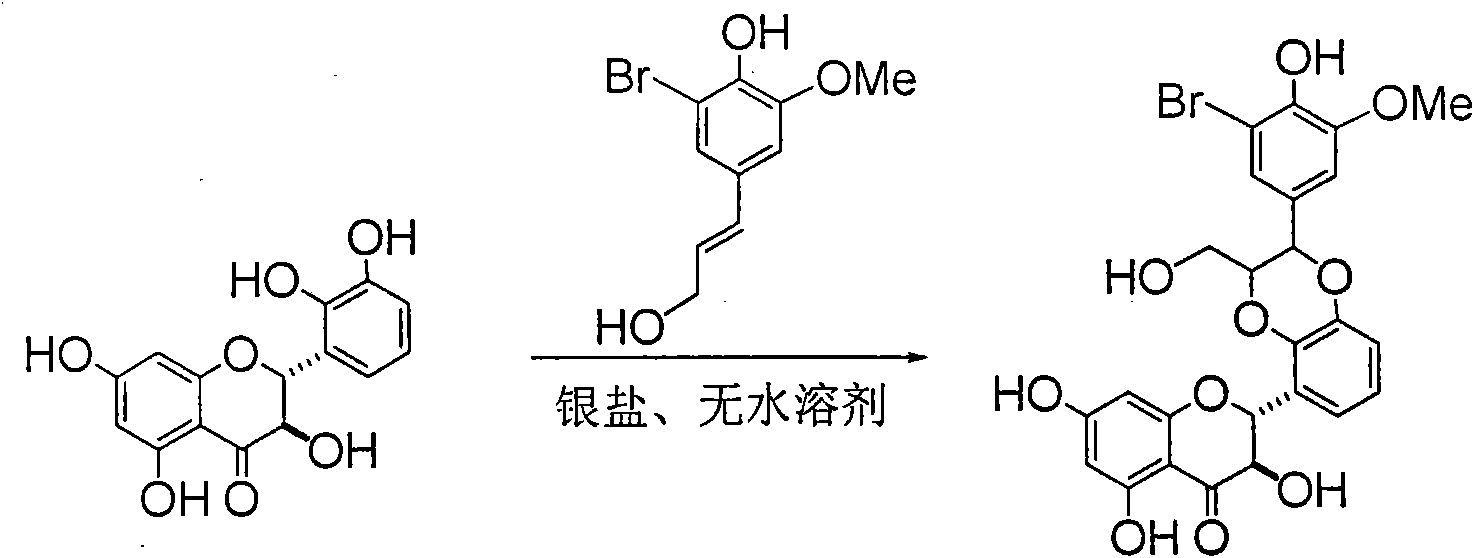
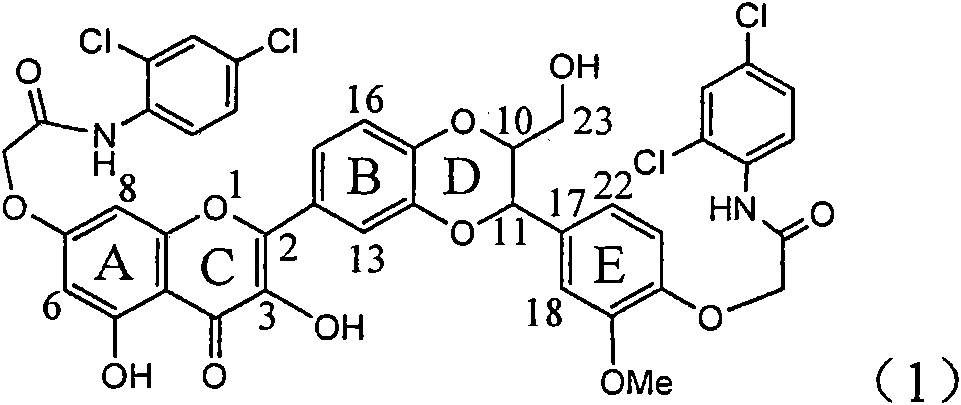
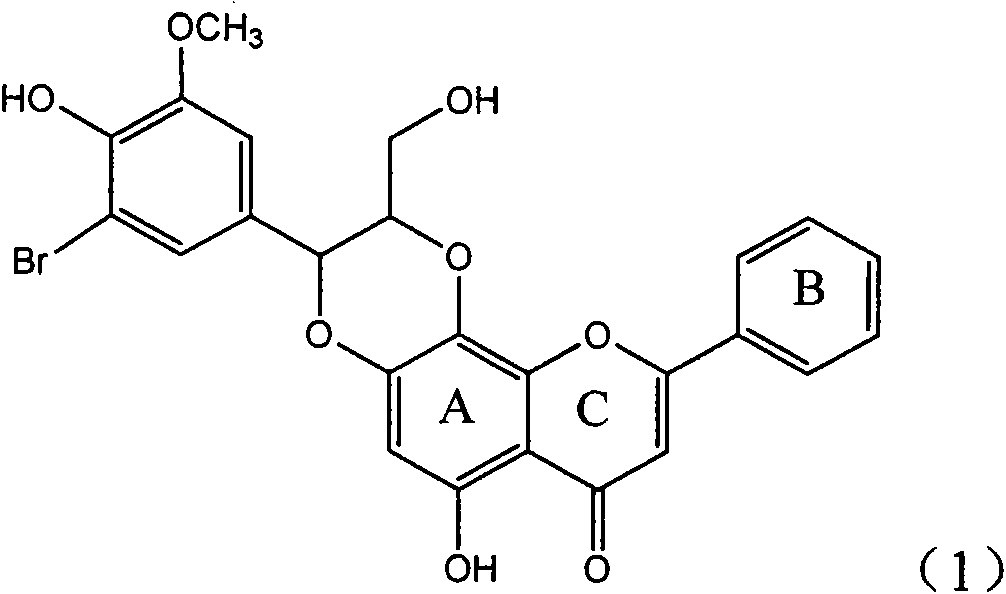
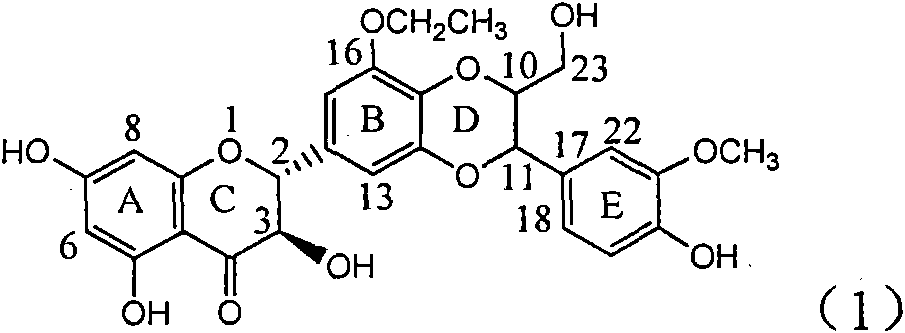
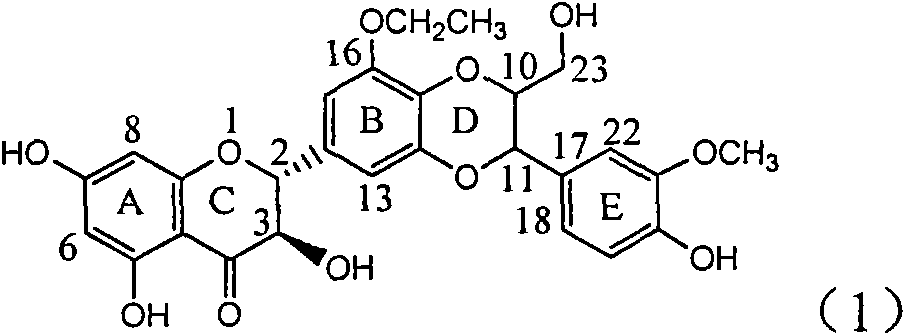
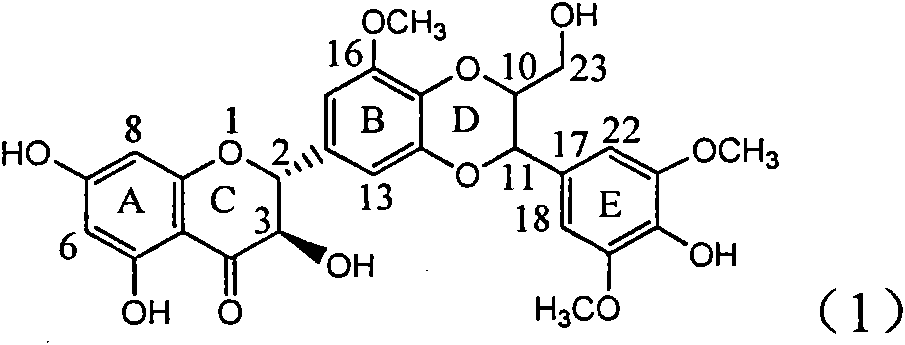
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
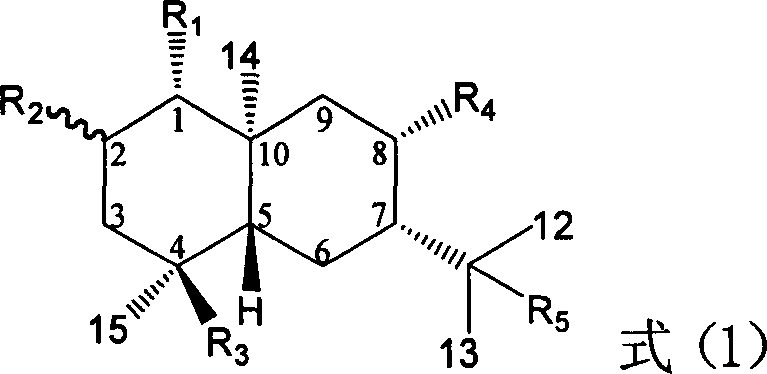
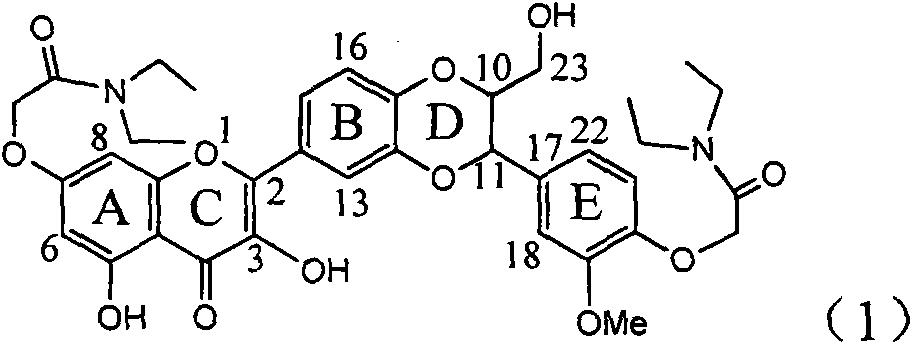
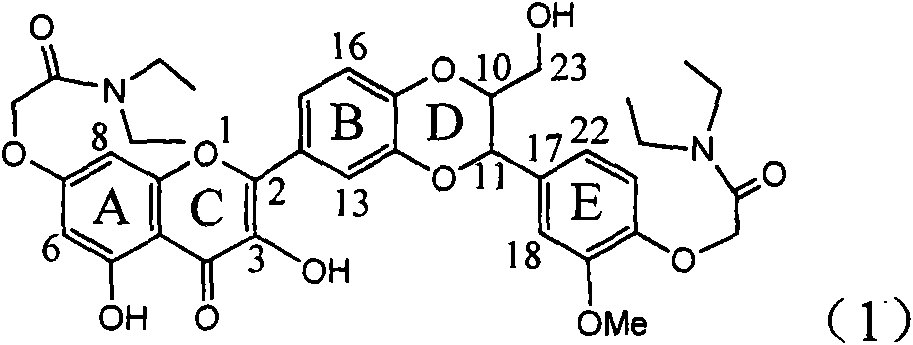
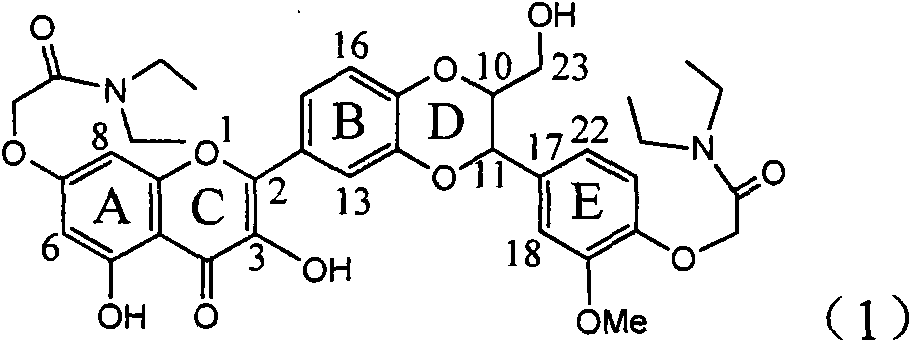
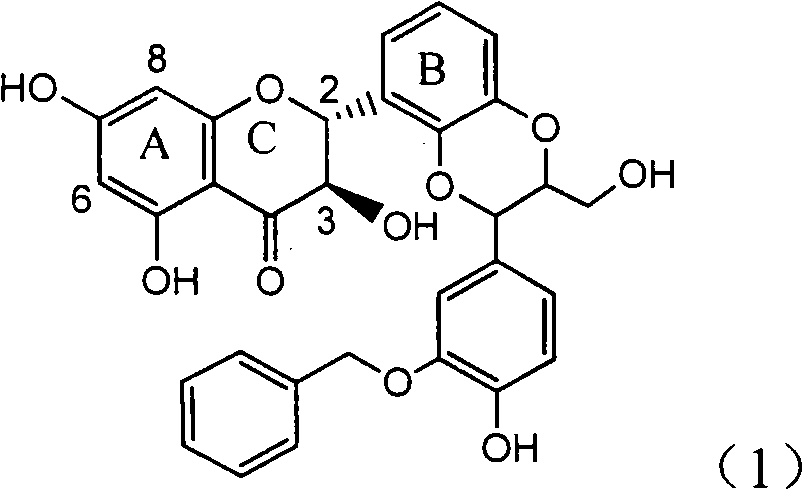
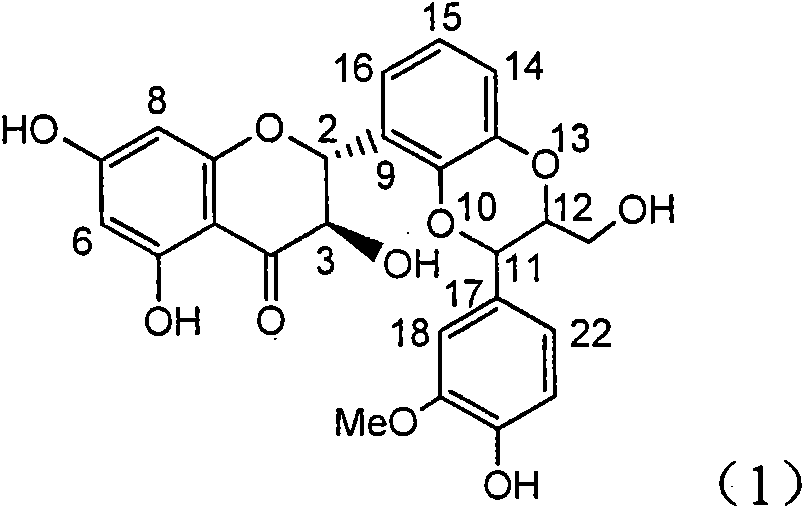
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
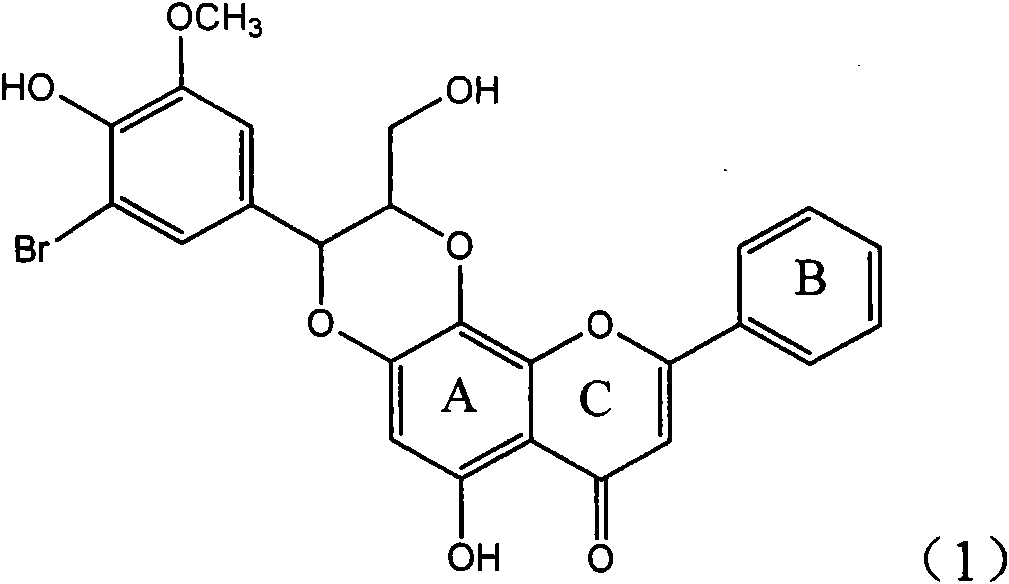
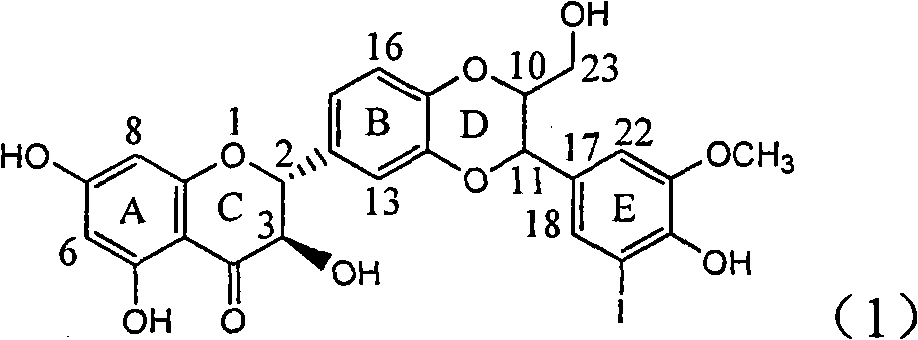
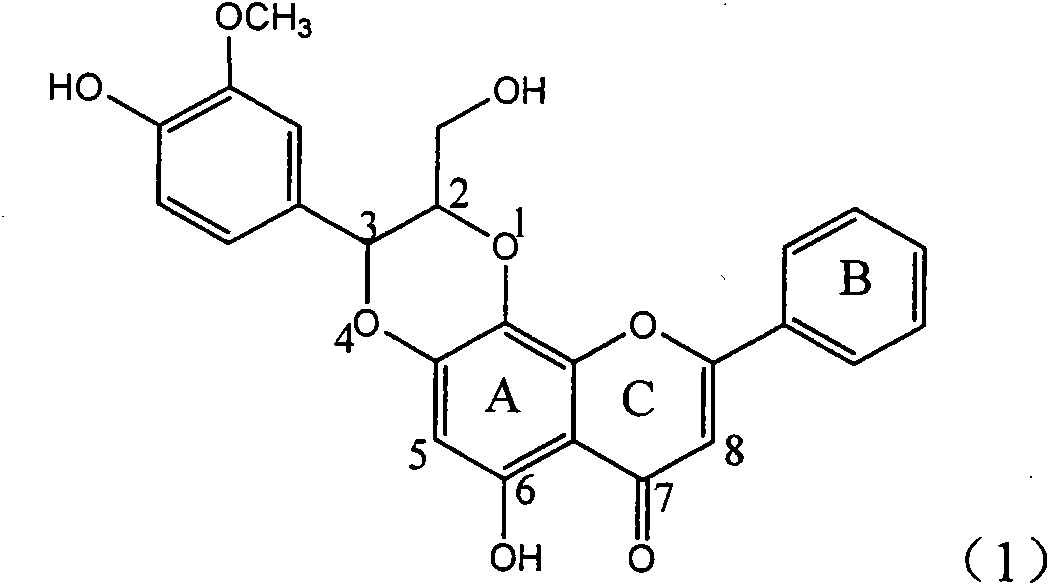
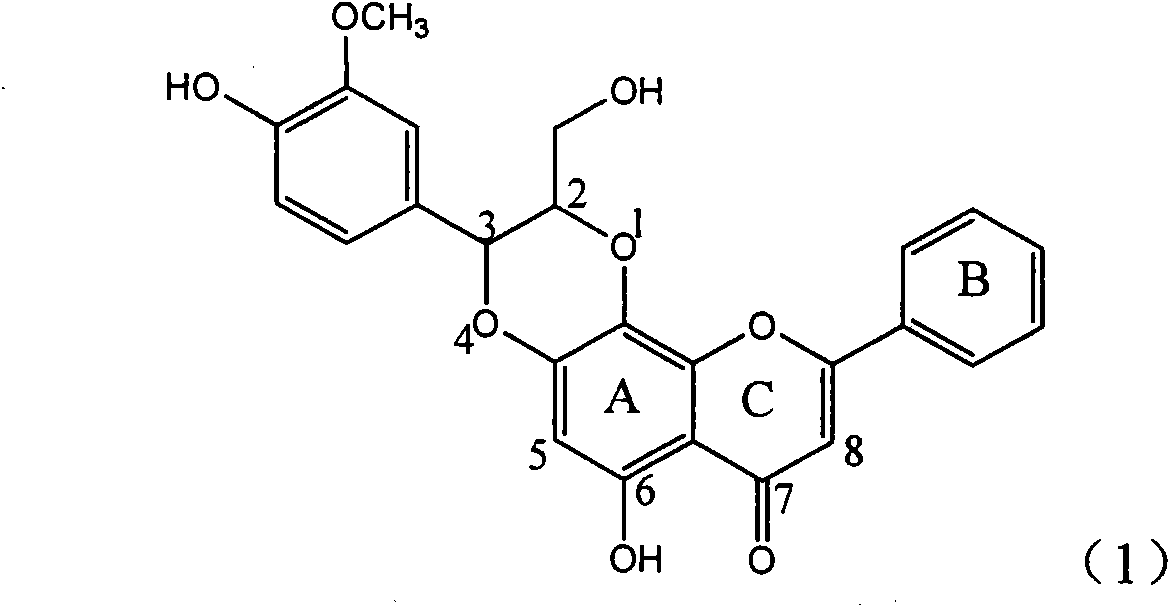
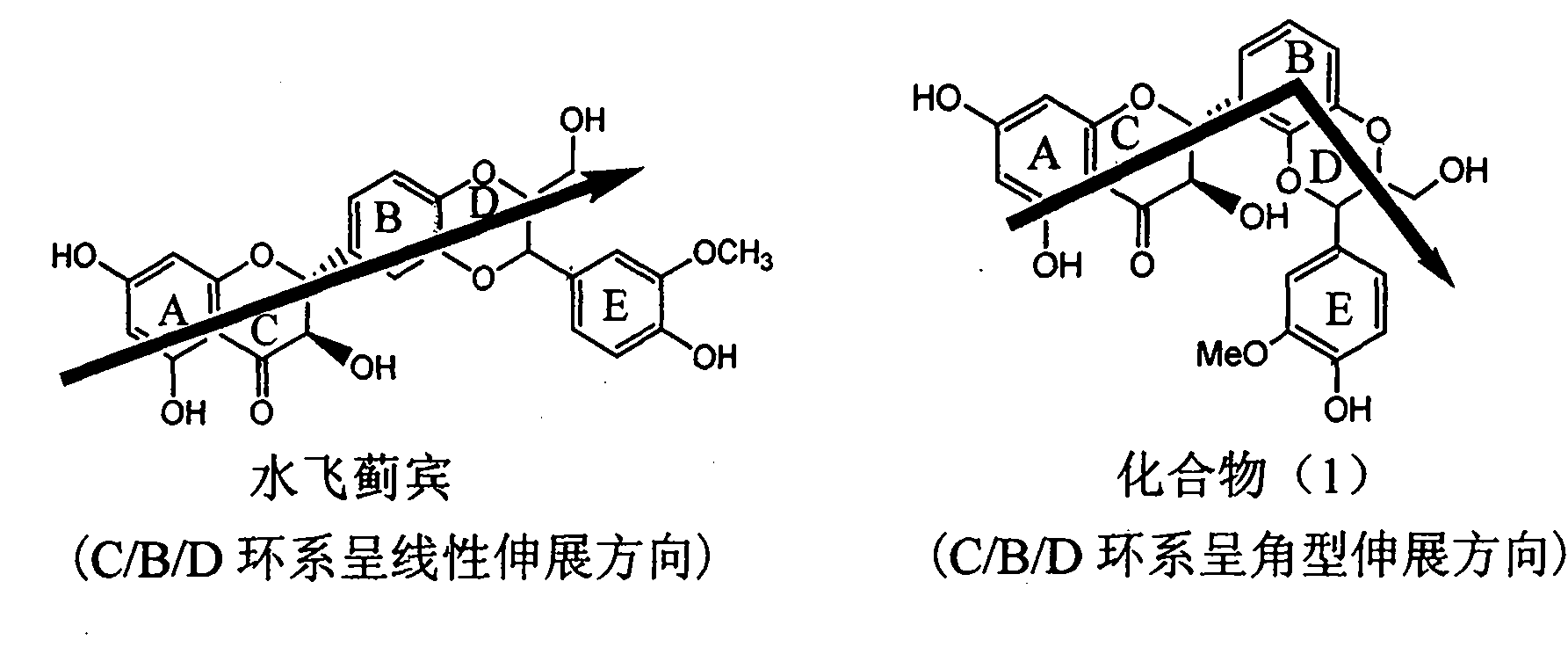
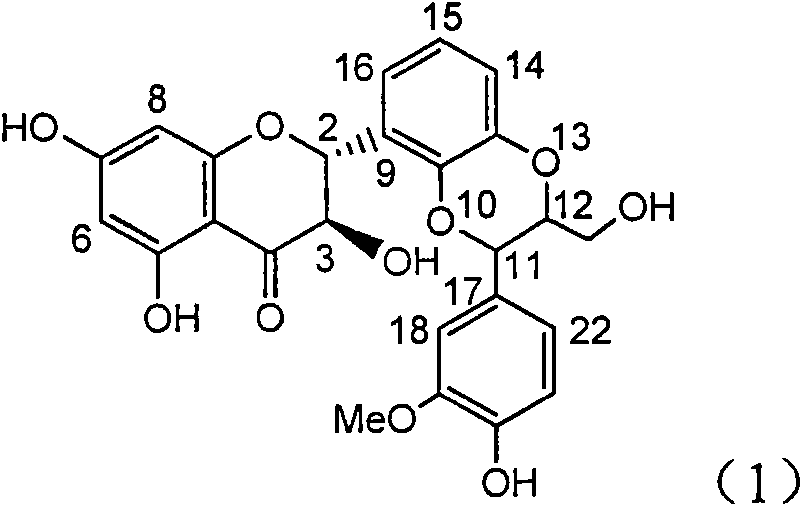
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
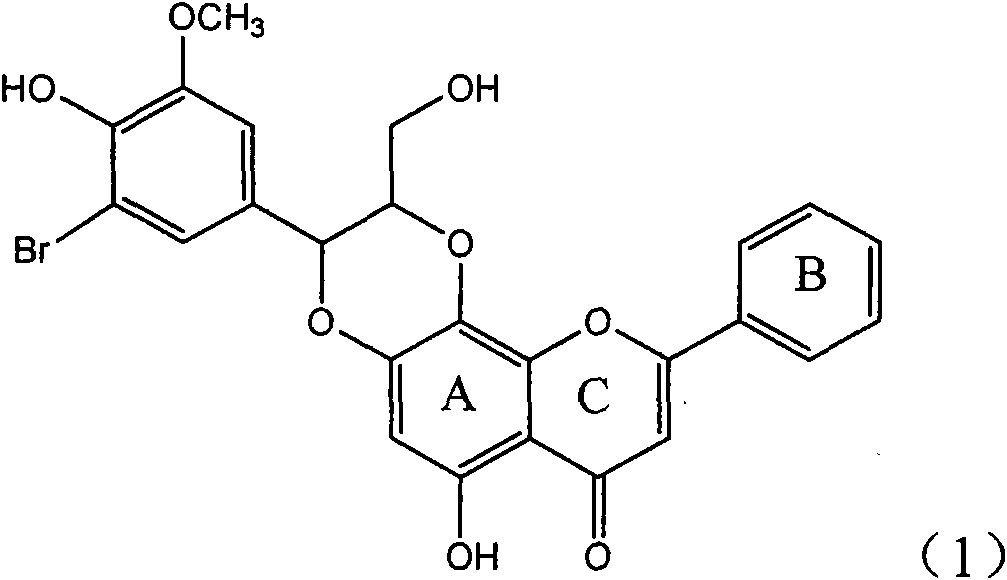
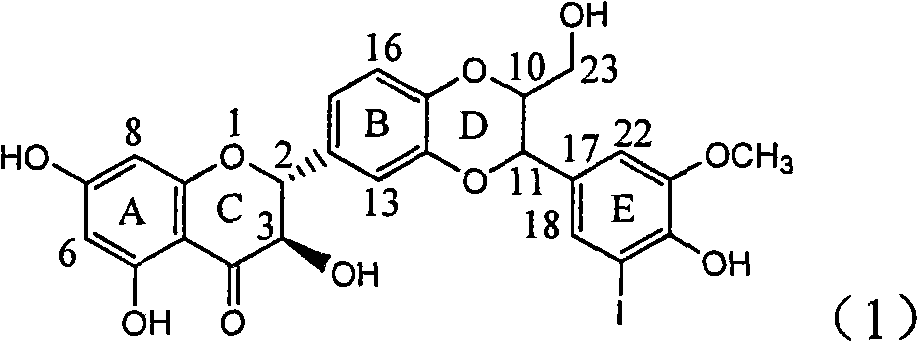
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
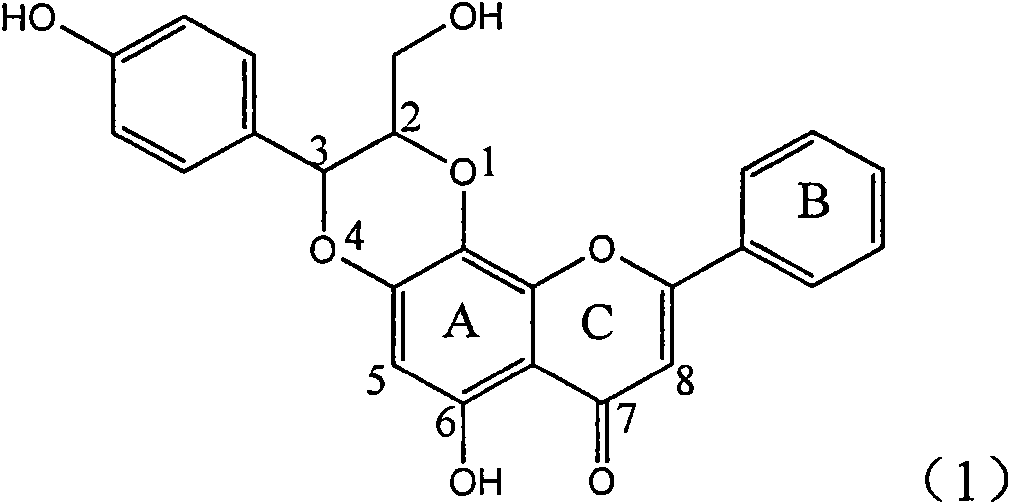
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
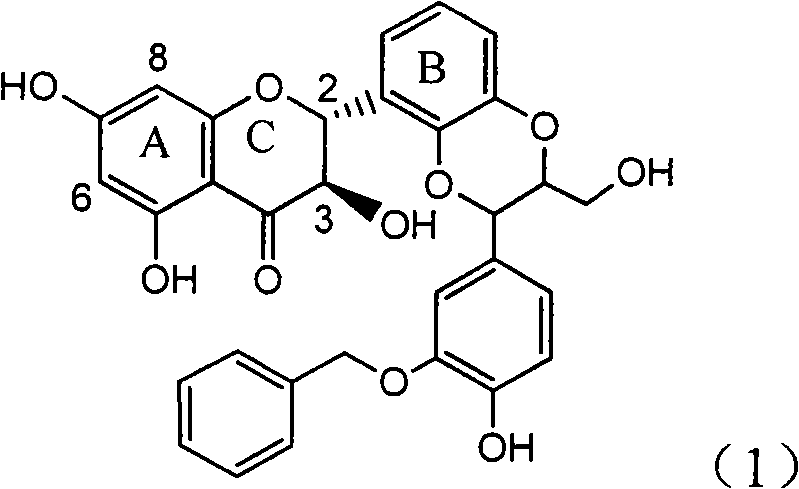
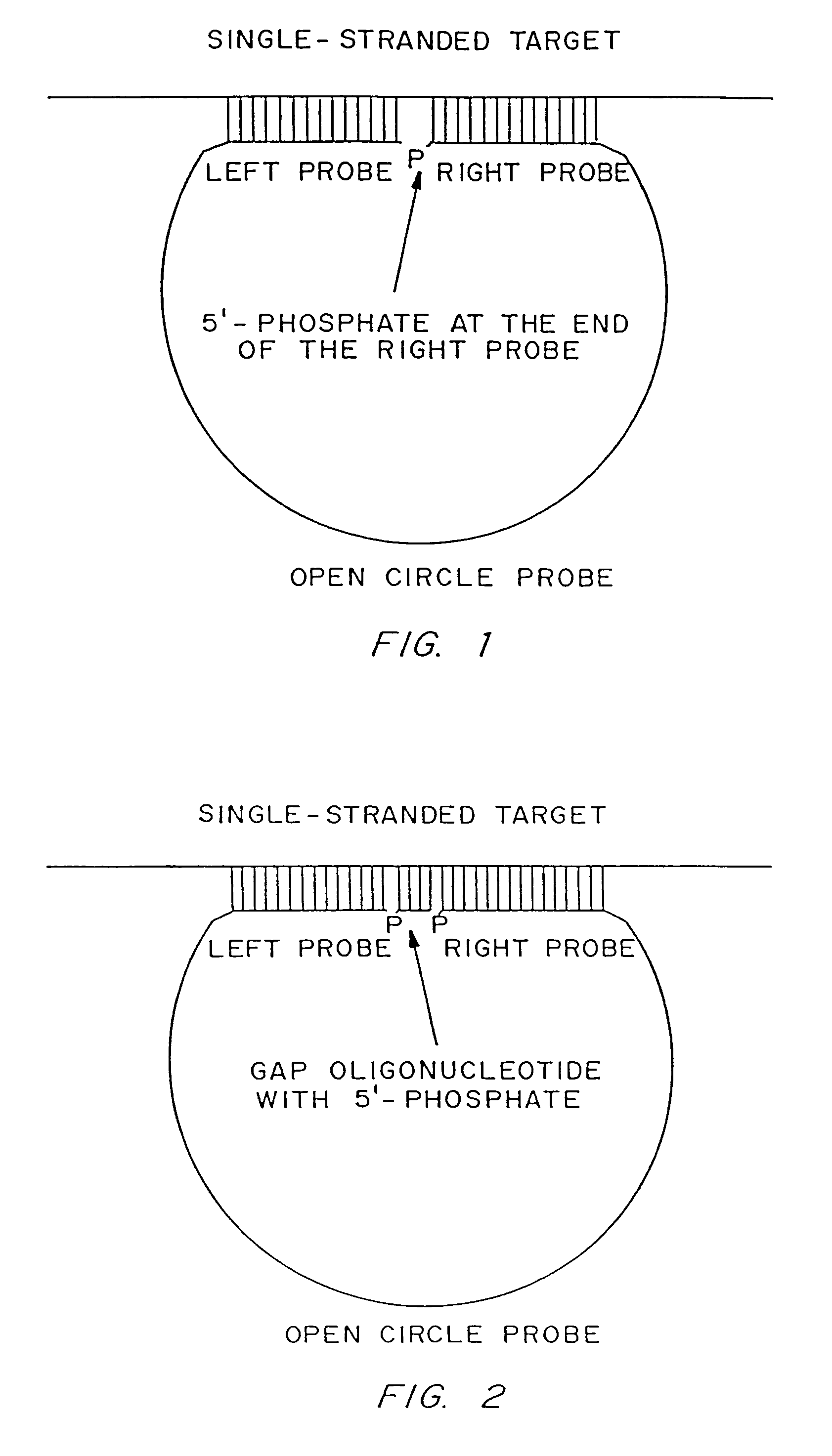
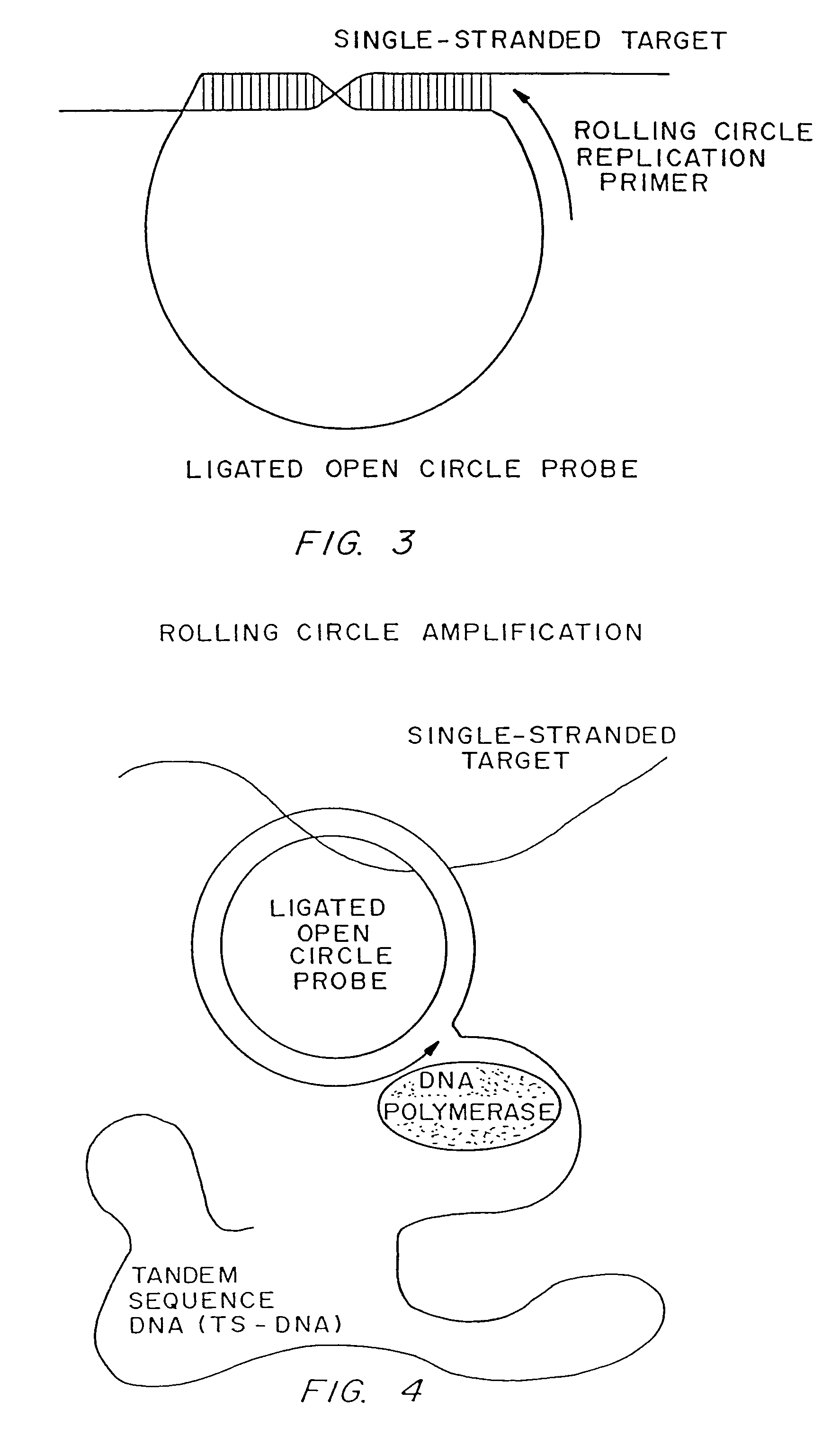
Rolling circle replication reporter systems
InactiveUS7618776B2Consistent outputAmplification less complicatedMicrobiological testing/measurementFermentationMultiplexNucleotide
Disclosed are compositions and a method for of amplifying nucleic acid sequences useful for detecting the presence of molecules of interest. The method is useful for detecting specific nucleic acids in a sample with high specificity and sensitivity. The method also has an inherently low level of background signal. A preferred form of the method consists of a DNA ligation operation, an amplification operation, and a detection operation. The DNA ligation operation circularizes a specially designed nucleic acid probe molecule. This operation is dependent on hybridization of the probe to a target sequence and forms circular probe molecules in proportion to the amount of target sequence present in a sample. The amplification operation is rolling circle replication of the circularized probe. A single round of amplification using rolling circle replication results in a large amplification of the circularized probe sequences. Following rolling circle replication, the amplified probe sequences are detected and quantified using any of the conventional detection systems for nucleic acids such as detection of fluorescent labels, enzyme-linked detection systems, antibody-mediated label detection, and detection of radioactive labels. Because, the amplified product is directly proportional to the amount of target sequence present in a sample, quantitative measurements reliably represent the amount of a target sequence in a sample. Major advantages of this method are that the ligation step can be manipulated to obtain allelic discrimination, the DNA replication step is isothermal, and signals are strictly quantitative because the amplification reaction is linear and is catalyzed by a highly processive enzyme. In multiplex assays, the primer oligonucleotide used for the DNA polymerase reaction can be the same for all probes. Also described are modes of the method in which additional amplification is obtained using a cascade of strand displacement reactions.
Owner:YALE UNIV
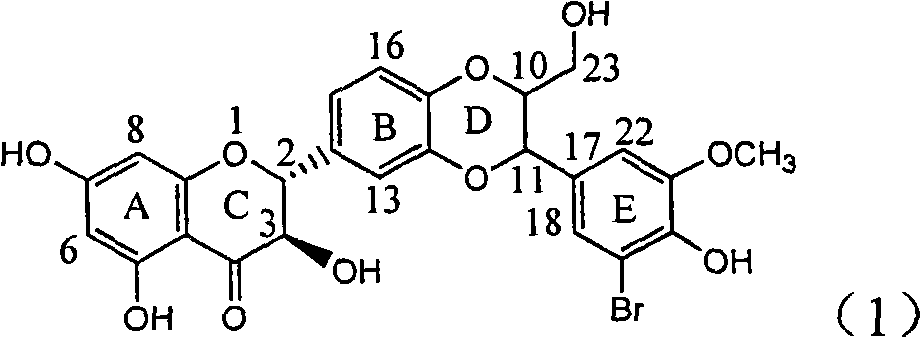
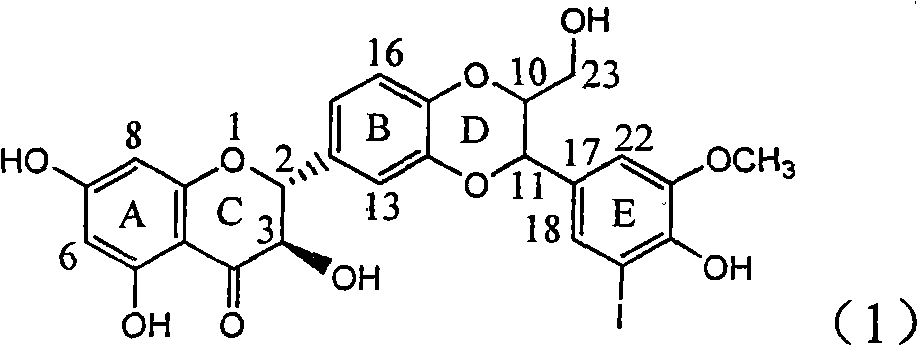
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
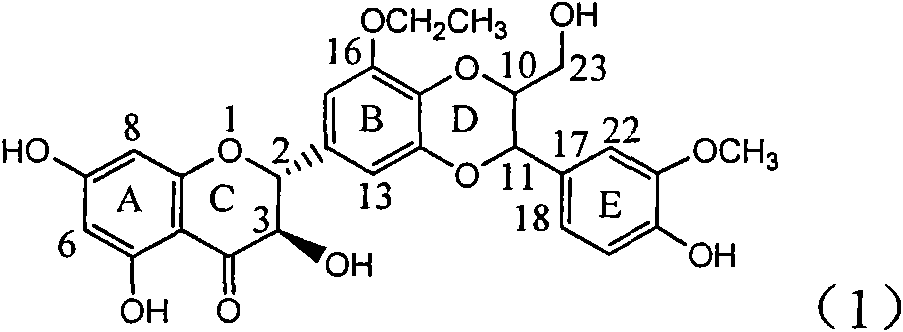
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
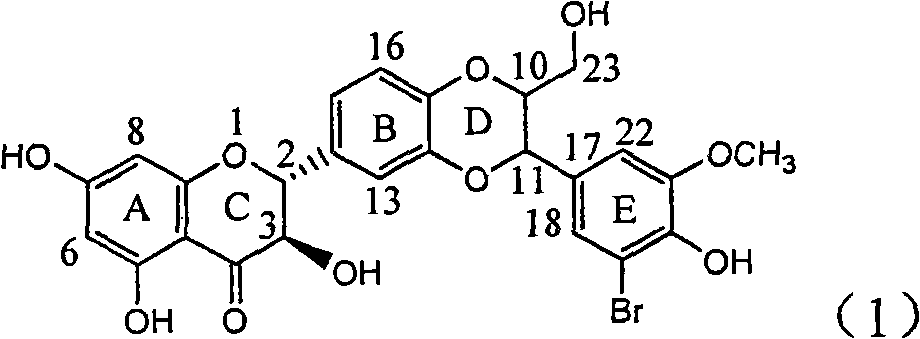
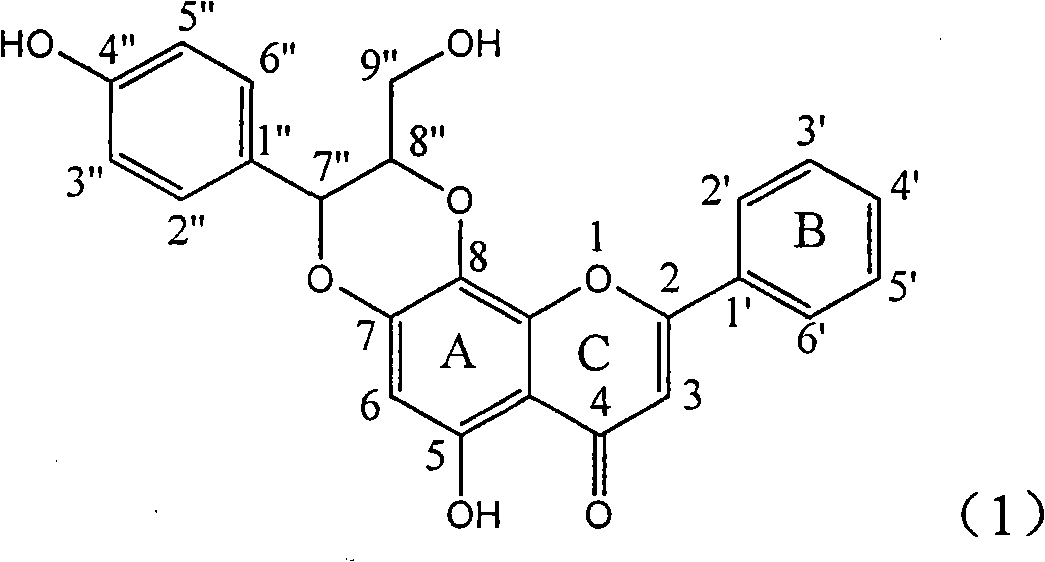
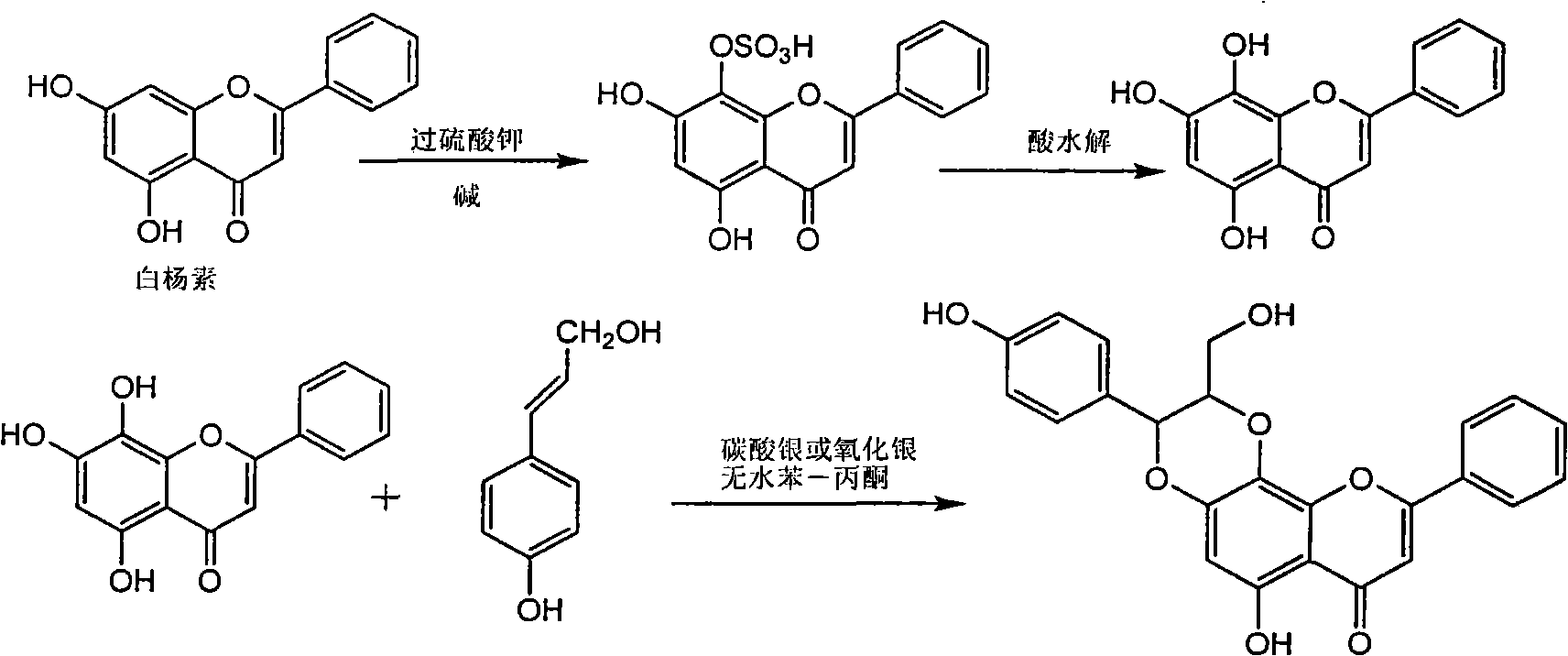
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B
InactiveCN101953828AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsLignanInterferon alpha
The invention relates to application of an angle flavonoids lignan to preparation of medicaments for treating viral hepatitis B, in particular to application of the angle flavonoids lignan or medicinal salts thereof to preparation of medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating HBV infection diseases. The flavonoids lignan can exactly inhibit HBV DNA activity; the inhibition activity of the flavonoids lignan with high dosage (20 microgram / ml) to the HBV DNA replication is 189 percent higher than that of alpha-interferon with the maximum concentration of 10,000 unit / ml; and the flavonoids lignan belongs to a strong-effect non-nucleosides inhibition HBV natural product. The pharmacological results show that the angle flavonoids lignan or the medicinal salts thereof can be expected to be used for preparing the medicaments for inhibiting hepatisis B virus (HBV) DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Corynebacterium glutamicum genes encoding proteins involved in DNA replication, protein synthesis, and pathogenesis
InactiveUS20060269975A1Efficient producerImprove efficiencyBacteriaSugar derivativesAntisense nucleic acidGenetic engineering
Isolated nucleic acid molecules, designated RRP nucleic acid molecules, which encode novel RRP proteins from Corynebacterium glutamicum are described. The invention also provides antisense nucleic acid molecules, recombinant expression vectors containing RRP nucleic acid molecules, and host cells into which the expression vectors have been introduced. The invention still further provides isolated RRP proteins, mutated RRP proteins, fusion proteins, antigenic peptides and methods for the improvement of production of a desired compound from C. glutamicum based on genetic engineering of RRP genes in this organism.
Owner:BASF AG
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
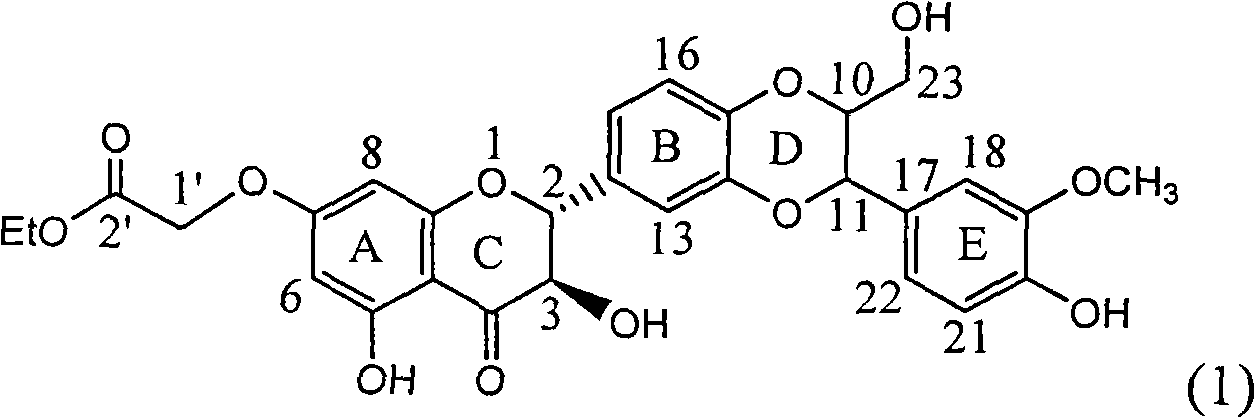
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com